Pharmacological Effects of Moringa oleifera (Lam.) Leaves Powder in the Treatment of Anaemia in Children Aged from 6 to 30 Months in Lissezoun in Central Benin
Received: 08-Dec-2017 / Accepted Date: 29-Jun-2018 / Published Date: 07-Sep-2018 DOI: 10.4172/2168-9652.1000244
Keywords: Center-benin; Infant; MoLP; Treatment of anaemia
Introduction
Malnutrition and associated deficiencies, particularly anemia, are one of the biggest public health problems in the poorest areas of developing countries. For WHO [1,2], anaemia is a widespread public health problem with major implications for human health and socio-economic development. It is associated with an increased risk of mortality in young children. Children with anaemia have a 4.3 times greater risk of death than non-anaemic children [3]. In tropical countries, this is mainly due to the fact that the local diet contains inhibitory factors for intestinal absorption of iron, so that the amounts of iron absorbed available for metabolic purposes are insufficient [4]. Benin is not immune to this global situation because nearly eight out of ten (78%) children have anaemia: 25% in a mild form, 46% in a moderate form and 8% in a severe form. Moreover, the disparities according to the place of residence have proved to be important; 82% in rural areas compared to 70% in urban areas [5]. The magnitude of the micronutrient deficiency problem and its impact on the health of the individual justifies the implementation of Public health interventions. Thus, programs to be implemented to control micronutrient deficiencies need to integrate different approaches such as supplementation, food diversification and food fortification, followed by comprehensive public health measures and control of infections and pathologies [6,7]. For infants and young children, enrichment of breast milk supplements is effective, but these foods are most often imported and economically inaccessible to populations with limited resources [6]. It would then be interesting to turn to the use of local products and economically accessible to all layers of the population.
Indeed, traditional vegetable leaves are now recognized as allies in the fight against macro and micro nutrient deficiencies although they have long been overshadowed by other green vegetables of European origin such as cabbage and lettuce, which have low nutritional content. One of these traditional vegetable leaves is the Moringa oleifera Lam.
This multi-purpose plant originated from India is produced and used in many African countries (Ghana, Senegal, Malawi), South America (Nicaragua, Bolivia) and in New Zealand. It also continues to be an important food crop in India [7]. For Dansi et al. [8], many vegetables leaves have medicinal properties and can serve as medicines. Their consumption, through the soup, could prevent or treat many diseases as well as nutritional deficiencies. Thus, the leaves of Moringa oleifera are effective against anemia, diabetes and high blood pressure. The infusion of leaves of bissap (Hibiscus sabdariffa) can be used in preventive fight against malaria. However, data on the effect of Moringa oleifera (Lam.) on reducing nutritional problems are not yet complete. This study, which is a first in Benin, has set itself the objective of testing the effectiveness of leaves powder of Moringa oleifera (Lam) in the reduction of anemia in children from 6 to 30 mon.
Population And Methods
Study zone
The intervention took place in the Nutritional Recovery Centers (CRN) in Lissèzoun (Centre-Benin), after an assessment of the nutritional status of children from 6 to 30 mon.
Experimental device: The study was conducted in a real-life setting and included a sample of 84 moderately malnourished children aged from 6-30 mon randomly assigned to two groups: A “control group” of 40 children and a study group of 44 children. The children of the “control” group are subjected to their usual diet, while those of the “Study” group, in addition to the usual diet, are supplemented with Moringa oleifer Leaves Powder (MoLP). The children of the “Study” group receive a quantity of MoLP corresponding to 10 g/dl of the product for a period of six mon fortnightly (stock for 15 days to be given to the mother). The powder is mixed with the ready-to-eat food, in two takings, between 8 and 9 o’clock in the morning and between 5 pm and 6 o’clock in the afternoon. Fifteen of these small bags are packed in a double black biodegradable plastic bag to protect them from light and moisture. Mothers were previously sensitized during the different weighing sessions on the objectives of the study. Local investigators were recruited for ambulatory follow-up of the regular and effective taking of the powder by the targeted children. The follow-up is done three times a week, one of which is an unannounced visit to ensure the effectiveness of the MoLP taking. It is worth noting that the children of 6-23 mon still breast fed.
Subjects and sampling criteria: The study included a sample of 84 moderately malnourished children aged from 6-30 mon. The age of the subjects was estimated from their date of birth as shown on the birth certificate, birth form or health booklet. The written consent of the parents has been received before the children were included in the study sample.
To be included in the study sample, the child should be at the time of recruitment, aged between 6 and 30 mon ± 7 days, be breastfed by his mother, be admitted to one of the Nutrition Recovery Centers ( CRN) with moderate malnutrition (Z-weight-height and height-to-weightbetween 3 and 2). In addition, children should be included in a family not planning a movement or trip more than one week throughout the study period. In addition, all children with chronic diseases such as renal insufficiency, diabetes, bronchopneumonia, homozygous hemoglobinopathies, etc. are excluded from the study. It should be mentioned that at the beginning of the study the size of the two samples was of 88 but the data were analyzed for 84 individuals because there have been four cases of “lost sight”. Among the “lost sight” there have been two children whose parents were assigned and two abandoned because the children got recovered.
Blood tests: The blood test was made to appreciate the anemia within the subjects. The subjects’ blood has been recovered in EDTA tubes with anticoagulant. The collection of 3 to 4 cubic cm of venous blood has been made by syringes with needles either at the level of the elbow crease or at the level of the head in the epicranial vein or the femoral vein by higher technicians in pediatrics. The mixture of the anticoagulant with the blood has been done by slow movements of the tube held between thumb and forefinger. No further precautions were taken and the children did not need to be fasting. The samples were placed in coolers containing carboglaces and sent to the laboratory where they have been stored in the refrigerator at 5°C for later use, in particular for NFS. In order to compare the final situation with the initial situation, the children have undergone two blood tests: one at the beginning and the second at the end of the intervention, 6 mon later. From these samples, parameters such as Hemoglobin (Hb), Average Globular Volume (AGV) and Average Global Hemoglobin Concentration (AGHC) have been measured out at the Biomedical Analysis Laboratory (CIRSAR-Cotonou) by an automaton of the type MEDONICTM M-series Lyse/5 L, Thinner/20 L.
Biochemical indicators
Anaemia was defined from the adjusted hemoglobin thresholds for Africans, ie, Hb<10 g/dl for children aged from 12 to 59 mon and Hb<10.5 g/dl for children aged from 5 to 11 yrs [9]. It was characterized as severe, moderate or mild when the Hb values were respectively less than 7 g/dl, 7-8.9 g/dl, and 9-9.9 g/dl, respectively. Or between 9 and 10.4 g/dl (for children between the ages of five and six yrs) [10]. AGHC was used to classify erythrocytes as either hypochromes (AGHC<32 g/l) or normochromes (CGMH ≥ 32 g/l) [9]. The AGV has enabled to distinguish microcytosis (VGM<67 fL for children from 12 to 23 mon, AGV<73 fL for children from 24 to 59 mon and AGV<74 fL for children from five to eight yrs old) Normocytosis (67 ≤ AGV<90 fL, 73 fL ≤ AGV<90 fLet 74 ≤ AGV<90 fL respectively for children from zero to two years, 24 to 59 mon and five to six years) and macrocytosis (AGV ≥ 90 fL) [9]. The threshold of 15.0.103 white globules/mL has been used to define leukocytosis.
Statistical analysis
In order to assess the evolution of anemia in the “Study” group and in the “control” group, two comparisons have been made. On the one hand, the parameters observed in each of the two groups at t=0 mon and at t=6 mon have been compared with the help of a Student t test with matched samples. The values of the two groups were then compared by a Student t test with independent samples in the same software. On the other hand, the percentage of children with anemia and children with leukocytosis between t=0 mon and t=6 mon has been compared between the intervention group and the control group. All tests have been performed with the help of MiniTabversion 14.0 software and the results are considered statistically different for probability values below 5% (p<0.05).
Results
Overall, the analyses have shown that the two groups, initially statistically equal (p>0.05), show significant differences at the end of the study (p<0.05). Table 1.presents the haematological parameters at the beginning and at the end of the intervention, six mon later, of the two groups.
| Parameters | Period | Witness | Study |
|---|---|---|---|
| White Blood cells (x103/mL) | Beginning intervention | 11.7 ± 3.56aA | 11.5 ± 2.72aA |
| End intervention | 12.6 ± 3.43aA | 13.2 ± 2.18aB | |
| Hemoglobin Level (g/dL) | Beginning intervention | 10.44 ± 1.25aA | 10,27 ± 1.33aA |
| End intervention | 9.82 ± 1.15aB | 11.86 ± 1.16bB | |
| AGHC (g/L) | Beginning intervention | 32.02 ± 1.33aA | 33.72 ± 1.22bA |
| End intervention | 26.87 ± 1.86aB | 33.78 ± 0.91bA | |
| AGV (fL) | Beginning intervention | 77.48 ± 6.17aA | 74.19 ± 6.28bA |
| End intervention | 75.38 ± 5.82aB | 76.59 ± 6.71aB |
Table 1: Hematologic parameters over the period of the intervention
For a parameter, the values assigned to the same lower-case letter on the same line are not significantly different from the 5% threshold.
For a parameter, the values assigned to the same uppercase letter in the same column are not significantly different at the 5% threshold.
White blood cells and hemoglobin levels
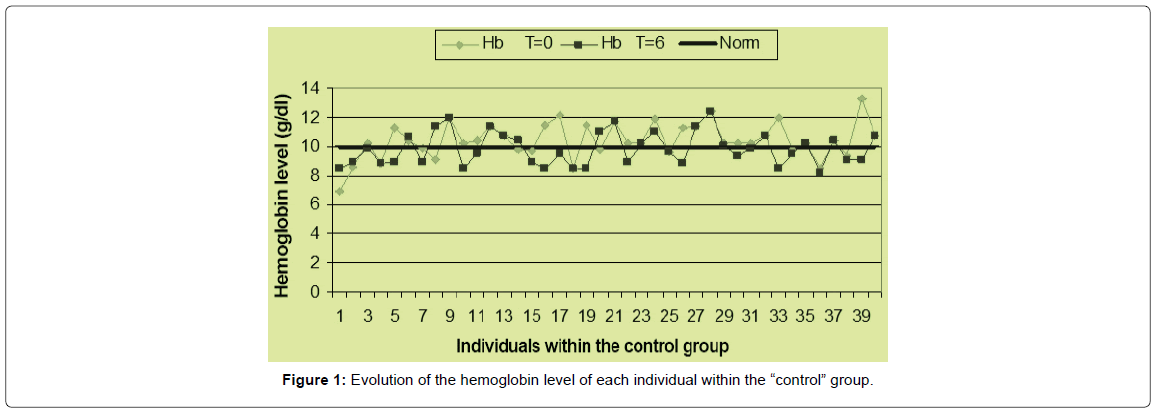
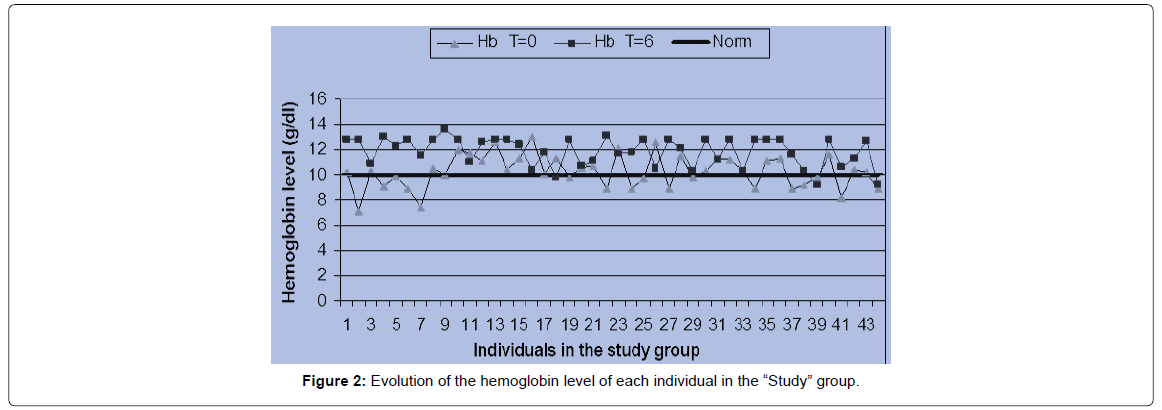
A comparison of the average number of white blood cells in the subjects has revealed a significant increase in the production of white blood cells in the MoLP supplementation group (p=0.001). In the “control” group, between the beginning and the end of the intervention, there has been no significant increase (p=0.151) in the production of white blood cells (Figures 1 & 2).
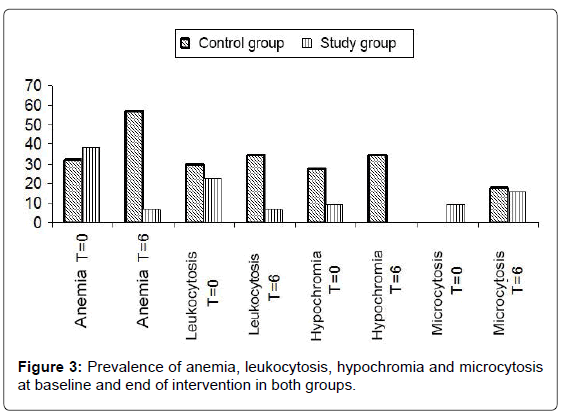
In the case of Hb, statistical analyzes show that the hemoglobin values in the children receiving supplementation with MoLP have showed a very significant increase (p=0.000). On the other hand, in the “control” group, between the start of the intervention and the 6th mon, the hemoglobin in children has decreased significantly (p=0.01). Thus, there has been an increase in hemoglobin of more than 13% in the “Study” group and a decrease in hemoglobin of more than 6% in the “control” group. This leads to a decrease in the prevalence of anaemia in the “Study” group and an increase in this prevalence in the “control” group (Figure 3).
A graphical representation (Figures 1 & 2) of the pre- and post-intervention situation in each group has reveals that most individuals in the “Study” group have their hemoglobin measured at the end of the intervention above the measured value at the start of the procedure. On the other hand, it can be seen that most of the individuals in the witness group have a hemoglobin at the end of the study less than or equal to the hemoglobin level at the start of the study. These different tendencies result in spacing between the two curves in the first case and a superposition of the two curves in the second case. At the beginning of the operation, the mean value of the hemoglobin level in the “Study” group was 10.27 ± 1.33 g/ dl with individual levels varying between 7.1 and 13 g/dl. At the end of the sixth mon, average hemoglobin of 11.86 ± 1.16 g/dl was obtained with variations ranging from 9.2 to 13.1 g/dl. In the control group, the hemoglobin level at baseline was 10.44 ± 1.25 g/dl on average with a change from 6.9 to 13.3 g/dl. This value increased to 9.82 ± 1.15 g/dl with a change from 8 to 12.4 g/dl. In the “Study” group, there was an improvement in the hematological status of the children, whereas in the “control” group, the hematological status of the children deteriorated relatively.
Average globular concentration in hemoglobin (AGCH)
For this parameter and within the “Study” group, there has been no significant improvement in the values (p=0.378) during the intervention period. However, between the beginning of the intervention and the 6th mon, the AGCH of the children in the witness group has decreased significantly (p=0.001). This results in no hypochromic cases in the “Study” group at the end of the procedure and an increase in the prevalence of hypochromia at the end of the study in the “control” group.
Average globular volume (AGV)
Among the children receiving supplementation with MoLP, the AGV values overall improved significantly (p=0.012) during the intervention period. On the other hand, in the “control” group, between the beginning of the intervention and the 6th mon, the children’s AGV has decreased very significantly (p=0.006). At the end of the 6 mon, we noticed that the AGVs of the two groups, which have been initially different (p=0.018), show no differences (p=0.379). These different observations result in an increase in the prevalence of microcytosis (decrease in the volume of red blood cells) in both groups. Indeed, at the beginning of the procedure, no cases of microcytosis were recorded in the “control” group. But at the end of the study, a prevalence of microcytosis was 17.5% in this group. In the study group, the prevalence of microcytosis has increased from 9.09% to 15.91% at the end of the intervention.
Prevalence of anemia, leukocytosis, microcytosis and hypochromia
The comparison of the number of white blood cells of each subject with the norm shows us that the prevalence of hyperleucocytosis has increased in the “control” group whereas it was reduced in the “Study” group.
In addition, referring to adjusted hemoglobin thresholds for Africans [9], the individuals in the study have been divided into two categories: anaemic and non anaemic. This distribution has given us at the beginning of the intervention and in the “control” group, an anemia prevalence of 32.5% against a prevalence of 38.64% in the “Study” group. At the end of the intervention, 23 children were anaemic in the “control” group, compared with 3 children who were anaemic in the “Study” group, with respective prevalences of 57.5% and 6.82%. Thus, the number of anaemic children has increased in the “control” group, while in the “Study” group, the number of anaemic children has decreased considerably (Figure 3). In addition, the number of children with leukocytosis has increased in the “control” group, while in the “Study” group there have been a decrease in cases of leukocytosis. Figure 3 shows the prevalence of anemia, hyperleucocytosis, microcytosis and hypochromia.
Discussion
This study assessed the effect of MoLP supplementation in reducing anemia in moderate malnourished children. In general, we can say that a 6-mon supplementation with MoLP has enabled to correct anaemia in children from 6 to 30 mon of age. This improvement results in an increase in hemoglobin and other parameters such as leukocyte level, AGHC and AGV. The values of these parameters, which at the start of the study were lower than the normal values, are at the end of the study, higher than norms in most children who received 10 g of MoLP daily. This confirms not only the improvement in the hematological status of individuals but also the strengthening of their immune systems and therefore better resistance to infections [11,12].These results are in agreement with those of Chwang et al. [13] and Fairweather-Tait [14], which showed that anaemic children have a smaller size and slower growth dynamics. Resistance to infections and immune competence are also impaired for these children [15-17].
This suggests, on the one hand, that MoLP not only provides an iron supplement to children’s diets, but also iron activators, albeit in small quantities. This improves the bioavailability of iron contained in the diet of children undergoing supplementation. Indeed, anaemia is a condition characterized by a reduction in the number of red blood cells and a weakening of the concentration of hemoglobin in the blood. It is usually the result of a deficiency in iron, vitamin B12. Although anaemia can be caused by parasitoses, haemorrhages, congenital ailments or chronic diseases, it is most often due to a deficiency in food, including iron deficiency [10,18]. The presence of iron absorption inhibitors largely explains the low bioavailability of dietary iron in tropical regions [19] and low-protein cereal and legume diets Animals and vitamin C present a low availability where iron absorption is about 5%. This is the typical food ration of many developing countries (DCs), particularly in Africa [4]. The contribution iron of foods from developing countries does not, therefore, cover the iron requirements of populations. Inhibitors are very present in foods of vegetable origin which are most often the basis of diet for the poorest populations in developing countries and or the absence of promoters of absorption such as vitamin C and Animal products. It was noted that the incorporation of MoLP in processed foods such as fermentation or germination increases the nutritional benefits of MoLP as one way to improve the bioavailability of iron and zinc is to reduce Phy/Zn and Phy/Fe molar ratios that minimize phytate levels [2,20].
On the other hand, degradation of the hematological status of children in the control group could be attributed to parasitic infections. Children in both groups during the intervention period (6 mon) have not undergone any treatment and were therefore vulnerable to infestations. Indeed, parasitic infections affect the nutritional status of the host and cause a delay in the growth of children. In tropical countries, anaemia is also caused by a number of other factors such as parasitic, infectious, genetic and nutritional [17,18,21,22]. Hookworm and malaria are major causes of anaemia in areas where these endemic parasitoses occur (Dillon, 2000). In addition, studies in Benin on nursery children have shown that 57% of these children were anaemic (Hb<11 g/dl) and severely infected with parasites such as Ascaris lumbricoides (53%), Trichuristrichiura (51%) and hookworms (12%) [23]. This may show the relationship between haematological status and parasite status of children and probably the effect of MoLP on parasites. This is because, although the children of the two groups were not immune to parasites during the study period, those in the “Study” group show a better haematological status. Previous studies show that saponin, steroids, carbohydrates, alkaloids, tannins, proteins and flavonoids are present in the ethanolic extracts of the leaves of M. oleifera [2]. In addition, ethanolic extracts of M. oleifera leaves have an anthelmintic activity which results in a loss of motility of these worms within 6 to 15 min [24]. However, these different hypotheses deserve to be investigated and further investigated by subsequent studies, including studies of protein markers of the inflammatory state.
It should be mentioned that the prevalence of anaemia observed in both groups at the start of the intervention is slightly below the WHO threshold of 40%, defining severe anaemia in a population [1,2]. These observed prevalences could be explained by the positive effects induced by nutritional recovery because 73.8% of children from 6 to 59 mon of Zou department in Benin are anaemic according to INSAE and Macro Int. Inc. [5]. At the end of the intervention, in the “Control” group, a prevalence of 57.5% has been observed, which exceeds the WHO threshold of 40%. It could then be said that children once in their homes have still been subjected to anaemia risk factors and that mothers do not or cannot put into practice the notions received in the nutritional recovery center. This would probably lead to degradation of haematological status [25,26].
Although hemoglobin is a commonly used measure of anaemia in a population, the concomitant measurement of erythrocyte indices provides guidance on the origin of anaemia. Among the globular indices, the average globular volume and the average globular hemoglobin concentration are the two most sensitive indices of iron deficiency. The decrease in average globular volume that occurs in parallel with anaemia is a delayed phenomenon accompanying iron deficiency [1,27].Thus, the decrease in this parameter in the control group and the prevalence associated with it confirm that these anaemias are probably due to an iron deficiency. Our results show that a non-negligible place should be given to other potential causes of anaemia. Indeed, for Sanou et al. [8], the level of hemoglobin taken alone gives the magnitude of the problem of anaemia, but does not provide sufficient information on the etiological factors that can guide the development of effective interventions or detect the various risks in order to initiate appropriate preventive actions. At the end of the procedure, 6 mon after recovery, 1 over 6 children, all groups combined, showed hypochromia. This result, while not giving any information on the proportion of anaemia attributable to iron deficiency, suggests that onesixth of the anaemias would have other origins.
Conclusion
This study reveals that MoLP can effectively reduce anaemia in children from 6 to 30 mon of age because supplementation has improved the hemoglobin level of children in this age group. MoLP could therefore be considered as a nutritional supplement in poor populations to prevent anaemia in children. However, the mechanism of the pharmacological effect of MoLP on erythropoiesis remains to be elucidated. In addition, it would be interesting to carry out further investigations and to deepen this study so that the impact of daily consumption of MoLP on iron status is known. This will provide sufficient data on the etiological factors that will guide the development of effective interventions or early detection of the various risks in order to initiate appropriate preventive actions.
References
- Houndji BVS. (2013). Use of Moringa oleifera leaf powder (Lam.) In the nutritional recovery of moderate acute malnourished children in the district of Lissèzoun in Center-Benin PhD, University of Lomé, Togo: 128.
- Jones G (2003) How many child deaths can we prevent this year? Lancet 362: 65-71.
- Dillon JC (2000) Prevention of iron deficiency and iron deficiency anemia in a tropical environment. Med Trop 60: 83-9.
- INSAE Macro International Inc. (2007) Benin Demographic and Health Survey (EDSB-III). http://www.measuredhs.com/pubs/pub_details.cfm?ID=733&ctry_id=52&SrchTp=ctry
- Berger J, Dillon JC (2002) Strategies for controlling iron deficiency in developing countries. Cah. Health 12: 22-30
- Houndji BVS, Ouetchehou R, Londji SBM, Amouzou KSE, Yehouenou B, et al. (2013) Microbiological and physicochemical characterization of leaf powder of Moringa oleifera (Lam.), A traditional leaf vegetable in Benin. Int J Biol Chem Sci 7: 75-85.
- Sanou D,Turgeon-O’Brien H, Desrosiers T (2008) Prevalence and non-dietary determinants of anemia and iron deficiency in orphans and vulnerable preschool children in Burkina Faso. Clin Nutr Metab 22: 10-19.
- Nestel P (2002) Adjusting hemoglobin values in program surveys. Int nutr anemia consult group (INACG): 6.
- DeMaeyer EM, Dallman P, Gurney JM, Hallberg L, Sood SK,et al. (1989) Preventing and controlling iron deficiency anemia through primary health care: A guide for health administrators and programme managers. WHO/Geneva; 61.
- Mistry AN, Brittin HC, Stoeker BJ (1998) Availability of iron from cooked in an iron utensil determined by an in vitro method. J Food Sci 53: 1546-1558.
- Guiro AT, Galan P, Cherouvrier F, Sall MG, Hercberg S (1992) Iron absorption from african pearl-millet and rice meals. Nutr Res 11: 885-893.
- Chwang L, Soemantri AG, Pollit E (1988) Iron supplementation and physical growth of rural indonesian children. Am J Clin Nutr 47: 496-501.
- Fairweather-Tait SJ (1992) Iron deficiency in infancy: Easy to prevent- or is it? Eur J Clin Nutr 46: 9-14.
- Berger J, Schneider D, Dyck JL, Joseph A, Aplogan A, et al. (1992) Iron deficiency, cell-mediated immunity and infection among 6-36 month old children living in rural Togo. Nutr Res 12: 39-49.
- Walter T, Olivares M, Pizarro F, Munoz C (1997) Iron, anemia, and infection. Nutr Rev 55: 111-124.
- Houndji BVS, Bodjrenou SF, Londji SBM, Ouetchehou R, Acakpo A, et al. (2013b) Improving the nutritional status of children aged 6 to 30 months in Lissèzoun (Central Benin) with Moringa oleifera leaf powder (Lam.) Int J Biol Chem Sci 7: 225-235.
- Hallberg L, Björn-Rasmussen E (1981) Measurement of iron from meals contamined with iron. Am J Clin Nutr 34: 2808-2815.
- Hallberg L (1981) Bioavailability of dietary iron in man. Annu Rev Nutr 1: 123-147.
- Solomons NW (1993) Pathways to impairment of human nutritional status by gastrointestinal pathogens. Parasitol 107: 19-35.
- Oikeh SO, Menkir A, Maziya-Dixon B, Welch R, Glahn RP (2003) Assessment of concentrations of iron and zinc and bioavailable iron in grain of early maturing tropical maize varieties. J Agri Food Chem 51: 3688-3694.
- Dossa RA, Ategbo EA, de Koning FI, van Raaij JM, Hautvast JG (2001) Impact of iron supplementation and deworming on growth performance in preschool Beninese children. Eur J Clin Nutr 55: 223-228.
- Forrester JE, Bailar III JC, Esrey SA, José MV, Castillejos BT, et al. (1998) Randomised trial of albendazole and pyrantel in symptomless trichuriasis in children. Lancet 352: 1103-1108.
- Tshikuka JG, Gray-Donald K, Scott M, Olela KN (1997) Relationship of childhood energy-protein malnutrition and parasite infections in urban African setting. Trop Med Int Health 2: 374-382.
- Stephensen CB (1999) Burden of infection on growth failure. J Nutr 129: 534-538.
- WHO (1997) Iron deficiency: Indicators for assessment and strategies for prevention. Document WHO/ NUT/ 96.12, Geneva.
Citation: Saturnin HBV, Mamatchi M, Eunice KN, Balbine FA, Lumo AK, et al. (2018) Pharmacological Effects of Moringa oleifera (Lam.) Leaves Powder in the Treatment of Anaemia in Children Aged from 6 to 30 Months in Lissezoun in Central Benin. Biochem Physiol 7:244. DOI: 10.4172/2168-9652.1000244
Copyright: © 2018 Saturnin HBV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5161
- [From(publication date): 0-2018 - Oct 04, 2025]
- Breakdown by view type
- HTML page views: 4247
- PDF downloads: 914