Research Article Open Access
Postoperative Accumulation of Cyr61/CCN1 in Surgical Wound Fluid Precedes Cytokine Activation and is Disparate from Systemic Alterations
| Claus Vinter B Hviid1,2,3*, Are Hugo Pripp4, Ansgar O Aasen1,2 and Claus Danckert-Krohn1 | ||
| 1Institute for Surgical Research, Oslo University Hospital–HF, Rikshospitalet, 0027 Oslo, Norway | ||
| 2Institute of Clinical Medicine, Faculty of Medicine, Oslo University, Blindern, 0318 Oslo, Norway | ||
| 3Department of Anesthesia, Operation, and Intensive Care, Vestre Viken Hospital Trust, Drammen Sygehus, 3004 Drammen, Norway | ||
| 4Department of Biostatistics, Epidemiology and Health Economics, Oslo University Hospital–HF, Ullevål, 0424 Oslo, Norway | ||
| Corresponding Author : | Claus Vinter B Hviid Institute for Surgical Research Oslo University Hospital–HF, Rikshospitalet P.O. Box 4950 Nydalen, 0424 Oslo, Norway Tel: +47 41178329 E-mail: Claus.vinter@rr-research.no |
|
| Received August 30, 2014; Accepted October 28, 2014; Published October 31, 2014 | ||
| Citation: Hviid CV, Pripp AH, Aasen AO, Danckert-Krohn C (2014) Postoperative Accumulation of Cyr61/CCN1 in Surgical Wound Fluid Precedes Cytokine Activation and is Disparate from Systemic Alterations. J Infect Dis Ther 2:181. doi:10.4172/2332-0877.1000181 | ||
| Copyright: © 2014 Hviid CVB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background: Cysteine-rich protein 61 (Cyr61/CCN1) is a multifunctional matricellular protein that has recently emerged as a potential player in injury-repair mechanisms involving the regulation of inflammatory responses. Experimental research has revealed the pro-inflammatory effects and chemotactic capacities of this protein, and clinical investigations have found it to be elevated in patients with chronic inflammatory conditions. However, its regulation at sites of acute tissue injury and inflammation in humans has not been investigated.
Methods: Ten otherwise healthy patients undergoing major orthopedic surgery for idiopathic thoracic scoliosis were recruited to the study. Fluid from the surgical drain and systemic blood was collected at 0, 1, 2, 4, and 6 hours following wound closure and analyzed for CCN1 levels, neutrophil counts, selected cytokines, and markers of primary hemostasis activation.
Results and Conclusions: In the drained fluid, CCN1 levels increased from 1 hour after wound closure, whereas its levels remained unaltered in systemic circulation. The platelet activation marker soluble P-selectin increased in the drained fluid but not in the systemic blood, resulting in a strong correlation between CCN1 and soluble P-selectin in the drained fluid (r=0.649, p=0.042). Levels of interleukin-6 and granulocyte-colony stimulating factor were elevated in the drained fluid only from two hours after wound closure, and neutrophil counts, tumor necrosis factor– alpha, interleukin-8 and interleukin-10 were elevated from 4 hours after wound closure. The present study demonstrated that CCN1 accumulates in fluid drained from surgical wounds following major surgery. It revealed that CCN1 levels increased simultaneously with soluble P-selectin, and prior to those of classical cytokine mediators, which suggests a connection between platelet activation and CCN1 accumulation. Collectively, these data suggest that CCN1 may play a role in the acute phases of human tissue injury and support its role as an early participant in acute inflammation in humans.
| Keywords |
| Cysteine-rich protein 61; Inflammation; Blood Platelets; Cytokines; Chemokines |
| Abbreviations |
| CCN1/Cyr61: Cysteine-rich protein 61; Ctgf: Connective tissue growth factor; CV: Coefficient of Variation; ECM: Extra Cellular Matrix; ELISA: Enzyme-Linked Immunoabsorbant Assay; G-CSF: Granulocyte-colony stimulating factor; Hb: Hemoglobin, IL: Interleukin; NF-κB: Nuclear Factor Kappa B; Nov: Nephroblastoma overexpressed; SAG-M: Sodium Chloride-Adenine-Glucose- and Mannitol; SD: Standard Deviation; SEM: Standard Error of the Mean; sP-selectin: soluble P-selectin; TNF-α: Tumor necrosis factor-alpha |
| Introduction |
| Acute inflammation is a nonspecific response of the host to tissue injury or infection, and it is the first event in a series of reactions that restore tissue integrity and function. This powerful response provides protection at the cost of some collateral damage but typically remains local and allows for the reinstating of homeostasis [1]. If the injury/infection is excessive, however, the response may disseminate into the systemic inflammatory response syndrome with the potential for causing distant organ dysfunction [1,2]. Hence, the mechanisms underlying this potentially lethal transition are, to a large extent, the consequence of the collateral damage imposed by an exaggerated host response [3,4]. Major surgical procedures and severe infections may provoke this transition from normal and beneficial inflammation to catastrophic scenarios [5], but the exact mechanisms underlying this transition remain poorly understood and warrant further investigation, particularly with regard to the molecular biology of acute inflammation. |
| CCN1 is the founding member of the CCN (Cyr61, Ctgf, Nov) family of matricellular proteins, which are dynamically expressed and secreted factors that reside in the extracellular matrix (ECM). In contrast to ordinary ECM proteins, the CCNs have no known structural functions but rather serve to support cellular responses to environmental perturbations in a context- and cell-type-dependent manner [6,7]. Experimental research on the multifunctional CCN1 protein have revealed that it is a potential key player in tissue injury repair [6,7]. It has diverse roles in regulating fibroblast behavior [7-9], promoting angiogenesis [10,11], and regulating vascular injury repair [12,13], and it displays organ-protective and regenerative capabilities in some contexts [14,15], while in others, it has been associated with detrimental effects to organ functioning [16]. |
| We have pursued the regulation of this factor in experimental rodent models of sepsis-induced organ dysfunction and recently demonstrated that its expression is altered in acutely inflamed organs presenting with biochemical signs of dysfunction [17,18]. Attempting to identify the potential mechanisms behind these regulations, we demonstrated that CCN1 expression in parenchymal cells is sensitive to cytokine exposure in vitro. However, accumulating evidence now suggests that CCN1 may also possess pro-inflammatory capabilities [19-21], indicating that this immediate early gene may be involved in the initial promotion of the inflammatory response. The present study was undertaken to investigate whether CCN1 is activated at local sites of acute tissue injury in humans and to assess its temporal relationship with the activation of the inflammatory response. |
| Materials and Methods |
| Patients and surgical procedure |
| The study was approved by the Regional Ethical Committee, Helse Sor-Ost (REK, S-00022), and all participants and their parents provided informed consent. Ten patients, ranging from 13-18 years of age, who were undergoing major orthopedic surgery for idiopathic thoracic scoliosis, were recruited to the study. The participants suffered from no comorbidities and denied taking daily medications. By selecting this population, the influence of comorbidity on the obtained results was judged as minimized. Furthermore, orthopedic surgery involving extensive musculoskeletal dissection is known to elicit a profound inflammatory response and is therefore considered an appropriate model for these investigations [22]. |
| The surgical procedure was performed through a posterior access, the column was straightened and dorsally fixated with rods according to the Cotrel/Debousset technique, as described previously [23,24]. At the end of the procedure, a drainage tube was placed centrally in the wound. The surgery was performed under general anesthesia, which was induced with thiopentone, fentanyl and vecuronium and maintained with a mixture of isoflurane and fentanyl. The patients were mechanically ventilated with a mixture of nitrous oxide and oxygen and extubated at the end of surgery. Post-operative pain relief was provided by continuous epidural bupivacaine/fentanyl infusion and supplementation with acetaminophen (paracetamol) and ketobemidone-morphine, as required. Subcutaneous injections of low-molecular-weight heparin (Fragmin, Pharmacia & Upjohn, Stockholm, Sweden) were used for thromboprophylaxis beginning on the night before surgery, and they were administered daily thereafter. For infectious prophylaxis, dicloxacillin (Diclocil, Bristol-Myers Squibb, NY, USA) was administered intravenously at a dosage of 1 g×3 daily, beginning during surgery and continuing for four days. |
| Autologous blood transfusion with washed erythrocytes (Haemolite 2 Cell Saver, Haemonetics Corporation, Braintree, MA, USA) was performed and supplemented with a homologous transfusion with sodium chloride-adenine-glucose- and mannitol (SAG-M)-suspended erythrocytes if required. Blood loss was estimated from the autologous transfusion suction unit, surgical swaps and dressings and was replaced with crystalloids (NaCl, Ringer solution, and 5% glucose) and colloids (Hemohes, Braun, Melsungen, Germany). |
| Blood Sampling |
| Reference samples were obtained from systemic circulation before the induction of anesthesia. The remaining samples were collected simultaneously from systemic circulation and the drain at wound closure (0 h) and 1, 2, 4, and 6 hours after closure into ethylenediaminetetraacetic acid (EDTA) and citrate-treated Vacutainers® (Becton Dickinson and Company, NJ, USA). Samples from systemic circulation were collected from the radial arterial cannula, and the drain samples were obtained from a nipple incorporated into the drainage tube in close proximity to the patient for this purpose, as described previously [23,24]. The drainage reservoir was closed during the collection of the drain fluid so that fresh samples could be obtained directly from the wound. The samples were centrifuged (1300×g for 10 min at room temperature [RT]), and the plasma was harvested and stored at -70°C until further analysis. |
| Analysis |
| Hemoglobin (Hb) levels and thrombocyte and neutrophil counts were measured using a Coulter Counter® (Beckman Coulter, Inc., CA, USA) that was optimized for routine patient analysis. Cyr61 levels were measured in citrate anti-coagulated plasma using a commercially available enzyme-linked immunosorbent assay (ELISA) (DRG Diagnostics, NY, USA) according to the manufacturer’s instructions. The detection limit of this assay was 14.9 pg/ml, with a range of 14.9-1000 pg/ml. The intra- and inter-assay coefficients of variation (CVs) were 7.4 and 8.5%, respectively. Soluble (s) P-selectin levels were measured by an ELISA (Life Technologies Corporation, MD, USA) according to manufacturer’s instructions. The detection limit and range of this assay were 0.2 ng/ml and 0.63-40 ng/ml, respectively, and the intra- and inter-assay CVs were 7.8 and 5.4%, respectively. Tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-8 and granulocyte-colony stimulating factor (G-CSF) levels were measured in the EDTA anti-coagulated plasma using DuoSet ELISAs (R&D systems, McKinley Place NE, MN, USA) according to manufacturer’s instructions. The ranges of these assays were as follows: 15.62-1000, 3.9-250, 9.4-600, 31.25-2000, and 31.25-2000 pg/ml, respectively. The inter-assay CVs were approximately 13, 15, 5, 8, and 5%, respectively. IL-10 levels were measured by a commercially available ELISA (Dianova GmbH, Hamburg, Germany) according to the manufacturer’s instructions. The range and inter-assay CV of this kit were 1.2-300 pg/ml and 11%, respectively. |
| For the DuoSet ELISAs, the detection limit was set at half of the value of the first standard (½Std1), and values below ½Std1 were assigned a value corresponding to 50% of ½Std1. For IL-10, Std1 represented the detection limit, and values below this limit were assigned a value 50% of Std1. The percentages of measurements that were assigned a value of 50% of the detection limit in the systemic blood were as follows: TNF-α, 45%, IL-1β, 83%, IL-6, 2%, IL-8, 60%, G-CSF, 17%, and IL-10, 8%. Additionally, these percentages in the drained fluid were as follows: TNF-α 7%, IL-1β, 8%, IL-6, 0%, IL-8, 0%, G-CSF, 3%, and IL-10, 0%. |
| Statistical Analysis |
| Demographic and clinical treatment data are expressed in absolute numbers or as median and inter-quartile ranges, whereas Hb is expressed as the mean ± standard deviation (SD). To adjust for inter-individual variations, all other markers were normalized against the reference sample and are expressed as the mean ± standard error of the mean (SEM) on this scale. Hb levels in the wound fluid and systemic blood at wound closure were compared by paired Student’s T-test, and changes over time were analyzed using a linear mixed model for repeated measurements, as described below. Adjusted values were analyzed by a linear mixed model with random intercepts. An initial attempt to include a random slope was abandoned due to the breaching of the convergence criteria. Time after wound closure was expressed as a fixed factor, and wound closure was set as the reference point. The cytokines had skewed distributions and were transformed by the natural logarithm (Ln) prior to the analysis, whereas the remaining parameters were not transformed. For the graphic presentation, all parameters were presented on the natural linear scale. To evaluate the coefficient of correlation between the investigated markers, subjects’ means over time were calculated for each marker and analyzed by Pearson`s coefficient of correlation [25]. All statistical analysis were performed using the SPSS 18.0 software, and the alpha level was set at 0.05. |
| Results |
| Patient demographics and clinical characteristics |
| Patient demographics are displayed in Table 1. Seven girls and three boys with a median age of 15 (2.25) years and weight of 57 (11) kg were recruited to the study. The median peroperative blood loss totaled 1550 (975) ml, and this was replaced by 5000 (1375) ml crystalloids, 1500 (625) ml colloids, and 450 (410) ml transfused autologous blood. One patient required an additional transfusion with two units of SAG-M during the surgical procedure. The median postoperative blood loss was 950 (495) ml, which was replaced by 1000 (2025) ml crystalloids, 450 (525) ml colloids, and 300 (465) ml transfused autologous blood. |
| The Hb level in the systemic blood was 11.9 ± 1.3 g/dl before surgery, which then declined to 8.4 ± 1.3 g/dl at wound closure (p<0.001) and remained steady throughout the observation period (8.9 ± 0.9 g/dl, p=0.177). The Hb level in the drained fluid was lower than the systemic level at wound closure (7.6 ± 1.4 g/dl, p=0.001 compared with systemic levels) and declined to 5.7 ± 3.0 g/dl during the observation period (p=0.019). Two patients showed elevated cytokine levels in their reference blood samples (>2x SD) and were screened for preoperative infections by a retrospective chart assessment, but neither individual presented with any recorded evidence of infection. None of the patients developed infectious complications during the first 48 hours after surgery. |
| CCN1 activation |
| CCN1 levels as measured in the systemic reference sample presented with broad inter-individual variations (69-1337 pg/ml, mean=272 ± 128 pg/ml). CCN1 levels and all other measured markers were therefore normalized against their reference values to facilitate more convenient comparisons. |
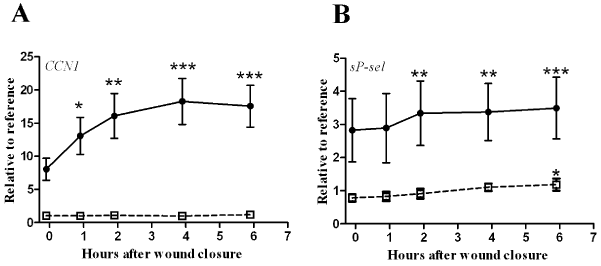
| In the drained fluid, the CCN1 level at wound closure was 8.0 ± 5.2 times higher than that of the reference sample and increased over time following wound closure, reaching a statistically significant level of elevation at 1 hour, which persisted throughout the observation period. By contrast, systemic CCN1 levels remained at reference levels throughout the observation period (p=0.210) (Figure 1A). |
| Primary haemostasis |
| The reference platelet counts and sP-selectin levels were 220 ± 13×109/l and 66 ± 16 ng/ml. Platelet counts did not demonstrate any statistically significant changes over time in the systemic blood or drained fluid (wound, p=0.866 and systemic, p=0.655), but sP-selectin levels increased over time in both compartments, reaching significance at 2 hours in the drained fluid and at 6 hours in systemic circulation (Figure 1B). |
| Inflammatory activation |
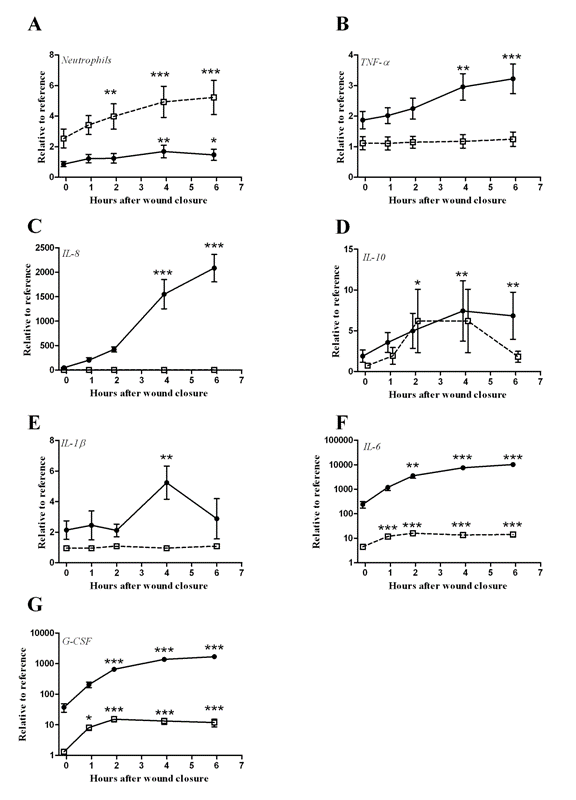
| Reference neutrophil counts were 2.8 ± 0.7×109/l, and the relative levels increased in both the systemic blood and the drained fluid during the observation period, reaching significance at 4 hours in the drained fluid and at 2 hours in the systemic blood (Figure 2A). The inflammatory cytokines TNF-α, IL-1β, IL-6, IL-8, IL-10 and G-CSF showed reference values of 36 ± 23, 2 ± 1, 6 ± 3, 8 ± 1, 20 ± 11, 18 ± 11 pg/ml, respectively. TNF-α and IL-8 levels were unaltered in the systemic blood during the observation period, and IL-10 was only elevated at 4 hours in this compartment. In contrast, the levels of these markers were increased beginning at 4 hours, and these increases persisted throughout the observation period in the drained fluid (Figure 2B-D). IL-1β levels remained at reference levels in the systemic blood and were observed to increase only at 4 hours in the drained blood, while IL-6 and G-CSF levels increased beginning at one hour in the systemic blood and at 2 hours in the drained fluid (Figure 2E-G). |
| The correlations between the analyzed markers and CCN1 are displayed in Table 2. Significant correlations were observed only with sP-selectin and IL-8. However, correlations between the evaluated cytokines were also analyzed and were significant for G-CSF vs. IL-6 (r=0.769, p<0.01) and G-CSF vs. IL-8 (r=0.825, p<0.01). |
| Discussion |
| The present study demonstrated that CCN1 was up-regulated in fluid drained from surgical wounds following major orthopedic surgery. We revealed a connection between CCN1 accumulation and levels of the platelet activation marker sP-selectin and demonstrated that CCN1 accumulation preceded the increase in cytokine levels in the drained fluid. |
| CCN1 is a multifunctional matricellular protein that has recently emerged as an important factor in the host response to tissue damage [7,26]. Studies have connected deregulations of CCN1 to a variety of diseases associated with chronic inflammation, such as rheumatoid arthritis [27], atherosclerosis [28], infectious myocarditis [29], inflammatory bowel disease [30], chronic obstructive pulmonary disorder [31] and several cancers [7]. However, experimental studies have also suggested a role for CCN1 in acute inflammation by demonstrating its ability to promote cytokine secretion [21,32] and support the adhesion of platelets [33], and monocytes/macrophages [21,34]. Acute tissue injury elicits a remarkably conserved response consisting of an instant hemostatic reaction followed by acute inflammation, which is essential for successful tissue recovery [35]. |
| Our data corroborate these local response patterns by demonstrating the release of the platelet activation marker sP-selectin, cytokine/chemokine secretion, and neutrophil accumulation and revealing that CCN1 levels increase during these early stages of tissue injury characterized by inflammatory activation. These findings suggest that CCN1 accumulates at sites of sterile tissue injury and imply that such accumulations may be general phenomena that occur in response to tissue injury in humans. |
| We recently provided evidence that CCN1 is a platelet-transported protein that is released from thrombocyte, isolated from healthy donors, upon their activation in vitro [18], and the correlation between CCN1 and sP-selectin may therefore suggest that platelet activation contributed to the observed CCN1 elevation in this study. In the drained fluid, levels of sP-selectin were on average three times higher than the systemic reference value at wound closure, and these levels increased further throughout the observation period, which is consistent with the presence of an ongoing hemostatic response. Similarly, CCN1 levels were substantially higher than reference levels at wound closure and increased throughout the study period. CCN1 is a ubiquitously expressed and secreted protein, and fibroblasts, endothelial cells, smooth and striated muscle cells, and immune cells have been reported to produce and secrete CCN1 in response to environmental stressors, such as coagulation factors, eicosanoids, hormones, hypoxia, and inflammatory cytokines [6,7,36]. All of these factors, which stimulate the aforementioned cell types, may have contributed cumulatively to the increased CCN1 levels in the drained fluid. Immune cells are especially interesting candidates because of their potential roles as both producers and target cells of CCN1 [21,36]. |
| The pro-inflammatory stimulation of human peripheral blood mononuclear cells and isolated monocytes provoke CCN1 transcriptional activity [37] and protein secretion [36,37], which suggests that monocytes/macrophages are potential contributors to the observed CCN1 accumulation in this study. However, the presence of a relatively limited number of such cells in the tissues during the early stages after injury, which was observed in the present study, combined with the lack of detectable CCN1 alterations in the systemic blood despite evidence of systemic inflammatory activity, suggest that they did not contribute substantially to CCN1 accumulation in this case. Altogether, our data may suggest that platelets play a role in the accumulation of CCN1 at the site of localized tissue injury in human patients. |
| The pro-inflammatory role of CCN1 has been confirmed in independent experimental studies, demonstrating its ability to induce cytokine secretion [21,32] and to affect chemotaxis [19,20,36]. As an immediate-early gene, CCN1 is activated within minutes of cellular stimulation without requiring de novo protein synthesis [38], which may suggest that it is able to act as an upstream mediator of cytokine activation. |
| Our data demonstrated that CCN1 levels increased before those of the majority of the investigated cytokines. These time-dependent regulations, in combination with the known inflammatory roles of CCN1, support its function as an early mediator of inflammatory activation. However, a large body of evidence has demonstrated that CCN1 is activated by cytokine exposure [39], and NF-B binding-sites have been reported in its promoter region [40]. While this would indicate the reverse to be true, i.e., that CCN1 is regulated by pro-inflammatory stimulation, it is crucial to emphasize that cytokines, in general, work in an interacting network of mutual promotion and inhibition to fine-tune the inflammatory response [1]. Thus, while our data suggest that CCN1 is an early mediator of inflammation, this does not preclude cytokine-induced CCN1 activation at later stages of inflammatory progression. |
| The investigations on the biological functions of CCN1 are still in an early phase of discovery. Whether this protein may serve as a biomarker of inflammatory activation or represent a future target point for development of therapy remains to be clarified. Investigations indicate an unusual functional diversity with involvement in regulation of inflammation [7,21,36] and in regulation of organ resistance to impairing insults [16,41] - akin the behavior of an alarmine [2,42]. |
| The present study has some clear limitations. It is purely observational, and the size of the studied population is small. The sample size did not allow for stratified analysis to control for a potential confounding effect of endothelial-derived sP-selectin on the correlation with CCN1. However, the combination of evidence suggesting that sP-selectin is primarily platelet-derived and our previous results demonstrating that platelets carry and release CCN1 suggests the risk of confounding to be limited [18,43]. Furthermore, It cannot be ruled out that blood loss, fluid replacement, or other factors, such as anesthesia, may have masked the responses of CCN1 to surgery. CCN1 levels in the wound fluid were normalized against a reference sample obtained from systemic circulation, and it is presently unknown whether systemic levels accurately reflect ECM levels. |
| Taken together, the present study demonstrates that CCN1 is regulated at sites of acute tissue injury in humans and may suggest that platelets deliver this protein to the site of injury, supporting its role as a proinflammatory agent. |
| Acknowledgement |
| The authors thank Signe Flood Kjeldsen for her excellent technical assistance. |
| This work received no specific funding from any funding agency in the public, commercial, or not-for-profit sector. All authors declare that there are no conflicts of interest. |
References
- Medzhitov R (2008) Origin and physiological roles of inflammation.Nature 454: 428-435.
- Manson J, Thiemermann C, Brohi K (2012) Trauma alarmins as activators of damage-induced inflammation.Br J Surg 99 Suppl 1: 12-20.
- Abraham E, Singer M (2007) Mechanisms of sepsis-induced organ dysfunction.Crit Care Med 35: 2408-2416.
- Cinel I, Opal SM (2009) Molecular biology of inflammation and sepsis: a primer.Crit Care Med 37: 291-304.
- Barie PS, Hydo LJ (2000) Epidemiology of multiple organ dysfunction syndrome in critical surgical illness.Surg Infect (Larchmt) 1: 173-185.
- Chen CC, Lau LF (2009) Functions and mechanisms of action of CCN matricellular proteins.Int J Biochem Cell Biol 41: 771-783.
- Lau LF (2011) CCN1/CYR61: the very model of a modern matricellular protein.Cell Mol Life Sci 68: 3149-3163.
- Jun JI, Lau LF (2010) The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing.Nat Cell Biol 12: 676-685.
- Chen CC, Lau LF (2010) Deadly liaisons: fatal attraction between CCN matricellular proteins and the tumor necrosis factor family of cytokines.J Cell Commun Signal 4: 63-69.
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth.ProcNatlAcadSci U S A 95: 6355-6360.
- Fataccioli V, Abergel V, Wingertsmann L, Neuville P, Spitz E, et al. (2002) Stimulation of angiogenesis by Cyr61 gene: a new therapeutic candidate.Hum Gene Ther 13: 1461-1470.
- Matsumae H, Yoshida Y, Ono K, Togi K, Inoue K, et al. (2008) CCN1 knockdown suppresses neointimal hyperplasia in a rat artery balloon injury model.ArteriosclerThrombVascBiol 28: 1077-1083.
- Yu Y, Gao Y, Qin J, Kuang CY, Song MB, et al. (2010) CCN1 promotes the differentiation of endothelial progenitor cells and reendothelialization in the early phase after vascular injury.Basic Res Cardiol 105: 713-724.
- Jin Y, Kim HP, Ifedigbo E, Lau LF, Choi AM (2005) Cyr61 protects against hyperoxia-induced cell death via Akt pathway in pulmonary epithelial cells.Am J Respir Cell MolBiol 33: 297-302.
- Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, et al. (2013) Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury.Am J Physiol Lung Cell MolPhysiol 304: L415-427.
- Hsu PL, Su BC, Kuok QY, Mo FE (2013) Extracellular matrix protein CCN1 regulates cardiomyocyte apoptosis in mice with stress-induced cardiac injury.Cardiovasc Res 98: 64-72.
- Hviid CV, Samulin EJ, Kunke D, Ahmed SM, Kjeldsen SF, et al. The matri-cellular proteins 'cysteine-rich, angiogenic-inducer, 61' and 'connective tissue growth factor' are regulated in experimentally-induced sepsis with multiple organ dysfunction. Innate Immun 2012; 18: 717-726.
- Hviid CV, Samulin EJ, Drechsler S, Weixelbaumer K, Ahmed MS, et al. The matricellular "cysteine-rich protein 61" is released from activated platelets and increased in the circulation during experimentally induced sepsis. Shock 2014; 41: 233-240.
- Bian Z, Peng Y, You Z, Wang Q, Miao Q, et al. (2013) CCN1 expression in hepatocytes contributes to macrophage infiltration in nonalcoholic fatty liver disease in mice.J Lipid Res 54: 44-54.
- Lai CF, Chen YM, Chiang WC, Lin SL, Kuo ML, et al. (2013) Cysteine-rich protein 61 plays a proinflammatory role in obstructive kidney fibrosis.PLoS One 8: e56481.
- Bai T, Chen CC, Lau LF (2010) Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages.J Immunol 184: 3223-3232.
- Krohn CD, Reikeras O, Aasen AO. The cytokines IL-1beta and IL-1 receptor antagonist, IL-2 and IL-2 soluble receptor-alpha, IL-6 and IL-6 soluble receptor, TNF-alpha and TNF soluble receptor I, and IL10 in drained and systemic blood after major orthopaedic surgery. Eur J Surg 1999; 165: 101-109.
- Krohn CD, Sørensen R, Lange JE, Riise R, Bjørnsen S, et al. (2003) Tranexamic acid given into the wound reduces postoperative blood loss by half in major orthopaedic surgery.Eur J SurgSuppl : 57-61.
- Krohn CD, Reikerås O, Bjørnsen S, Brosstad F (2000) Tissue factor antigen and activity in serum of postoperatively shed blood used for autologous transfusion.Blood Coagul Fibrinolysis 11: 219-223.
- Bland JM, Altman DG (1995) Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects.BMJ 310: 633.
- Jun JI, Lau LF (2011) Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets.Nat Rev Drug Discov 10: 945-963.
- Haas CS, Creighton CJ, Pi X, Maine I, Koch AE, et al. Identification of genes modulated in rheumatoid arthritis using complementary DNA microarray analysis of lymphoblastoid B cell lines from disease-discordant monozygotic twins. Arthritis Rheum 2006; 54: 2047-2060.
- Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, et al. (2002) Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II.Circulation 106: 254-260.
- Wittchen F, Suckau L, Witt H, Skurk C, Lassner D, et al. Genomic expression profiling of human inflammatory cardiomyopathy (DCMi) suggests novel therapeutic targets. J Mol Med 2007; 85: 257-271.
- Koon HW, Zhao D, Xu H, Bowe C, Moss A, et al. (2008) Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis.Am J Pathol 173: 400-410.
- Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. ProcNatlAcadSci U S A 2004; 101: 14895-14900.
- Lin J, Zhou Z, Huo R, Xiao L, Ouyang G, et al. (2012) Cyr61 induces IL-6 production by fibroblast-like synoviocytes promoting Th17 differentiation in rheumatoid arthritis.J Immunol 188: 5776-5784.
- Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3). J BiolChem 1999; 274: 24321-24327.
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, et al. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood 2002; 99: 4457-4465.
- Eming SA, Krieg T, Davidson JM (2007) Inflammation in wound repair: molecular and cellular mechanisms.J Invest Dermatol 127: 514-525.
- Löbel M, Bauer S, Meisel C, Eisenreich A, Kudernatsch R, et al. (2012) CCN1: a novel inflammation-regulated biphasic immune cell migration modulator.Cell Mol Life Sci 69: 3101-3113.
- Panutsopulos D, Arvanitis DL, Tsatsanis C, Papalambros E, Sigala F, et al. (2005) Expression of heregulin in human coronary atherosclerotic lesions.J Vasc Res 42: 463-474.
- O'Donnell A, Odrowaz Z, Sharrocks AD (2012) Immediate-early gene activation by the MAPK pathways: what do and don't we know?BiochemSoc Trans 40: 58-66.
- Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C (2011) The CCN family: a new class of inflammation modulators?Biochimie 93: 377-388.
- Latinkic BV, O'Brien TP, Lau LF (1991) Promoter function and structure of the growth factor-inducible immediate early gene cyr61.Nucleic Acids Res 19: 3261-3267.
- Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, et al. (2010) Matricellularsignaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator. Circulation 122: 2688-2698.
- Bianchi ME, Manfredi AA (2009) Immunology. Dangers in and out.Science 323: 1683-1684.
- Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, et al. (2009) Soluble P-selectin as a marker of in vivo platelet activation.ClinChimActa 399: 88-91.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14503
- [From(publication date):
December-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 9843
- PDF downloads : 4660
