Postweaning Consumption of Soy isoflavones Induced Alterations on Some Reproductive Parameters of Prepubertal and Postpubertal Male Wistar Rats
Received: 10-Sep-2018 / Accepted Date: 20-Nov-2018 / Published Date: 28-Nov-2018 DOI: 10.4172/2168-9652.1000247
Abstract
Background: Soy contains phytoestrogens which are potent endocrine disruptors. Soy mainly contains phytoestrogens called isoflavones predominantly daizein and genistein.
Main body: The present study determined the effects of isoflavones consumed post-weaning on prepubertal and postpubertal Leydig and Sertoli cell numbers, spermatozoa parameters and body weight to paired testicular weight ratio of Wistar rats. In this study, three diets were formulated containing different amounts of isoflavones. The diets were formulated by adding to the base diet different quantities of novasoy which is an isoflavone concentrate. The formulated diets contained 74.5, 235.6 and 1046.6 mg total isoflavones/kg pelleted diet, representing the isoflavones content less, equivalent or greater than that found in soy infant formula respectively. The results obtained showed that; administration of all doses of isoflavones significantly (p<0.05) increased Leydig and Sertoli cell numbers in both prepubertal and post pubertal rats as compared to the control groups. Administration of all doses of isoflavones significantly (p<0.05) decreased sperm count as compared to the control group. Administration of low doses (74.5 mg/ kg) of isoflavones significantly increased sperm motility as compared to the control group. Administration of moderate (235.6 mg/kg) and high (1046.6 mg/kg) doses of isoflavones significantly (p<0.05) increased sperm deformation as compared to the control group. Administration of moderate (235.6 mg/kg) and high (1046.6 mg/kg) doses of isoflavones significantly (p<0.05) increased body weight to paired testicular weight ratio as compared with the control groups.
Conclusion: Overall, the alterations brought about by isoflavones at all doses are indications of adverse effects on the male rat testicular function and this may adversely affect the functional capacities of the testes.
Keywords: Soy isoflavones; Post weaning; Sertoli cell; Leydig cell; Sperm; Prepubertal; Postpubertal; Testicular function
Introduction
Soy isoflavones have estrogenic activities and are structurally and functionally related to 17β-estradiol [1]. Genistein and daidzein plus their derivatives tend to mimic estrogen through binding to estrogens receptors [1]. Different studies have been carried out in humans indicating that exposure to endocrine disruptors such as phytoestrogens where isoflavones belong has led to decline in the quality of semen [2]. The studies have further shown that a decline in sperm count and semen volume among men have been associated with phytoestrogens [3].
Phytoestrogens have been associated with different reproductive conditions in male animals, exemplified by metaplasia of male accessory gland in cattle due to consumption of high levels of coumestrol [4], a decrease in testosterone levels and disorders of erection in rats due to consumption of daidzein [5], the effects being associated with steroid regulation disruption of the epididymis [6]. Consumption of genistein and vinclozolin caused a decline in sperm motility and count as well as litter size in rats [7].
Other studies carried out in mice and humans have shown that genistein and daidzein could lead to acrosome loss and thus impair fertility in the males [8]. The alpha estrogen and androgen receptors in the testes of adult rats had their expression reduced following the neonatal exposure of the rats to genistein but sperm count and motility were not affected [9], while penile erection was reported to be affected by administration of daizein to the rats [10]. Utero-trophic effects of soy-based diets were compared to cow milk formula by Ashby JH [11], where all experimental feeds were presented in drinking bottles to the rats from PND 21/22 to 24/25. In addition, animals were also allowed ad libitum feed and fluid intake. The results showed that the rats had more preference for the soy-based diets in comparison to the normal rodent feeds and it was then concluded by the authors that the high preference for the soy-based diets increased the rats’ isoflavone intake about three times the recommended amount for human infants. Thus, that limited the application of the results to what is seen in human infants. Soy infant formula was fed to marmosets during the period of lactation, an experiment carried out by Sharpe [12] and Tan [13].
After the experiment, soy formula fed rats had significantly lower plasma testosterone and increase Leydig cell abundance per testis in the absence of significant testicular weight. Since the animals were allowed to nurse from their mothers while being fed on soy infant formula, the authors suggested that the studies may underestimate the effects of soy formula on testicular development. Thus, the NTP experts concluded that the research did not give sufficient evidence to what is seen in infants fed with soy based infant formula [14] and this suggested further research. The NTP consideration was as a result of technical limitations in the carrying out of the researches which made them not to depict the conditions (period, concentration, and route of exposure) as seen in human infants [14].
This study was therefore aimed at determining the effect of oral administration during the post-weaning period on the pre-pubertal and post-pubertal testicular cells as well as sperm parameters of Wistar rats.
The route and mode of administration as well as age of consumption would simulate the real situation that occurs in humans, and the parameters determined later in life after the cessation of soy consumption, for both prepubertal and post pubertal stages.
Materials and Methods
Experimental design
Forty male rats aged 21 days were used in this experiment. The rats were fed on the diets containing different amounts of soy isoflavones, formulated by adding novasoy, an isoflavones concentrate to the rat base diet. The experiment was carried out at kampala international university animal house. These rats were provided with water and respective feeds adlibitum.
Four experimental diets were formulated and these included one which was isoflavone free and three of which contained varying doses of isoflavones that were 74.5, 235.6 and 1046.6 mg/kg of rat base diet representing low, moderate and high isoflavone doses respectively. These concentrations were in line with those used by McVey [15].
The rats were randomly divided into eight groups each containing five rats (Table 1). These were fed on the respective experimental diets from post-natal day 22 up to post-natal day 29. The rats were fed as follows:
| Pre-pubertal groups | Post-pubertal groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | |
| Quantity of isoflavones given | 0 mg/kg | 74.5 mg/kg | 235.6 mg/kg | 1046.6 mg/kg | 0 mg/kg | 74.5 mg/kg | 235.6 mg/kg | 1046.6 mg/kg |
| Post-natal day of sacrifice | 30 | 30 | 30 | 30 | 45 | 45 | 45 | 45 |
Table 1: Experimental Groups
With the dead rat on dorsal recumbence, the incision was made along the linear alba down the abdomen into the pelvic cavity using a pair of scissors. The surrounding tissues were removed to expose the testes on each side. The testes were then retrieved, spermatic cords severed and the testes removed. The testicular weights were then taken using a digital weighing scale. These were then stored in tissue containers with Davidson fixative and transported for testicular histology.
Sperm analysis was done with the aid of a microscope, a method employed by Faqi [16]. Processing of testicular tissues was done by a method previously described by Nagao [17]. The Sertoli and Leydig cell numbers per testis were obtained by procedure clearly described by McCoard [18] and Wreford [19].
Data Analysis
The means were written with standard deviations and one way ANOVA was used to get the statistical analysis across the groups at 0.5% level of significance. The students’t-test was then used to determine the statistical significance within groups.
Results
Leydig cell numbers of prepubertal rats fed on different amounts of isoflavones
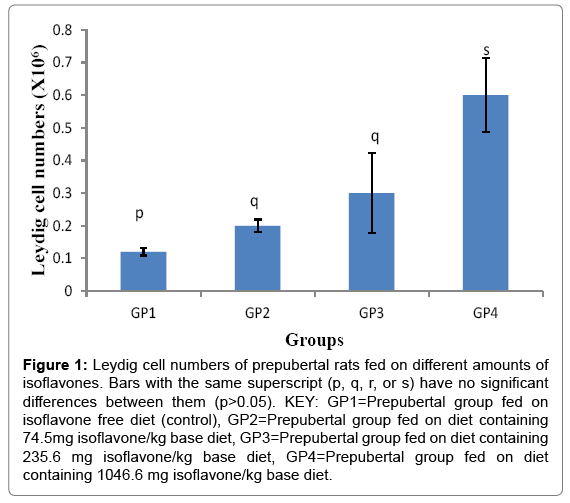
The Leydig cell numbers (Mean ± SD) were 0.122 ± 0.011, 0.222 ± 0.019, 0.300 ± 0.122 and 0.660 ± 0.114 ×106 cells for groups 1-4 respectively. The results indicated that isoflavones caused significant (p<0.05) increase in the Leydig cell numbers as shown in Figure 1.
Figure 1: Leydig cell numbers of prepubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r, or s) have no significant differences between them (p>0.05). KEY: GP1=Prepubertal group fed on isoflavone free diet (control), GP2=Prepubertal group fed on diet containing 74.5mg isoflavone/kg base diet, GP3=Prepubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP4=Prepubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
Sertoli cell numbers of prepubertal rats fed on different amounts of isoflavones
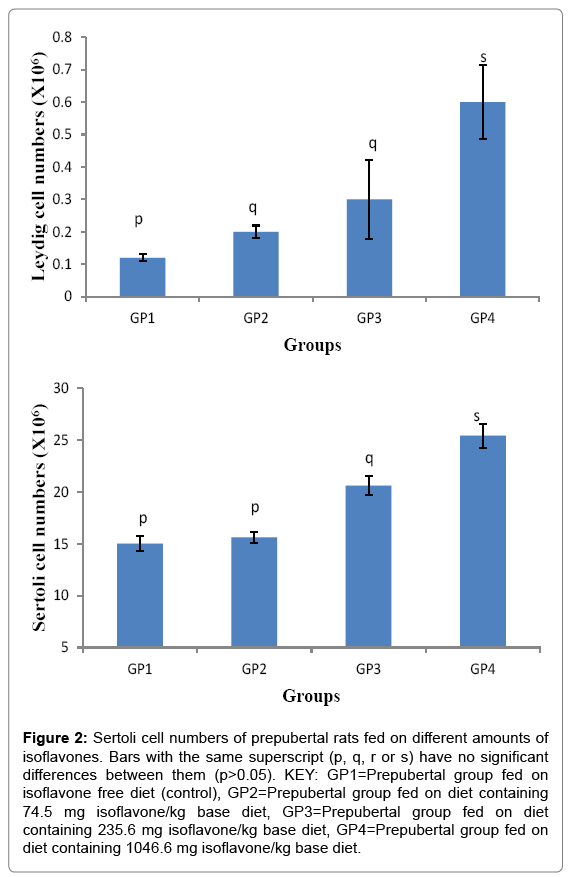
The Sertoli cell numbers (Mean ± SD) were 15.00 ± 0.71, 15.60 ± 0.55, 20.60 ± 0.89, 25.40 ± 1.14 ×106 cells for groups 1-4 respectively. The results indicated that the moderate and high amounts of isoflavones led to a significant (p<0.05) increase in sertoli cell numbers as shown in Figure 2.
Figure 2: Sertoli cell numbers of prepubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP1=Prepubertal group fed on isoflavone free diet (control), GP2=Prepubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP3=Prepubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP4=Prepubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
Leydig cell numbers for post pubertal rats fed on different amounts of isoflavones
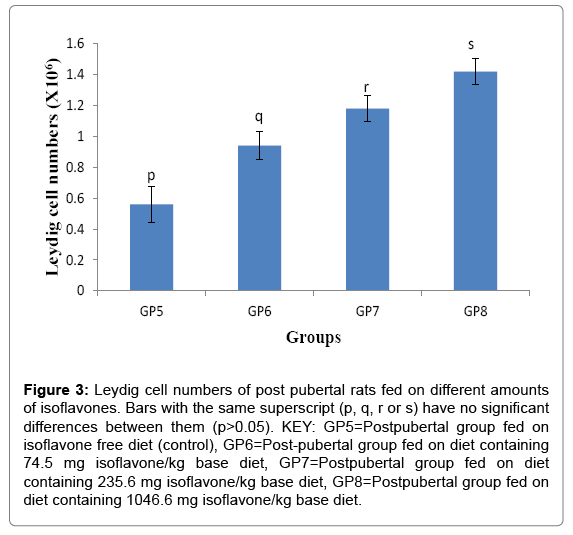
The Leydig cell numbers (Mean ± SD) for the post pubertal rats were 0.560 ± 0.114, 0.940 ± 0.089, 1.180 ± 0.084 and 1.42 ± 0.084 ×106 cells for groups 5-8 respectively. The results indicated that consumption of isoflavones led to the post-pubertal increase (p<0.05) in Leydig cell numbers, as shown in Figure 3.
Figure 3: Leydig cell numbers of post pubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP5=Postpubertal group fed on isoflavone free diet (control), GP6=Post-pubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP7=Postpubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP8=Postpubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
Sertoli cell numbers post pubertal rats fed on different of isoflavones
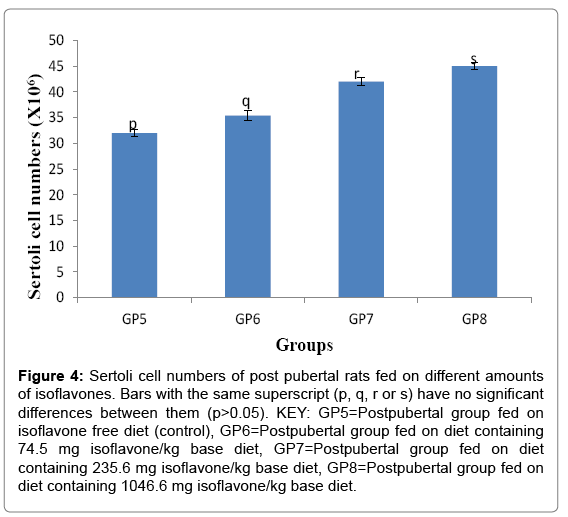
Sertoli cell number (Mean ± SD) for the prepubertal rats were 32.000 ± 0.707, 35.400 ± 0.894 42.000 ± 0.707 and 45.000 ± 0.707 ×106 cells for groups 5-8 respectively. The results in the current study indicated the consumption of soy isoflavones led to a significant increase in the postpubertal Sertoli cells as shown in Figure 4.
Figure 4: Sertoli cell numbers of post pubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP5=Postpubertal group fed on isoflavone free diet (control), GP6=Postpubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP7=Postpubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP8=Postpubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
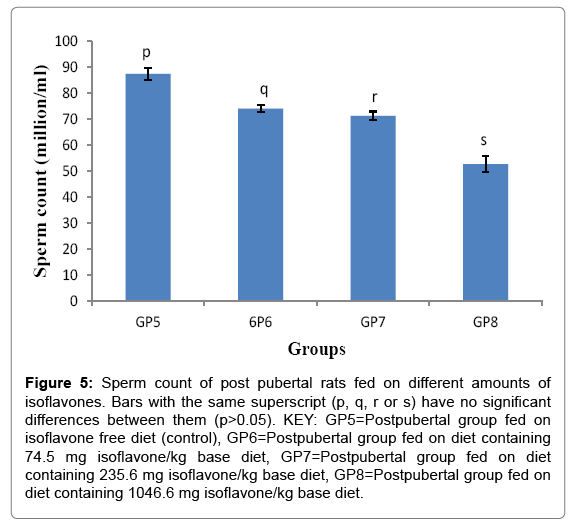
Sperm count of post pubertal rats fed on different amounts of isoflavones
Sperm counts (Mean ± SD) for the post-pubertal rats were 87.400 ± 2.302, 74.000 ± 1.414, 71.200 ± 1.643 and 52.600 ± 3.130 million/ mL for groups 5-8 respectively. The results of the study indicated that post-weaning consumption of isoflavones led to a significant decrease (p<0.05) in sperm count, as shown in Figure 5.
Figure 5: Sperm count of post pubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP5=Postpubertal group fed on isoflavone free diet (control), GP6=Postpubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP7=Postpubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP8=Postpubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
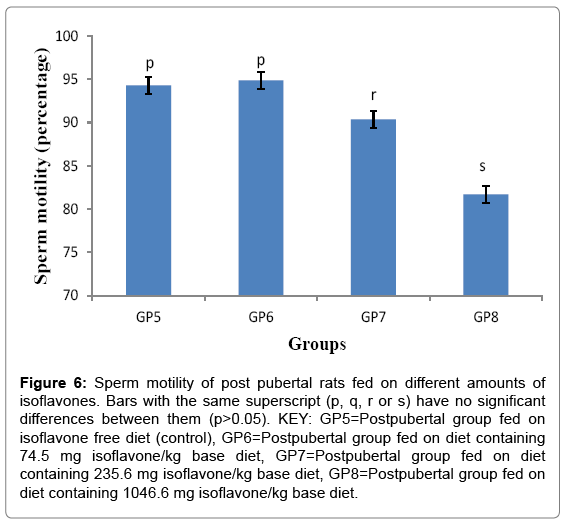
Sperm motility of post pubertal rats fed on different amounts of isoflavones
Sperm motility (Mean ± SD) for post-pubertal rats were 94.300 ± 2.427, 94.880 ± 2.149, 90.360 ± 2.158 and 81.700 ± 5.347% for groups 5-8 respectively. The results obtained in this study showed that postweaning consumption of low and high amounts led to a significant (p<0.05) decrease in sperm motility as shown in Figure 6.
Figure 6: Sperm motility of post pubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP5=Postpubertal group fed on isoflavone free diet (control), GP6=Postpubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP7=Postpubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP8=Postpubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
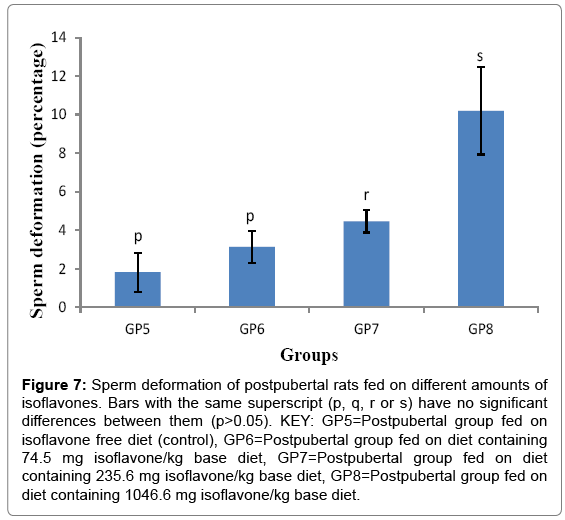
Sperm deformation of post pubertal rats fed on different amounts of isoflavones
The sperm deformation percentages (Mean ± SD) for the postpubertal rats were 1.820 ± 1.018, 3.14 ± 0.835, 4.460 ± 0.586 and 10.200 ± 2.270% for groups 5-8 respectively. The results in obtained in the current study indicated that post-weaning consumption of moderate and high amounts of isoflavones led to a significant (p<0.05) increase in sperm deformation, as shown in Figure 7.
Figure 7: Sperm deformation of postpubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP5=Postpubertal group fed on isoflavone free diet (control), GP6=Postpubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP7=Postpubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP8=Postpubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
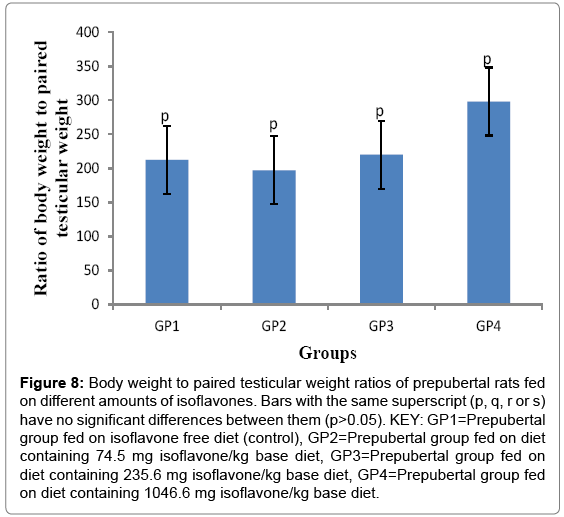
Body weight to paired testicular weight ratio of prepubertal rats fed on different amounts of isoflavones
The ratios of body weight to paired testicular weight (Mean ± SD) were 212.340 ± 141.100, 197.150 ± 57.191, 220.250 ± 63.834, 298.320 ± 216.672 for groups 1-4 respectively.
The results obtained in the current study indicated that postweaning consumption of soy isoflavones led to a non-significant (p>0.05) changes in the body weight to paired testicular weight ratios in all groups, as shown in Figure 8.
Figure 8: Body weight to paired testicular weight ratios of prepubertal rats fed on different amounts of isoflavones. Bars with the same superscript (p, q, r or s) have no significant differences between them (p>0.05). KEY: GP1=Prepubertal group fed on isoflavone free diet (control), GP2=Prepubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP3=Prepubertal group fed on diet containing 235.6 mg isoflavone/kg base diet, GP4=Prepubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
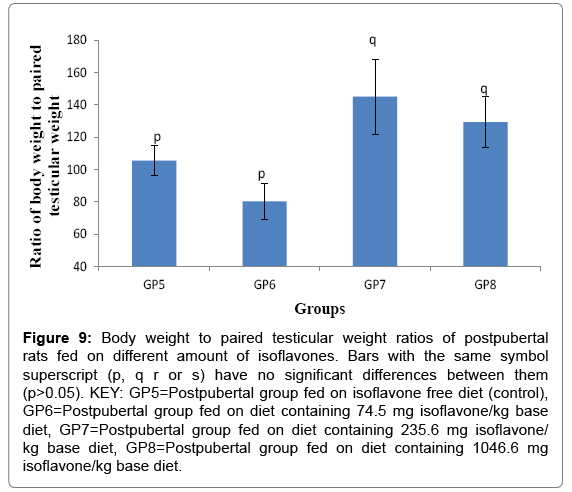
Body weight to paired testicular weight ratio of post pubertal rats fed on different amounts of isoflavones
The body weight to paired testicular weight ratios (Mean ± SD) for the post pubertal rats were 105.870 ± 9.374, 92.386 ± 10.941, 143.680 ± 23.193 and 127.360 ± 15.710 for groups 5-8 respectively. The results obtained in the current study indicated that post-weaning consumption of moderate and high amounts of soy isoflavones led to a significant (p<0.05) increase in the body weight to paired testicular ration of postpubertal rats, as shown in Figure 9.
Figure 9: Body weight to paired testicular weight ratios of postpubertal rats fed on different amount of isoflavones. Bars with the same symbol superscript (p, q r or s) have no significant differences between them (p>0.05). KEY: GP5=Postpubertal group fed on isoflavone free diet (control), GP6=Postpubertal group fed on diet containing 74.5 mg isoflavone/kg base diet, GP7=Postpubertal group fed on diet containing 235.6 mg isoflavone/ kg base diet, GP8=Postpubertal group fed on diet containing 1046.6 mg isoflavone/kg base diet.
Discussion
Leydig cells form 20% of mass of adult testis and they secrete androgens [20]. These cells are clustered near blood vessels in the testicular interstitium [21]. They are characterized by possession of many mitochondria, large smooth endoplasmic reticulum, much lipids and prominent droplets [22]. These cells are subjected to changes both in number and function as the animal matures [22]. Results of the current study have indicated a significant increase (p<0.05) in Leydig cell numbers following soy isoflavones administration. The findings are in line with those reported by Kumi-Diaka [23].
Sertoli cells are described as nurse cells of the testis and these are the supporting cells for the developing spermatozoa [24]. They provide attachment to the semiferous tubules where the spermatozoa develop from as well as provision of nutrients [25]. The Sertoli cells have fundamental importance to the development and maintenance of spermatogenesis, as well as numerical relationship to sperm production [25].
The present study showed that consumption of low doses of isoflavones (74.5 mg/kg) produced a non-significant increase in Sertoli cell numbers while the moderate (235.6 mg/kg) and high (1046.6 mg/ kg) recorded a significant increase in Sertoli cell numbers in prepubertal rats.
The results have also indicated an increase in sertoli cell numbers in post-pubertal rats following isoflavones administration. The increase in both the seroli and leydig cell numbers may be explained by the proliferative effect of isoflavones as stated by Socorro [26].
In males, sperm count is one of the parameters considered during assessment of male fertility [27]. Many factors including ejaculate failure, genital tract obstruction, genetic disturbances, exposure to too much heat as well as some drugs can lead to reduction in sperm count [28]. The complete absence of spermatozoa, which is also described as azoospermia may be brought about by factors such as hinderance of transportation of spermatozoa due to obstruction of the passages, inadequate androgens, drugs, as well as genetic causes [29].
Results of this study have revealed a significant (p<0.05) decrease in sperm count of the post-pubertal rats. The findings are supported by the study carried out [30] and [31] which showed a reduction in sperm count following administration of isoflavones.
Normal linear progressive sperm motility and normal morphology are major factors on which male fertility depends [32]. Sperm motility is the best predictor of fertility potential in males [32].
Sperm motility is considered low when more than 50% of the sperms in the semen sample cannot move progressively within 60 mins [33]. Factors such as mistakes during semen processing, contaminated equipment, and excessive heat can be predisposing to reduction in sperm motility [34]. Several confounding factors related to diet, life style, stress and socioeconomic status also affect semen quality [34]. In the current study, a significant (p<0.05) decrease in sperm motility was shown following consumption of moderate (235.6 mg/kg) and high (1046.6 mg/kg) amounts of soy isoflavones.
Sperm morphology is among factors that affect male fertility. Among factors that may bring about abnormalities in sperm morphology include toxins such as tar in tobacco, genetic abnormalities, as well as idiopathic causes [34]. Sperm morphology is another important indicator of the reproductive ability in males since very high morphological abnormalities is an indicator of progressive reproductive failure [35]. Researchers have got different views about the magnitude of sperm morphology as an indicator of male fertility. The study done by Dada [36] described sperm morphology as a very good indicator of male fertility while the one carried out by Schmahl [37] showed it to be a poor indicator.
In the prstudy, moderate (235.6 mg/kg) and high (1046.6 mg/kg) are significantly (p<0.05) increased sperm deformity. The fact that isoflavones have been associated with decreased synthesis of steroidal hormones [38] and high levels of phytoestrogens being associated with cell death and inhibiting spermatogenesis, these factors may have contributed to the low sperm count, low sperm motility and high sperm deformation following administration of isoflavones.
The increase in testicular weights improves male fertility [39]. The reduced size and weight of testes is associated with inability in semen production [40]. However, once testicular weight becomes too great, improvement in fertility tends to be small [41]. Therefore, it is important to carefully control the weight gains to achieve optimum body and testicular weights [41].
The results of the current study indicated that a significant (p<0.05) decrease in the body weight to paired testicular weight ratio in prepubertal rats was caused by low doses of soy isoflavones (74.5 mg/kg) while in post-pubertal rats, moderate (235.6 mg/kg) and high (1046.6 mg/kg) amounts caused significant (p<0.05) increase in body weight to paired testicular weight ratios.
Conclusion
Our results have indicated that isoflavones variously altered the testicular function indices investigated and this may adversely affect the functional ability of the rat testes.
Acknowledgements
The authors thank the financial support provided by Kampala International University management towards the completion of this research work. Great thanks also go Mr. Bukenya Robert, a laboratory technician at Mulago, Kampala, Uganda for all support rendered. The authors also thank all staff of physiology department, kampala international university, Uganda for all their input in this research.
References
- Gardner CD, Chatterjee LM, Franke AA(2009) Effects of isoflavone supplements versus soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem 20: 227-234.
- Jana K, Sen PC (2010) Environmental toxicants induced male reproductive disorders: Identification and mechanism of action. Toxicity Drug Testing2: 1-20.
- Alwis ID, Maroni DM, Hendry IR, Roy SK, May JV, et al. (2011) Neonatal diethylstilbestrol exposure disrupts female reproductive tract structure and function via both direct and indirect mechanisms in the hamster. Reprod Toxicol 32: 472–83.
- Hess RA, Carnes K (2004) The role of estrogen in testis and the male reproductive tract: A review and species comparison. Animal Reprod 7: 5-30.
- Kim SH, Park MJ (2012) Effects of phytoestrogen on sexual development. Korean pediat55: 265-271.
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs JrDR, et al. (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and non-monotonic dose responses. Endocr Rev 33: 378-455.
- Lewis JG, Nakajin S, Ohno S, Warnock A, Florkowski CM, et al. (2005) Circulating levels of isoflavones and markers of 5-reductase activity are higher in Japanese compared with New Zealand males: What is the role of circulating steroids in prostate disease?  Steroids 70: 974-979.
- Cummings AM, Laws SC (2000) Assessment of estrogenicity by using the delayed implanting rat model and examples. Reprod Toxicol 14: 111-117.
- Ferguson SA, Law CD, Abshire JS (2012) Developmental treatment with bisphenolA causes few alterations on measures of postweaning activity and learning. Neurotoxicol Teratol 34: 598-606.
- Irfan S, Wistuba J, Ehmcke J, Shahab M, Schlatt S (2015) Pubertal and testicular development in the common marmoset (Callithrix jacchus) shows high individual. Primate Bio 2: 1-8.
- Ashby JH, Tinwell J, Odum I, Kimber AN, Brooks I, et al. (2000) Diet and the Aetiology of Temporal Advances in Human and Rodent Sexual Development. J Appl Toxicol 20: 343-347.
- Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, et al. (2002) Infant feeding with soy formula milk: Effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod 17: 1692-1703.
- Tan KAL, Marion W, Keith M, Irene GJ, Ian M, et al. (2006) Infant feeding with soy formula milk: effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Hum Reprod 21: 896-904.
- McCarver G, Bhatia J, Chambers C, Clarke R, Etzel R, et al. (2011) NTPCERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res B DevReprod Toxicol 92: 421-468.
- McVey MJ, Cooke GM, Curran IH (2004) Increased serum and testicular androgen levels in F1 rats with lifetime exposure to soy isoflavones. Reprod Toxicol 18: 677-685.
- Faqi S Ali, Johnson WD, Morrissey RL, Cormic DL (2004) Reproductive toxicity assessment of chronic dietary exposure to soy isoflavones in male rats. Reprod Toxicol 18: 605-611.
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, et al. (2001) Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol 15: 399-411.
- McCoard SD, Lunstra DD, Wise TH, Ford JJ (2001) Specif staining of sertoli cell nuclei and evaluation of sertoli cell number and proliferative activity in Meishan and whit composite boars during the neonatal period. Biol Reprod 64: 689-695.
- Wreford NG (1995) Theory and practice of stereological techniques applied to the estimation of cell number and nuclear volume in the testis. Microsc Res Tech32:423-436.
- Mohammad EB, Sayyed MM, Majid P, Esmail A, Vahid Y, et al. (2014) Effects of silver nano-particles on sperm parameters, number of Leydig cells and sex hormones in rats. Iran J Reprod Med 12: 139-144.
- Wu N, Murono EP (1994) A sertoli cell secreted paracrine factor(s) stimulated proliferation and inhibits steroidogenesis of rat Leydig cells. Mol Cell Endocrinol 106: 99-109.
- Da-Nian Q, Lung MA (2002) Morphometric study on Leydig cells in capsulotomized testis of rats. Asian J Androl4:49-53.
- Kumi-Diaka J, Rosanna R, Gould G (1998) Influence of genistein on the growth and proliferation of testicular cell lines. Biol Cell 90: 349-354.
- Fernanda MC, Gian CD, Sandra MT, Hamada AJ, Esteves SC, et al. (2013) A comprehensive review of genetics and genetic testing in azoospermia. Clinics 68: 39-60.
- Griswold MD (1998) The central role of Sertoli cells in spermatogenesis. Semin CellDev Biol 9: 411- 416.
- Socorro R, Horacio H, José AF, Minerva M, Gerardo D, et al. (2013) No relationship between biopsy sites near the main testicular vessels or rete testis and successful sperm retrieval using conventional or microdissection biopsies in 220 non-obstructive azoospermic men. Asian J Androl 15: 795-798.
- Petrova E, Ormandzhieva V (2012) Hypoxia and the male reproductive system. Andrology 21: 7-14.
- Jungwirth AT, Diemer GR, Dohle A, Giwercman Z, Kopa C, et al. (2014) Guidelines on male infertility. Eur Asso Urol 32: 5-24.
- Schwarzer JU (2013) No relationship between biopsy sites near the main testicular vessels or rete testis and successful sperm retrieval using conventional or microdissection biopsies in 220 non-obstructive azoospermic men. Asian J Androl 15: 795-798.
- Ilhem FZ, Samia A, Youcef  B, Omar K, Djamel S (2014) Effect of the consumption of milk of soya on the male fertility of swiss mice. Int J Pharm Pharm Sci 6:669-674.
- Chavarro, JE, Toth TL, Sadio SM, Hauser R (2008) Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod 23:2584-2590.
- Hamada AJ, Esteves SC, Agarwal A (2013) A comprehensive review of genetics and genetic testing in azoospermia. Clinics 68: 39-60.
- World Health Organisation (1999) Laboratory Manual for the Examination of Human Semen and Semen-cervical Mucus Interaction. (4th Eds.), Cambridge University Press, United Kingdom.
- World Health Organization (2010) WHO laboratory manual for the Examination and processing of human semen. (5th Eds.), WHO Switzerland.
- Kamal A, Gubartallah A, Ahmed A, Bakhiet O, Babiker A (2005) Comparative studies on reproductive performance of Nubian and Sannen bucks under the climatic conditions of Khartoum. J Anim Vet Adv 4: 942-944.
- Dada R, Gupta NP, Kucheria K (2001) Deterioration of sperm morphology in men exposed to high temperature. J Anat Soc India 50: 107-111.
- Schmahl J, Capel B (2003) Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol 258: 264-276.
- Svechnikov K, Izzo G, Landreh L, Weisser J, Soder O (2010) Endocrine disruptors and Leydig cell function. J Biomed Biotechnol 2010: 1-10.
- Assinder S, Davis R, Fenwick M, Glover A (2007) Adult-only exposure of male rats to a diet of high phytoestrogen content increases apoptosis of meiotic and post-meiotic germ cells. Reproduction 133:11-19.
- Hocking PM (1990) The relationship between dietary crude protein, body weight and fertility in naturally mated broiler breeder male. Br Poult Sci 31:743-757.
- Pavlova ED, Dimova E, Petrova Y, Gluhcheva, Atanassova N (2013) Changes in Rat Testis and Sperm Count after Acute Treatment with Sodium Nitrite. Bulgarian J Agric Sci 19:186-189.
Citation: Ssimbwa G, Eze ED, Sheu OS, Okpanachi OA, Afodun AM, et al. (2018) Postweaning Consumption of Soy isoflavones Induced Alterations on Some Reproductive Parameters of Prepubertal and Postpubertal Male Wistar Rats. Biochem Physiol 7: 247. DOI: 10.4172/2168-9652.1000247
Copyright: © 2018 Ssimbwa G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4681
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 3763
- PDF downloads: 918