Predictors of Seven-Day Mortality in Patients with Advanced Oncologic Liver Disease Admitted to a Palliative Care Unit
Received: 18-Jun-2018 / Accepted Date: 20-Jun-2018 / Published Date: 27-Jun-2018 DOI: 10.4172/2165-7386.1000339
Abstract
Context and objectives: A specific prognostic tool is lacking for end-stage oncological liver diseases. In addition to various factors, bilirubin, which reflects the severity of liver dysfunction, may be associated with mortality in this population. We aimed to assess how bilirubin influence survival in patients admitted in palliative care units with advanced oncologic liver diseases and to develop a prognostic model combining bilirubin with other factors. Methods: Data were collected retrospectively from 652 patients with oncologic liver diseases, accounting for 25% of all admissions in our palliative care units from 2011 to 2016. Age, gender, chronic liver diseases, infections including spontaneous bacterial peritonitis, gastrointestinal bleeding, encephalopathy, Eastern Cooperative Oncology Group score (ECOG), oral intake, jaundice, dyspnea, bilirubin, albumin and urea variables collected within 24 hours before or after admission were analyzed. Univariate and multivariate survival analyses were performed to identify the predictive value of bilirubin and other variables for 7-day survival. Results: Bilirubin value was collected in 398 patients. Univariate analysis showed that male sex, chronic liver diseases, encephalopathy, ECOG, oral intake, jaundice, bilirubin and urea blood levels, were associated with 7-day survival. Multivariate analysis showed that bilirubin>25 μ mol/L, urea>15 mmol/L, ECOG=4 and reduced oral intake, were independently correlated with survival. Accuracy of the model based on these factors to predict 7-day mortality is high (AUC=0.90). Conclusion: Bilirubin is an independent prognostic factor for 7 day-survival among patients with end-stage oncologic liver disease. Combining bilirubin, urea, ECOG and oral intake increases short term prognostication accuracy in this subgroup of patients.
Keywords: Prognosis; Palliative care; Liver cancer; Liver metastases; Bilirubin
Background
Oncologic liver disease, either secondary or primary, is a leading cause of death in the world [1]. Firstly; the liver remains the second organ most commonly affected by metastases after lymph nodes. Secondly, primary liver cancer is responsible for more than 750,000 deaths every year and its incidence is increasing worldwide, in relation to risk factors for cirrhosis: alcoholism, viral hepatitis and nonalcoholic fatty liver disease. By 2030, the number of deaths due to HCV should increase by 70% and the number of hepatocellular carcinoma by 85% [2]. Non-alcoholic fatty liver disease is also highly prevalent, being present in more than 20% of the population in Europe and North America and even higher in the Middle East and South Asia [3].
Patients with end-stage liver disease experiment frequent, severe and distressful physical and psychological symptoms. Moreover, complications due to hepatocellular insufficiency may come to the fore in patients with advanced liver cancer, including encephalopathy, gastrointestinal bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome and various respiratory symptoms [4,5]. However, according to the WHO, less than 15% of people needing palliative care currently receive it, although palliative care and hospice enrollment are associated with less aggressive end-of-life care and increased patient’s quality of life and perceived family satisfaction [6]. To reduce the proportion of late referral to palliative care during the last days of the last week of life is one quality outcome included in ASCO’s Quality Oncology Initiative [7]. Survival prediction is thus essential to consider palliative care referral and end-of-life decisions in patients with terminal cancer. In advanced stage cancer affecting the liver, survival depends not only on general performance status but also residual hepatic function.
In this context, general prognostic scores are of limited interest. These scores are based on the assessment of the ability to eat, consciousness disorders, dyspnea and general performance status [8]. The performance assessment tools of the Palliative Prognostic Index and the Palliative Prognostic Score, respectively the Palliative Performance Score and the Karnofsky Scale, indeed combine criteria which are difficult to measure accurately and subjects to interobserver variation [9].
Prognostic scores specific to liver diseases are not validated in endof- life palliative situations. The ten or so existing scores for the staging of hepatocellular cancers include criteria to measure liver function, such as Child Pugh’s score, presence of ascites, serum albumin and bilirubin levels. Whereas the Child Pugh’s score is the most widely used tool to estimate prognosis in liver diseases, this score is not relevant for patients in advanced palliative situations, being validated to predict 1- year survival [10-12]. The ALBI score was introduced and validated in the predictive survival scores of hepatocellular carcinomas, such as the Cancer of the Liver Italian Program, the Barcelona Clinic Liver Cancer, the Japan Integrated Staging [13-16]. Combining serum albumin and bilirubin, the ALBI score, is superior to the Child Pugh’s in estimating hepatic function remaining in patients with hepatocarcinoma and primary biliary cirrhosis. However, the ALBI score was validated to make a medium-term prognosis (60 months) in liver cancer situations and is therefore not useful for predicting the risk of death in the short term in the context of palliative care units, where survival is rather in weeks [17-19].
It is a remarkable fact that serum bilirubin is part of parameters shared among Child-Pugh's, ALBI prognostic scores. Although it remains unclear how bilirubin may shorten the survival of patients with end stage oncologic liver, serum bilirubin level is a marker of great interest in chronic liver diseases, whether oncological or not, as well as in cancers of the biliary tract and head of pancreas, accounting for significant causes of mixed hyperbilirubinemia. Consequently, we hypothesized that serum bilirubin could be a significant factor to considerate when developing a specific prognostic tool for short-term prognostication in advanced oncologic liver diseases.
The primary objective of this study was to show how bilirubinemia is associated with mortality in patients with advanced oncological liver diseases, admitted in palliative care units. The secondary objective was to develop a short-term prognostic tool, useful in these situations, by exploring the relevance of a score aggregating different factors including the bilirubin.
Patients and Methods
This study was a retrospective longitudinal analysis of all patients with primary or secondary cancer liver disease, admitted at Geneva University Hospitals Department of palliative care, between 2011 and 2017. Eligibility criteria included: an age more than 18 years, with or without previous chronic liver disease pre-existing advanced cancer liver disease, amid these 3 types of cancer:
• primary liver cancer,
• extended secondary liver cancer or
• biliary duct/pancreas cancer, responsible for intra-hepatic cholestasis with obstructive mixt hyperbilirubinemia, with or without liver metastases.
Exclusion criteria included: exclusively non-malignant liver diseases. A total of 652 patients (amid a total of 2600 admitted) fulfilling to inclusion criteria, were retrospectively evaluated.
A prior request to the Swiss Ethics Commission for research on human beings has been made to enable this research (N°2016-01474). This study was approved by the Institutional Ethical Board of Geneva, Switzerland. Written informed consent was not obtained from patients, who were deceased at the moment of inclusion.
Patients characteristics (age, gender), diagnoses (cancer’s type and localization; preexisting liver disease: hepatitis B and/or C, nonalcoholic steatohepatitis (NASH), alcoholic cirrhosis, other), as well as clinical and laboratory parameters were reviewed from the hospital database. Clinical parameters investigated in the analysis assessed at admission, namely: hepatic encephalopathy, delirium, consciousness disorders, dyspnea, and jaundice. Recent episodes of bacterial spontaneous peritonitis as well as other infections and gastrointestinal bleeding occurring within 7 days before admission were retrieved. Data concerning feeding ability (oral intake according to Palliative Performance Score’ criteria: normal or reduced/minimal sips/mouth care only) and general performance status (ECOG score; range, 0-4) at admission were gathered. Total bilirubin, albumin and urea blood levels, dosed on the day of admission were used [20].
Blood parameters
Routine laboratory measurements of bilirubin, albumin and urea concentrations were performed in our hospital’s laboratory with a coefficient of variation <5% over the range of measurement for these methods, as established by routine quality procedures.
Endpoints
Patients were followed up until death or last follow-up.
Statistical Analysis
Frequency (percent) for categorical variables, mean ± standard deviation, and median (interquartile range) values are given as descriptive statistics. Encephalopathy (defined as consciousness disorders, hepatic encephalopathy or delirium), spontaneous bacterial peritonitis or other infections, dyspnea, gastrointestinal bleeding, jaundice, liver chronic diseases preexisting cancer were dichotomized as absent (no) or present (yes). The serum total bilirubin levels were categorized as 0-25 μmol/L, >25 to 100 μmol/L, or >100 μmol/L (x4 upper normal value). The ECOG performance status was categorized as 1-2, 3 and 4. The serum urea levels were categorized as ≤ 7.5 mmol/L, 7.5-15 mmol/L or >15 mmol/L (x 2 upper normal value). The serum albumin levels were categorized as <28 g/L, 28-35 g/L or >35 g/L (thresholds values being the same than Child Pugh’s) [12].
Univariate analyses were used to demonstrate the association between survival and bilirubin level as well as for other possible factors. Overall survival from the day of admission into the palliative medicine service and seven day-survival of the subjects were estimated by Kaplan-Meier method. The log-rank test was used to compare the survival curves according to independent variables. A p-value <0.05 was considered as statistically significant.
A Cox proportional hazards regression model was used to investigate whether the relationship between 7-day survival and bilirubin with adjustment for potential cofounders collected at admission. Following adjustment factors were considered for inclusion in the model, including demographic, clinical, laboratory markers and diagnoses. Firstly, age, infections, dyspnea, encephalopathy, oral intake, ECOG score, serum bilirubin and urea levels. Secondly diagnoses: primary cancer sites and chronic liver diseases preexisting cancer. Graphical methods were used to check that the proportional hazards assumption was reasonably supported by data. The 7-day mortality was predicted from the factors previously mentioned using a multivariable logistic regression model. A backward selection procedure of variable was applied to obtain a parsimonious model. The predictive accuracy of the model has been expressed as a Harrell C index. The C index corrected for the optimism has also been reported.
Sample size calculation
Assuming a risk of type 1 error of 0.05 and that hyperbilirubinemia is found in 75% of patients admitted to the palliative care service, a sample size of 314 patients is needed to reach a power of 80% in detecting a hazard ratio of 1.5 for the association between bilirubin and mortality.
Results
Patients’ characteristics
We retrospectively included 652 patients admitted in palliative medicine from January 2011 to December 2016, known for advanced oncologic liver disease, either primary liver cancer (n=69, 10.6%), biliary duct cancer (n=36, 5.5%), pancreas cancer (n=128, 19.6%) or extended secondary liver cancer (n=419, 64.3%). The primary site of cancer responsible for liver metastases in descending order of frequency was pulmonary, colorectal and breast (Table 1). A total of 86.5% had no chronic liver disease preexisting cancer, whereas others were known for alcoholic cirrhosis (n=47, 7.2%), viral hepatitis (n=29, 4.2%) or NASH (n=4, 0.6%). Recent gastrointestinal bleeding, spontaneous bacterial peritonitis were less common than other infections, jaundice (n=173, 26.6%) or dyspnea (n=226, 34.8%) in our sample.
| n (%) or Median (interquartile range) | |
|---|---|
| Primary cancer sites | |
| Hepatobiliary | 105 (16.1) |
| Pancreas | 128 (19.6) |
| Lung | 103 (15.8) |
| Colorectal | 102 (15.6) |
| Breast | 61 (9.3) |
| Urinarytract | 40 (6.1) |
| Ovary/cervix/uterus | 31 (4.7) |
| Melanoma | 17 (2.6) |
| Chronicliverdisease | 88 (13.5) |
| Alcoholiccirrhosis | 47 (7.2) |
| HepatitisC | 16 (2.4) |
| HepatitisC+B | 5 (0.7) |
| HepatitisC+alcoholiccirrhosis | 3 (0.4) |
| HepatitisB | 5 (0.7) |
| Non-alcoholic steatohepatitis | 4 (0.6) |
| Other | 8 (1.2) |
| Female | 334 (51.2) |
| Age (years) | 73 (64-81) |
| Encephalopathy | 163 (25.2) |
| Missing data | 4 |
| Infection (including spontaneous bacterial peritoniti) | 145 (22.2%) |
| Spontaneous bacterial peritonitis | 16 (2.5%) |
| Missing data | 1 |
| Gastrointestinal bleeding | 27 (4.2%) |
| Missing data | 5 |
| Jaundice | 173 (26.6%) |
| Missing data | 1 |
| Dyspnea | 226 (34.8%) |
| Missing data | 3 |
| ECOG score | |
| 1 or 2 | 30 (4.6%) |
| 3 | 387 (59.8%) |
| 4 | 230 (35.5%) |
| Missing data | 5 |
| Oral intake | |
| Normal | 441 (68.7%) |
| Sips | 151 (23.5%) |
| Nothing | 50 (7.8%) |
| Missing data | 10 |
| Bilirubin, µmol/L | 19.0 (10.0 to 55.5) |
| Missing data | 253 |
| Albumin, g/L | 25 (21 to 30) |
| Missing data | 255 |
| Urea, mmol/L | 7.3 (4.9 to 11.6) |
| Missing data | 230 |
Table 1: Detailed patients’ characteristics.
The median survival time was 13 days (range 1-180). Nine patients died outside our service and were censored in survival analyses. The survival was 65.4% (95% CI 61.9-69.2) at 7 days, 17.7% (95% CI 15.0-20.9) at 30 days and 1.9% (95% CI 1.1-3.3) at 90 days.
399 patients had been tested for serum bilirubin at the time of admission. Bilirubin was less frequently collected in patients with encephalopathy (p=0.0011), reduced oral intake (p=0.0002) and a high ECOG score (p=0.0069) (Appendix 1). The frequency of jaundice was similar in patients with and without bilirubin test (26.6% in both groups). The survival was significantly better in the group of patients with bilirubin dosed than in the group with no collected value of bilirubin (p<0.0001) (Appendix 2). The 7-day survival was respectively 70.1% (95% CI 65.7 to 74.8) and 58.1% (95% CI 52.3 to 64.5) in both groups.
Associations with survival over entire follow-up
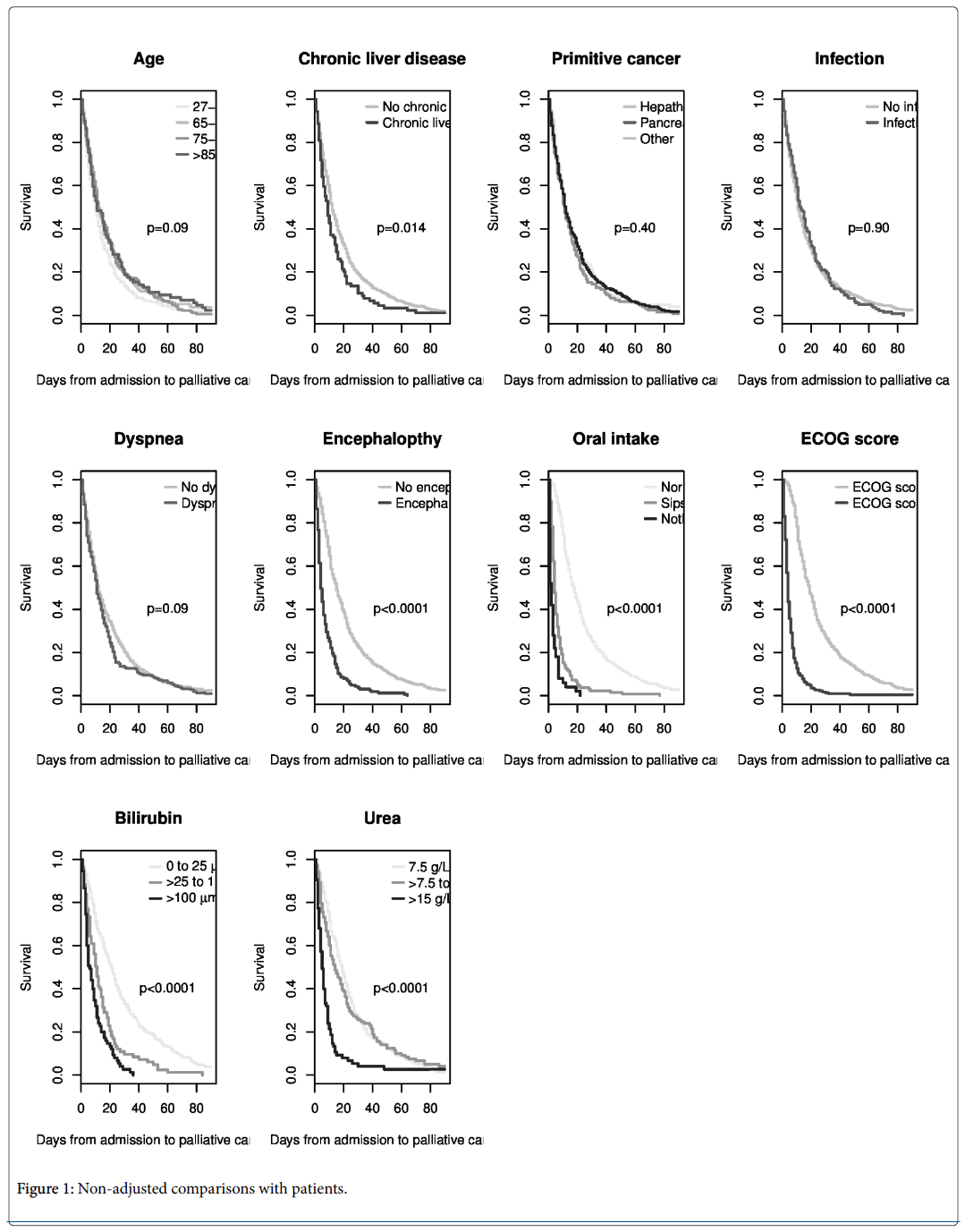
In non-adjusted comparisons (Figure 1), the associations with mortality were statistically significant for chronic liver disease, encephalopathy, oral intake, ECOG score, and bilirubin and urea levels. There were no statistically significant differences in survival according to age, localization of primary cancer, dyspnea, gastrointestinal bleeding, infections or albumin level. After adjustment, bilirubin and urea were still significantly associated with mortality (Table 2).
| Variables | 7-day mortality (95% CI) | Adjusted hazard ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Age (years) | <65 | 40.7 (33.1 to 47.4) | Ref | 0.0283* |
| 65-74 | 31.9 (25.0 to 38.2) | 0.78 (0.59 to 1.04) | 0.0949 | |
| 75-84 | 31.2 (24.3 to 37.5) | 1.07 (0.81 to 1.42) | 0.6325 | |
| ≥ 85 | 34.8 (24.1 to 44.0) | 0.70 (0.48 to 1.00) | 0.0481 | |
| Chronic liver disease | No | 32.9 (28.9 to 36.7) | Ref | - |
| Yes | 44.9 (33.6 to 54.4) | 0.92 (0.64 to 1.31) | 0.634 | |
| Primary site of cancer | Hepatic, biliary | 30.5 (21.1 to 38.7) | Ref | 0.0514* |
| Pancreatic | 36.7 (27.8 to 44.5) | 1.53 (1.04 to 2.25) | 0.0319 | |
| Other | 34.9 (30.2 to 39.3) | 1.47 (1.05 to 2.06) | 0.0247 | |
| Infection | No | 35.8 (31.4 to 39.8) | Ref | - |
| Yes | 30.3 (22.4 to 37.4) | 0.89 (0.69 to 1.14) | 0.3568 | |
| Dyspnea | No | 33.4 (28.7 to 37.8) | Ref | |
| Yes | 35.8 (29.3 to 41.8) | 1.31 (1.04 to 1.64) | 0.0203 | |
| Encephalopathy | No | 23.8 (19.9 to 27.5) | Ref | - |
| Yes | 66.9 (58.8 to 73.4) | 1.53 (1.11 to 2.11) | 0.0102 | |
| Oral intake | Normal | 14.3 (11.0 to 17.5) | Ref | <0.0001* |
| Sips | 72.8 (64.7 to 79.1) | 1.74 (1.25 to 2.41) | 0.0009 | |
| Nothing | 92.0 (79.5 to 96.9) | 5.90 (3.33 to 10.45) | <0.0001 | |
| ECOG score | 1, 2 or 3 | 10.3 (7.4 to 13.2) | Ref | - |
| 4 | 77.8 (71.8 to 82.6) | 4.12 (3.00 to 5.66) | <0.0001 | |
| Bilirubin | ≤25 | 18.2 (13.1 to 23.0) | Ref | <0.0001* |
| 25 to 99 | 37.9 (24.1 to 49.2) | 2.10 (1.59 to 2.79) | <0.0001 | |
| ≥100 | 51.9 (41.3 to 60.6) | 2.28 (1.62 to 3.21) | <0.0001 | |
| Urea | ≤7.5 | 18.8 (13.5 to 23.8) | Ref | <0.0001* |
| 7.5 to <15 | 29.3 (20.8 to 36.9) | 1.05 (0.82 to 1.34) | 0.7163 | |
| ≥15 | 66.7 (54.1 to 75.8) | 2.15 (1.58 to 2.93) | <0.0001 | |
Table 2: Association of bilirubin and urea with mortality.
Compared with patients with a level of bilirubin less than 25 μmol/L, the increase of mortality in patients with a level from 25-99 μmol/L and higher than 100 μmol/L was similar. For urea, the increase of mortality was statistically significant only for the higher category (more than 15 mmol/L). The highest hazard ratios were found for poor oral intake and high ECOG score.
Prediction of the 7-day mortality
Ten factors were investigated to predict the 7-day mortality. Levels of bilirubin and urea were collapsed into two categories (bilirubin ≤ 25 and >25 μmol/L, urea<15 and ≥ 15 mmol/L). The multivariable model without any selection of variables identified elevated bilirubin>25 μmol/L, urea >15 mmol/L, ECOG=4 and poor oral intake as factors associated with 7-day mortality. The backward procedure of selection retained these four factors. Odds ratios before and after the selection of factors are represented in Table 3.
| Variables | Without procedure of selection | With procedure of selection | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | ||
| Age (years) | <65 | Ref | 0.8165* | not retained | |
| 65-74 | 0.84 (0.33 to 2.14) | 0.717 | |||
| 75-84 | 1.08 (0.44 to 2.61) | 0.8724 | |||
| ≥ 85 | 0.63 (0.18 to 2.14) | 0.4564 | |||
| Chronic liver disease | No | Ref | not retained | ||
| Yes | 1.61 (0.58 to 4.50) | 0.3595 | |||
| Primary cancer | Hepatic, biliary | Ref | 0.1024* | ||
| Pancreatic | 2.40 (0.68 to 8.49) | 0.1742 | |||
| Other | 3.16 (1.06 to 9.45) | 0.0392 | not retained | ||
| Infection | No | Ref | |||
| Yes (incl. PBS) | 0.48 (0.20 to 1.14) | 0.095 | |||
| Dyspnea | No | Ref | |||
| Yes | 1.56 (0.75 to 3.25) | 0.2345 | not retained | ||
| Encephalopathy | No | Ref | |||
| Yes | 2.05 (0.88 to 4.80) | 0.0977 | |||
| Oral intake | Normal | Ref | 0.0020* | ||
| Sips | 3.01 (1.27 to 7.15) | 0.0126 | 3.40 (1.51 to 7.63) | 0.003 | |
| Nothing | 12.78 (2.04 to 79.94) | 0.0064 | 14.65 (2.57 to 83.61) | 0.0025 | |
| ECOG score | 1, 2 or 3 | Ref | Ref | ||
| 4 | 12.97 (5.72 to 29.39) | <0.0001 | 12.41 (5.87 to 26.25) | <0.0001 | |
| Bilirubin | ≤25 | Ref | Ref | ||
| >25 | 2.60 (1.26 to 5.36) | 0.0097 | 2.46 (1.26 to 4.81) | 0.0082 | |
| Urea | <15 | Ref | Ref | ||
| ≥15 | 4.19 (1.82 to 9.66) | 0.0008 | 4.33 (1.93 to 9.70) | 0.0004 | |
| *: A backward procedure for the selection of variables was used to obtain a parcimonious model. | |||||
Table 3: Odds ratios before and after the selection of factors.
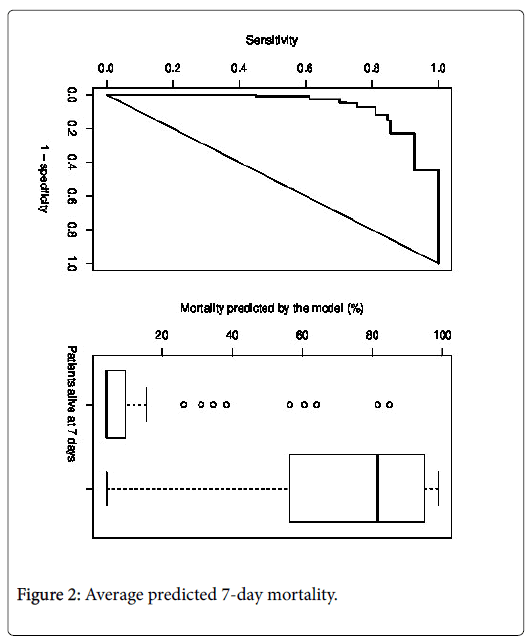
The C index of the retained model was 0.90 (95% CI: 0.86 to 0.94). The corresponding ROC curve is shown in Figure 2.
The correction of the optimism did not sensitively modify the C index (corrected C index: 0.89). The equation of the model is presented in Appendix 3.
More than three quarters of patients had a predicted 7-day mortality either lower than 10% (n=224, 58.8%) or higher than 80% (n=76, 19.9%) (Table 4). The 7-day mortality predicted by the model and the mortality observed in data were similar.
| Category of predicted 7-day mortality (%) | n (%) | Average predicted 7-day mortality (%) | Observed 7-day mortality, n (%) |
|---|---|---|---|
| < 10 | 224 (58.8) | 5.7 | 16 (7.1) |
| 10 to 50 | 55 (14.4) | 26.7 | 11 (20.0) |
| 51-80 | 26 (6.8) | 61.6 | 16 (61.5) |
| >80 | 76 (19.9) | 88.9 | 68 (89.5) |
Table 4: Representation of 7-day mortality (%).
The average predicted 7-day mortality was 13.1% in patients alive 7 days after admission in palliative care service and 68.1% in patients deceased in the 7 days following admission (Figure 2). The survival curves up to 90 days of follow-up over according to the predicted 7- day mortality are shown in Appendix 4.
Discussion
With increasing incidence of oncologic liver disease worldwide in relation with primary liver cancer and considering its high mortality, it is feared that the number of patients with oncologic liver disease admitted in palliative care units will continue to grow. Although there are already a number of general prognostic scoring systems for patients with oncologic diseases at the end-of-life, most of these scores consider only clinical factors subject to semi-quantitative assessment and lack accuracy. A specific prognostic tool was lacking for these situations, which accounted for 25% of all admissions in our population study.
This current study is the first to show that elevated serum bilirubin (>25 μmol/L), is an independent prognostic factor for 7 day-survival among patients with end-stage primary or secondary oncologic liver disease. Previous studies revealed the importance of bilirubin prognostic value in palliative care context, but they were conducted on non-specific oncologic populations, like those leading to validate the Objective Prognostic Score for 3 weeks survival prediction. The Objective Prognostic Score (OPS), was developed in a study including patients with all kinds of cancer, including 20% of hepatobiliary cancers. This score combines the same general performance score (ECOG score), than the one in our model. OPS biological criteria include leukocyte count, LDH and lymphocyte counts, serum creatinine and bilirubin levels above 34 μmol/L (or 2 mg/dL) [21,22].
The prognostic role of high concentrations of bilirubin could be mediated by numerous deleterious mechanisms. Bilirubin induces inflammation, oxydative stress and cellular apoptosis, particularly in erythrocytes, hepatocytes, glial cells and neurons [23-27]. It has been suggested that unconjugated bilirubin is involved with inflammatory signaling pathways leading to astroglial activation. Bilirubin neurotoxicity to glial cells could lead to encephalopathy and thus to death [28,29]. Study of populations with end-stage oncologic liver diseases could be of importance when clarifying bilirubin neurologic and general toxicity.
End-stage liver disease is indeed especially complicated by hepatorenal syndrome [4]. Considering renal complications impact on short-term survival, we chose not to consider whereas creatinine, one of the parameters in the OPS, was a predictive factor. Indeed serum creatinine reflects not only acute but also chronic poor renal function as well as muscle loss which is very common in end-stage oncologic situations.
We f ound high urea blood levels (>7.5 mmol/L) to be a poor 7-day survival prognostic factor. Because the cases of gastrointestinal bleeding accounted for less than 5% in our sample, urea reflected mainly acute kidney injury.
Thus, bilirubin and urea dosages are simple helpful laboratory tests, which should be considered when questioning survival of patients with oncologic liver disease, as this study indicated that these factors are reliable objective prognostic criteria in those situations.
Among the significant prognostic factors of survival, ECOG score=4 and reduced oral intake might contribute to short term prognostication’s accuracy in patients with oncologic liver diseases admitted in palliative care units. As previously reported in a wide variety of oncologic situations, these factors reflect a sharp decline in the overall general performance status [8,9]. ECOG score and poor oral intake criteria are also found in Palliative Performance Score (PPS) in the Palliative Prognostic Index and in Karnofsky’s scale in Palliative Prognostic Score score. But while the main criteria of the PPS and Karnosfsky scores are difficult to measure accurately and may be subject to inter-observer rating variations, it has been shown in this current study that including biological tests make the prediction more accurate.
Whereas previous studies leading to ALBI score validation showed that albumin level was a predictive factor in early stage oncologic liver diseases, in our study, albumin was found not to be a predictive factor in multivariate analyses [17-19]. In last months of life a majority of patients (90% in our sample) with oncologic situations, have nonspecific lower serum albumin levels due to poor nutritional status, systemic inflammation, not only to poor liver function. Our results suggest that near end-of-life albumin is no longer useful to measure liver function.
Complications such as recent gastrointestinal bleeding, spontaneous bacterial peritonitis was less common than other infections (1 patient of 5) or dyspnea (1 patient of 3) in our sample. But none of them were found to be significant prognostic factors.
We chose not to consider jaundice, as a predictive factor in our future model. Jaundice commonly appears when bilirubin blood level exceeds 40 μmol/L. But jaundice assessment is subject to inter-observer variation especially in mild jaundice cases. Otherwise, jaundice assessment could have been influenced by knowledge of bilirubin levels.
Limitations
First, we did not exclude patients that had diagnosis according to imaging criteria without biopsy. But clinical and radiologic presentation of patients made no doubt about existence of end-stage oncological liver diseases.
Second, situations in which bilirubin was not dosed were associated with poor prognosis. Indeed, bilirubin was less frequently collected in patients with encephalopathy, reduced oral intake and an high ECOG score (see Appendix 1). This reflects real life in palliative care context, where it is likely that in the presence of these factors, which are already known to be of poor prognosis, clinicians decided deliberately not taking blood samples and not making futile tests to the patients concerned. In the same manner, frail patients and proxy could have refute the utility of doing those tests. However, bilirubin dosing does not appear to have been influenced by whether the patients had jaundice or not, as the frequency of jaundice was similar in patients with and without bilirubin test (26.6% in both groups).
Finally, this was a retrospective, single-center study. However our study was enough powered for the analysis to be statistically significant. Future multicenter prospective cohort studies are required to confirm our results and validate a clinically usable score. Such a score could help facilitate end-of-life decisions and simplify patients' clinical pathways by facilitating the transition to palliative care.
One of the strengths of the study is the generalizability of the results, because we did not focus on one sole type of primary cancer such as hepatocellular carcinoma but on end-stage oncologic diseases causing liver dysfunction at the end-of-life. The proportion of preexisting chronic liver disease due to viral hepatitis or alcohol was low in our study population (viral hepatitis B and/or C<5%) compared with those reported in other countries especially in Asian geographic regions. Thus, the analysis of our sample might reflect mainly oncologic liver diseases influence.
Overall, the greatest strength of this study appears to be that the model we developed helps to identify 80% of patients with very high (>80%) or very low (<10%) 7-day-mortality risk.
Conclusion
This retrospective study demonstrated that bilirubin is an independent prognostic factor for survival among patients with endstage oncologic liver diseases, when admitted in palliative care units. Bilirubin >25 μmol/, urea >15 mmol/L, Eastern Cooperative Oncology Group score=4 and reduced oral intake are predictive of worse 7 daysurvival, in this subgroup of patients. As the population with liver cancer is increasing and although this model needs to be further validated in a multicenter trial, this information will be helpful when discussing end-of-life issues.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, et al. (2014) Cancer incidence and mortality worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer; 2014.
- Hatzakis A, Chulanov V, Gadano AC, Bergin C, Ben-Ari Z, et al. (2015) The present and future disease burden of hepatitis C virus infection with today’s treatment paradigm. J Viral Hepatitis 22: 26-45.
- Non-Alcoholic Fatty Liver Disease: Assessment and management. NICE Guideline (2016) National Guideline Centre (UK).London: National Institute for Health and Care Excellence (UK): 49.
- Larson AM (2015) Palliative care for patients with end-stage liver disease. Curr Gastroenterol Rep 17: 440.
- Surani SR, Mendez Y, Anjum H, Varon J (2016) Pulmonary complications of hepatic diseases. World J Gastroenterol 22: 6008-6015.
- Choi JY, Kong KA, Chang YJ, Jho HJ, Ahn EM, et al. (2017) Effect of the duration of hospice and palliative care on the quality of dying and death in patients with terminal cancer: A nationwide multicenter study. Eur J Cancer: e12771.
- The Quality Oncology Practice Initiative, Measures summary, spring: 2014.
- Maltoni M, Scarpi E, Pittureri C, Martini F, Montanari L, et al. (2012) Prospective comparison of prognostic scores in palliative care cancer populations. Oncologist 17: 446-454.
- Anderson F, Downing GM, Hill J, Lerch N (1996) Palliative performance scale (PPS): A new tool. J Pall Care 12: 5-11.
- Durand F, Valla D (2008) Assessment of prognosis of cirrhosis. Semin Liver Dis 28: 110-122.
- Peng Y, Qi X, Guo X (2016) Child-Pugh versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Fan H ed Medicine 95: e2877.
- Child CG, Turcotte JG (1964) Surgery and portal hypertension. Major Probl Clin Surg 1: 1-85.
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60: 646-649.
- Kudo M, Chung H, Osaki Y (2003) Prognostic scoring system for hepatocellular carcinoma (CLIP score): Its value and limitations and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 38: 207-215.
- Manghisi G, Elba S, Mossa A, Giorgio A, Aloisio V (1998) The Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology 28: 751-755.
- Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis 19: 329-338.
- Toyoda H, Kumada T, Osaki Y, Oka H, Urano F, et al. (2006) Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol 4: 1528-1536.
- Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, et al. (2015) Assessment of liver function in patients
with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol 33: 550-558.
- Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, et al. (2016) Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol 31: 1031-1036.
- Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, et al. (2015) New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol Hepatol 30: 1391-1396.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-655.
- Suh SY, Choi YS, Shim JY, Kim YS, Yeom CH, et al. (2010) Construction of a new, objective prognostic score for terminally ill cancer patients: A multicenter study. Support Care Cancer 18: 151-157.
- Jho HJ, Suh SY, Yoon SJ, Lee SS, Ahn HY, et al. (2016) Prospective Validation of the Objective Prognostic Score for Advanced Cancer Patients in Diverse Palliative Settings. J Pain Symptom Manage 52: 420-427.
- Trauner M, Meier PJ, Boyer JL (1998) Molecular pathogenesis of cholestasis. N Engl J Med 339: 1217-1227.
- Rodrigues CM, Sola S, Brito MA, Brites D, Moura JJ (2002) Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J Hepatol 36: 335-341.
- Alexandra Brito M, Silva RF, Brites D (2006) Bilirubin toxicity to human erythrocytes: A review. Clin Chim Acta 374: 46-56.
- Lang E, Gatidis S, Freise NF, Bock H, Kubitz R, et al. (2015) Conjugated bilirubin triggers anemia by inducing erythrocyte death. Hepatology 61: 275-284.
- Fernandes A, Falcao AS, Silva RF, Gordo AC, Gama MJ, et al. (2006) Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin. J Neurochem 96: 1667-1679.
- Brites D (2012) The evolving landscape of neurotoxicity by un-conjugated bilirubin: Role of glial cells and inflammation. Front Pharmacol 3: 88.
Citation: Rahm N, Calmant A, Combescure C (2018) Predictors of Seven-Day Mortality in Patients with Advanced Oncologic Liver Disease Admitted to a Palliative Care Unit. J Palliat Care Med 8: 339. DOI: 10.4172/2165-7386.1000339
Copyright: © 2018 Rahm N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 23123
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 22171
- PDF downloads: 952