Case Report Open Access
Prolonged Use of Dexmedetomidine Infusion in an Infant for Sedation as Adjuvant Therapy
Paola Genovese1*, Joseph Tobias1 and Anjana Kundu21Department of Anesthesiology and Pain Medicine, Nationwide Children's Hospital, Columbus, Ohio, USA
2Department of Anesthesiology, Dayton Children's Hospital, Ohio, USA
- *Corresponding Author:
- Paola Genovese
Department of Anesthesiology and Pain Medicine
Nationwide Children's Hospital, Columbus, Ohio, USA
Tel: (614) 722-4200
Fax: (614) 722-4203
E-mail: paogenovese@gmail.com
Received date: December 08, 2016; Accepted date: September 11, 2017; Published date: September 16, 2017
Citation: Genovese P, Tobias J, Kundu A (2017) Prolonged Use of Dexmedetomidine Infusion in an Infant for Sedation as Adjuvant Therapy. J Palliat Care Med 7:319. doi:10.4172/2165-7386.1000319
Copyright: © 2017 Genovese P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Dexmedetomidine, a central and peripheral alpha-2 receptor agonist approved by the FDA for the use in patients 18 years old and older, it has been increasingly used in the pediatric population for sedation and analgesia due to its advantage of minimal respiratory depression and lack of abuse/dependence. This is a case report of a successful use of dexmedetomidine in an infant for 15 weeks achieving; symptom control, reduction in the dose of opioids and benzodiazepines by 50% and a safe transition to oral equivalents.
Keywords
Dexmedetomidine; Pediatrics; Dexmedetomidine prolonged use; Opioid adjuvant
Introduction
Dexmedetomidine is a central and peripheral alpha-2 receptor agonist; its analgesic therapeutic effects are mediated centrally. The alpha-2 receptor is located on the vascular pre-synaptic neuron terminals where it inhibits the release of norepinephrine via negative feedback [1].
The medication is approved by the FDA for 24 hours or less for sedation in patients 18 y/o and older [2] in intubated and nonintubated patients in the Intensive Care setting and/or during surgical procedures. Another quality is its analgesic properties, although it may not be sufficient to use it alone, it has been used more and more frequently as an adjunctive agent along opioids.
It has been increasingly used off-label in the pediatric population for sedation and analgesia due to its advantage in safety, minimal to none respiratory depression and lack of dependence/abuse potential compared to other more traditional options (opioids/benzodiazepines) [3-5].
We report the efficacy and safety of the use of Dexmedetomidine infusion for a prolonged duration in an infant beyond current manufacturer and FDA recommendations.
Case Presentation
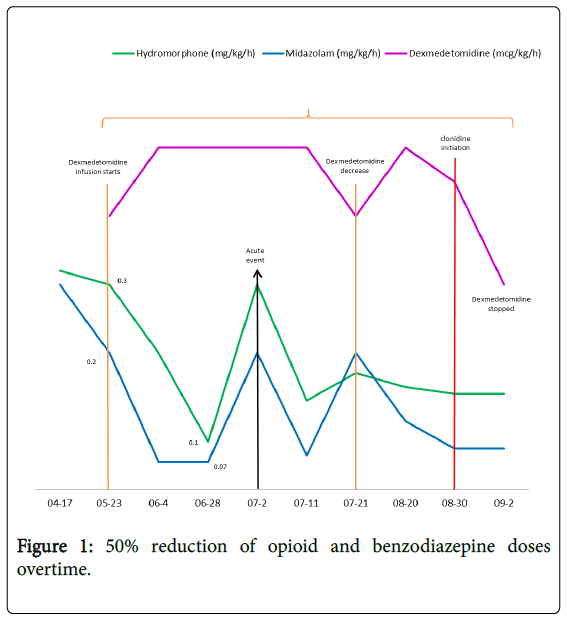
The patient was a male infant born at 34 weeks of gestational age due to preterm labor with the following diagnosis at birth: Trisomy 21, Esophageal stenosis, Duodenal atresia, Annular pancreas, complete balanced Atrio-Ventricular septal defect. With the following major complications during the hospitalization; Mixed apneas, tracheostomy placement, mechanical ventilation dependence, secondary pulmonary hypertension, venous and arterial clotting episodes leading to Leftabove the knee amputation, multifocal brain infarcts and seizures (Figure 1).
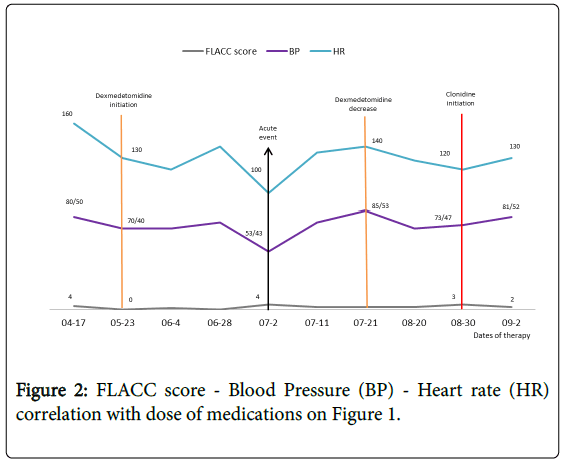
He required for sedation and analgesia the continuous infusion of; a benzodiazepine (Midazolam – maximum used dose 0.4 mg/kg/h) and an opioid together (Hydromorphone –maximum used dose: 0.7 mg/kg/h). After 11 weeks of unsuccessful pain control/unacceptable comfort level and inability to wean benzodiazepines and opioids dexmedetomidine was added to his regimen. The clinical goals were to decrease pain, improve comfort level and to wean opioids and benzodiazepines. The clinical parameters we followed were; Heart rate (HR), blood pressure (BP) and pain scale (FLACC score) as subrogate for sedation/comfort. The patient was ventilator-dependent therefore the effects of dexmedetomidine on the respiratory rate could not be evaluated (Figure 2).
Results
In 5 weeks, while on Dexmedetomidine the doses of benzodiazepine and opioid were decreased by at least 50% achieving the same or better degree of pain control/comfort. The doses decreased from 0.2 to 0.1 mg/kg/h for midazolam and from 0.3 to 0.07 mg/kg/h for hydromorphone.
In the 15 weeks of infusion of Dexmedetomidine, the patient did not show side-effects that required medical intervention (Bradycardia, Hypotension) he had one event of bradycardia/hypotension due to sepsis, but not related to the medication proved by the fact he recover his baseline parameters when the infection was controlled without changes in the dose of dexmedetomidine.
The patient was successfully transitioned to another alpha-2 agonist; oral clonidine over a 5-day transition without rebound of hypertension, tachycardia or an increase of the dose of opioids or benzodiazepines.
Discussion
Dexmedetomidine was safely used for longer than 24 hours (15 weeks, in this case) in a pediatric patient without major side-effects showing a potential expansion in the time and age limit of use of Dexmedetomidine. Another potential use of Dexmedetomidine is as an opioid and benzodiazepine adjuvant, allowing reduction to the dose of these medications, decreasing the potential side-effects and dependence on them, along with speeding up the weaning of these two potentially dangerous medications without creating dependence on its substitute. Besides the lack of dependence, the easy transition from an intravenous to an oral medication with similar properties (Clonidine, another alpha-2 agonist) makes the decision to introduce and use dexmedetomidine as an opioid/benzodiazepine adjuvant even more tempting in the ICU setting when aiming for faster transition off of continuous infusions and decreasing the hospital stay in ICU.
References
- Phan H, Nahata MC (2008) Clinical uses of dexmedetomidine in pediatric patients. Pediatric Drugs 10: 49-69.
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021038s021lbl.pdf
- Guinter JR, Kristeller JL (2010) Prolonged infusions of dexmedetomidine in critically ill patients. Am J Heath Syst Pharm 67: 1247-1253
- Oschman A, McCabe T, Kuhn RJ (2012) Dexmedetomidine for opioid and benzodiazepine withdrawal in pediatric patients. Am J health Syst Pharm 68: 1233-1238.
- Prommer E (2011) Dexmedetomidine: Does it have potential in palliative medicine? Am J Hosp Palliat Care 28: 276-283.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 2370
- [From(publication date):
September-2017 - Aug 15, 2025] - Breakdown by view type
- HTML page views : 1550
- PDF downloads : 820