Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Commentary Open Access
Provenance of Computers in Pharmacy
| Ramu Bandameedi* | |
| Department of Pharmacy, KVK College of Pharmacy, Hyderabad, Telangana, India | |
| Corresponding Author : | Ramu Bandameedi Department of Pharmacy, KVK College of Pharmacy Hyderabad, Telangana, India Tel: 919989078708 E-mail: bandameedi.ramu@gmail.com |
| Received: December 28, 2015; Accepted: January 27, 2016; Published: February 03, 2016 | |
| Citation: Bandameedi R (2016) Provenance of Computers in Pharmacy. Clin Pharmacol Biopharm 5:153. doi:10.4172/2167-065X.1000153 | |
| Copyright: © 2016 Bandameedi R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
| Introduction |
| Computers in pharmacy are used for the information of drug data, records and files, drug management (creating, modifying, adding and deleting data in patient files to generate reports), business details. The field of pharmacy is awe fully benefitted by use of computers getting and comparing the information to yield an accurate study. In field of operation like new drug discovery, drug design analysis, and manufacturing of drugs and in hospital pharmacy computers are widely used. The drug discovery, designing, manufacturing and analysis have become virtually possible only through the development of upcoming various hard wares and soft wares. Receiving the details, storing it and processing it and its dissemination is the main role of computers and this continuous flow of information shows effective functioning of any system [1]. |
| Applications of Computers in Pharmacy |
| 1. Usage of computers in the retail pharmacy |
| 2. Computer aided design of drugs (CADD) |
| 3. Use of Computers in Hospital Pharmacy |
| 4. Data storage and retrieval |
| 5. Information system in Pharmaceutical Industry |
| 6. Diagnostic laboratories |
| 7. Computer aided learning |
| 8. Clinical trial management |
| 9. Adverse drug events control |
| 10. Computers in pharmaceutical formulations |
| 11. Computers in Toxicology and Risk Assessment |
| 12. Computational modeling of drug disposition |
| 13. Recent development in bio computation of drug development |
| 14. In Research Publication |
| 15. Digital Libraries |
| Usage of computers in the retail pharmacy [2,3] |
| • Providing a receipt for the patient |
| • Record of transaction of money |
| • Ordering low quantity of products via electronic transitions |
| • Generation of multiple analysis for day, week, month for number of prescription handles and amounts of cash |
| • Estimation of profits and financial rational analysis |
| • Printing of billing and payment details |
| • Inventory control purpose |
| • Whenever the drugs or medicaments are added to the stock or else removed from stock; the position of stock gets updated instantaneously |
| • Records of various drug data, i.e., drug data information |
| • Computers are useful for getting the complete drug information which is used to satisfy the queries by patients about toxicology, adverse drug reactions, and drug-drug and drug-food interactions. |
| • Drug Bank Data Base gives complete and detailed description of drug (pharmacological and pharmaceutical action) and also involves bioinformatics and cheminformation. |
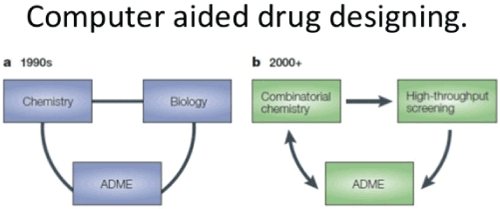
| Computer aided design of drugs (CADD) [4] (Figure 1) |
| • CADD is referred as a distinct and advanced drug designing process |
| • It is a process of pronouncement of new medications |
| • With a base of the refined graphics software existing or feed data the medicinal chemist have a scope to design the new molecules and improve their efficiency of the action (Figure 2) [5,6]. |
| Use of computers in hospital pharmacy [7] |
| • In receiving and allotment of drugs |
| • Storing the details of every individual |
| • Professional supplies |
| • Records of dispensed drugs to inpatient and outpatient |
| • Information of patients records |
| • Patient monitoring (blood pressure, pulse rate, temperature) |
| Data storage and retrieval [8] |
| • Hospital administration computers help in storage of data and recovery of data (retrieval) as there would be persistent changes coming up. It is frequently observed in the process of admission of patient their clinical and nursing staff, bed, operation theatre, intensive care unit, pharmacy department, radiological services, etc. |
| • As soon as the patient gets admitted computer records and reserves information like diagnosis, medication, demography, clinical information etc. |
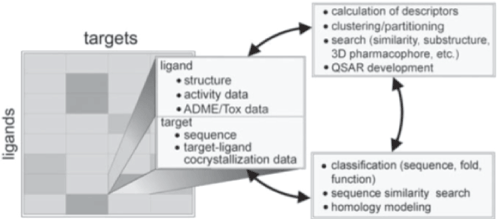
| Information system in pharmaceutical industry [9,10] |
| • Information system is the system which aggregates information technology with public which is very desirable and useful to them |
| • Many information managing technologies are implemented in their operations by pharmaceutical companies to amplify the chances of success and intensifying the efficiency in their production runs. |
| • With the advances in computer technology applications lead to the structured analysis and management of biological information which is useful for the exploration of biological processes with an aim to enrich in healthcare sector. |
| • Bioinformatics helps in data implementation and out benefits the data into reliable therapeutics thus emerging as an important tool for the development of standardized computer organizations [11]. |
| • Bioinformatics also developed novel drug molecules by using receptor based pharmacophore tool; generating pharmacophores by optimization of structural targets at the protein level at the specific binding site which were used in virtual screening and to get the set of ligand that show considerable activities [12,13] (Figure 3). |
| Pharmacoinformatics [14] |
| • It is the new originating information technology |
| • It includes neuroinformatics, bioinformatics, immunoinformatics, genoinformatics, metaboloinformatics, healthinformatics which are used as a base for the discovery of drugs [15-17]. |
| • The computers are needed to spread this pharmacoinformatics, i.e., transferring the data/information and knowledge to the public |
| • This evolving or emerging technology is becoming a fundamental component to pharmaceutical sciences. |
| • Medical informatics focuses on using information processing with in the clinical setting for medical billing, patient and resource scheduling, and patient care. |
| • Clinical informatics in the use of clinical decision support systems which provide feedback and instructions to healthcare service providers for maximizing patient compliance at the pharmaceutical care. |
| • “In silico” research informatics developed a pathway for medical discovery and investigation at every aspect of healthcare from basic investigation research to care delivery [18]. |
| • With the in depth analysis of medical information advances in informatics to primary health care sector creates the opportunity for rapid learning health applications to aid in bio medical research with safety as primary concern. |
| • With open access data and data sources among the health care information will build a digital platform for medical research assuring the proper evaluation of medical data and draw meaningful conclusions. |
| Diagnostic laboratories [19] |
| • Manual procedures were tiresome and time consuming whereas automated computerized instruments accomplish number of tasks with accurate results in diagnosis. |
| • Laboratory Information System (LIS) is used to preside huge amount of data. |
| • Instruments function as preprocessors, i.e., which will convert the raw data to digital format and finally gives the numerical values in the reports. |
| • The advancement of persuasive computers offer better and enhanced view and perception in radiology department |
| • In this many of the current imaging techniques such as Computerized Tomography (CT) and Magnetic Resonance Imaging (MRI) are inherently digital. In this computer conceives a “functional image” by executing intricate calculations on measured data [20,21]. |
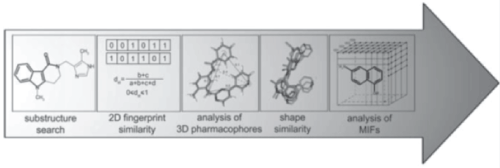
| Computer aided learning [22] |
| To update the latest advances in drug discovery and new therapies associated with the diseases web based education, digital libraries are playing a key role in pharmacy field education.to readily access and structure the information in a well-organized way computer aided learning is becoming as a part of pharmacy education. Simulative techniques, two way communication videos are helping to self-evaluating the process involved in decision making. As the presentations are retrieved and can be used many number of times will definitely help the learners to perform their operations effectively, safely and numerically judicious (Figure 4). |
| Clinical trial management [23,24] |
| Preclinical studies and clinical trials are the important part of current drug development which evaluates pharmacological benefits along with the toxicological risk associated with the medication. Computer software’s helping to ensure the safety therapeutic window for most of the drug candidates are listed below few examples. |
| 1. Oracle clinical V4i® from Oracle Corporation. |
| 2. Data LabsXC® from Data labs, Inc. |
| 3. Trial master® from Omnicomm systems. |
| 4. Cliniplus® Data management from DZC software solution, Inc. |
| 5. Openclinica by Akaza research (Cambridge, MA) |
| 6. Electronic data capture from DSG,Inc. |
| Adverse drug events control |
| Race among the pharmaceutical companies and huge demand to develop the new drug molecules for challenging diseases lead to exploration of new therapeutic approaches, but due to improper access of the post marketed data of the newly launched drug molecules. Several adverse drug reactions were not identified at the initial stages of treatment. With the implementation of food, drug and cosmetics act in 1938 improved drug safety [25]. However with lack of proper access and interpretation of drug safety data challenges in real time persisted and continue to persist to the present day. With development of computerized repository of post marketing voluntary adverse drug events reports helped to analyze adverse drug events .however, there were still no common platform for drug standards and interoperable system in place. To rectify this situation, the drug regulatory agencies and pharmaceutical industry began constructing a computerized repository of premarketing and post marketing clinical trial data that would ensure effective data analysis and decision making which lead to the implementation of new software for evaluating and monitor the adverse drug events [26]. |
| Reconfiguration of data bases and those validation is utmost important aspect before analysis of adverse drug events data .several database were designed with the application of computer such as adverse event reporting systems, spontaneous reporting system which is coded with COSTART (coding symbol for thesaurus of adverse reaction terms) dictionary which is further upgraded with MedDRA (medical dictionary for regulatory activities) system for coding [27,28]. |
| Computers in pharmaceutical formulations |
| Before a new drug can be released on the market developing in to a quality product with optimized formulation is an essence for approval from regulatory bodies. Correct choice of additives or excipients is paramount in the provision of efficacy, safety, stability. With developing advanced techniques scientists can experiment in multidimensional way; and process data to explore relationships with in the data set and optimize the formulation; to predict the outcome computer simulation for the development of mathematical models of the interaction between the experiment and formulations [29]. |
| Recent advances in mathematics and computer science resulted in development of these technologies that can be used to remedy the situation |
| Neural networks (an attempt to mimic the processing of human brain) [30] |
| Genetic algorithm (an attempt to mimic the evolutionary process by which biological systems self-organize and adapt) (Figure 5) [31] |
| Fuzzy logic (an attempt to mimic the ability of human brain to draw conclusions and generate responses based on incomplete or imprecise information) [32]. |
| The FDA suggests the use of design of experiments (DOE) because it provides a structured, organized method for determining the relationship between factors affecting a process and the response of that process.âÂ?Â? While it is possible to perform DOE with general statistical software, most users in the pharmaceutical industry look for software designed especially for DOE because it is generally much easier for non-statisticians to use. |
| DOE software enables the user to easily choose from a range of experimental designs. For example, mixture experiments are useful in many pharmaceutical applications. A typical mixture experiment might be used to investigate the effect of changing the proportions of polymer, drug and three excipients on four responses in a sustained release tablet based on a hydrophilic polymer. DOE software makes it easy to define the design space by entering low and high values for components. |
| The pharmaceutical Quality by Design (QbD) is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management. Quality by Design (QbD) is emerging to enhance the assurance of safe, effective drug supply to the consumer, and also offers promise to significantly improve manufacturing quality performance. |
| QbD software tool process includes |
| • Begin with a target product profile that describes the use, safety and efficacy of the product |
| • Define a target product quality profile that will be used by formulators and process engineers as a quantitative surrogate for aspects of clinical safety and efficacy during product development |
| • Gather relevant prior knowledge about the drug substance, potential excipients and process operations into a knowledge space. Use risk assessment to prioritize knowledge gaps for further investigation |
| • Design a formulation and identify the critical material (quality) attributes of the final product that must be controlled to meet the target product quality profile. |
| • Design a manufacturing process to produce a final product having these critical materials attributes. |
| • Identify the critical process parameters and input (raw) material attributes that must be controlled to achieve these critical material attributes of the final product. Use risk assessment to prioritize process parameters and material attributes for experimental verification. Combine prior knowledge with experiments to establish a design space or other representation of process understanding. |
| • Establish a control strategy for the entire process that may include input material controls, process controls and monitors, design spaces around individual or multiple unit operations, and/or final product tests. The control strategy should encompass expected changes in scale and can be guided by a risk assessment. |
| • Continually monitor and update the process to assure consistent quality. |
| ANN (Artificial neural network): It is a learning system based on a computational technique which can simulate the neurological processing ability of the human brain. The ANN has been applied to solving various problems such as product development QSARs (quantitative structure–activity relationships, QSPRs (quantitative structure–pharmacokinetic relationships), estimating diffusion coefficient, predicting the skin permeability and predicting the mechanism of drug action. |
| Computers in toxicology and risk assessment |
| In silico toxicity prediction have made great strides since its inception in 1962 [33-35] and to overcome the many problems following recommendation are made |
| • More toxicity data, of greater consistency are required |
| • A better mechanistic appreciation of clear toxicity is needed. |
| • The model user should consider use of a variety of techniques instead of relying on a single prediction. |
| Few software used in toxicology were listed below |
| DEREK Nexus: Predicts toxicological profiles by evaluating evidence for and against a broad collection of end points |
| • Literature look-up functionality for examination of data underlying a prediction. |
| HazardExpert: Identifies toxic molecules based on fragments |
| • Also calculates bioavailability, accumulation, and other parameters. |
| VirtualToxLab: Docking and QSAR hybrid approach for predicting activity on hERG, hormonal receptors, drug-metabolizing enzymes and their modulators. |
| MetaDrug: Systems pharmacology platform built on data from MetaBase |
| • Predicts mechanism of action, toxicity, and off-target effects. |
| Leadscope toxicity models: Collection of models for adverse craniological, hepatobiliary and urinary tract effects, as well as developmental, genetic, neurotoxic and reproductive toxicity and carcinogenicity. |
| TOPKAT: Predicts toxicity measures in a variety of in vitro assays and animal models. |
| Percepta Toxicity modules: Prediction of acute toxicity, aquatic toxicity, endocrine disruption, genotoxicity, hERG channel inhibition, irritation and health effects. |
| ADMET Predictor: Prediction of endocrine disruption, hERG channel inhibition, skin sensitization, phospholipidosis, and so on. |
| Computational modeling of drug disposition (Figure 6) |
| Several reviewers pointed out data quantity is the most limiting factor in ADMET modeling recent versions of gastro plus (simulation plus), Lancaster CA, PK-SIM (Bayer technology services, Germany), and ADME/TOX web (Pharma algorithms, Toronto, ON, Canada) advanced ADMET modeling [36-40]. |
| Recent development in bio computation of drug development |
| The major challenge that seems to emerge is the need for qualitative, testable and validated frame work for the joint analysis of large data sets available in disparate formats and focused on different biological facts. Integration of many disciplines biology, pharmacology, bioengineering, computer science, applied mathematics, physics and biostatistics can find out a solution to this problem [41]. |
| In research publication [42,43] |
| Submission and publication of research works of scholars in the form of manuscript is not an easy task, however with increased access to the research depositories and reviewing through various journals available online .computers helped as an effective tool in improving the high quality publication possible with the different stages of manuscript for authors, publishers and reviewers. Publications of investigations, examinations, scrutiny, and research work are the important countenances in every field including pharmacy too. The main aspect of the researchers is the publications of their own research work and computers play a key role in publishing their research work. When it comes to publish a paper or article it is dismayed and uneasy. But the computers are helpful in making corrections editing and making it very facile and multifaceted to assemble and publish the article. Computers provide different software s like notepad, word pad and especially Microsoft word for the accessible typing and processing. Microsoft word chiefly aids in correction of spellings grammatical mistakes and also administer primary options like insertion of page numbers table’s footnotes etc. By usage of computers which is also in one way helps in management of internet. Internet can exhibit or flourish different number of journals articles by a one click and also provides the information about submission process. Submission of papers is nowa- days very easy through internet by a website “electronic submission” which is also known as E-submission. After publishers publish the papers or articles online they are free to review by everyone shown in Figure 7. |
| Few softwares useful in research publication are given below: |
| Ithenticate: right now it is the one of the prominently used plagiarism detection software |
| Graph pad prism: for illustrating experimental data in to graphical representations and for analysis of kinetic data very useful. |
| Chem Draw and Corel Draw: these softwares are effetely utilized to draw chemical structures, and to view them as three dimensional (3D) models. |
| IBM SPSS Statistics is an integrated family of products that addresses the entire analytical process, from planning to data collection to analysis, reporting and deployment. With more than a dozen fully integrated modules to choose from, you can find the specialized capabilities you need to increase revenue, outperform competitors, conduct research and make better decisions. |
| Digital libraries [44,45] |
| Electronic collection of real or virtual resources known as digital libraries which accessed data anywhere in the world now widely accepted in all areas of education. Global expansion of internet and the increasing interest in development of digital library related technologies and collection helped to pace up the digitalisation of printed documents in the past few years (Figure 8). |
| Several advantages include |
| • Effective utilization of space without wastage. |
| • Expenses lowered for library maintenance and resource distribution. |
| • Information is stored in audio and video formats without the need of printed format |
| • Multiple access of a data by many users at a time. |
| • Less time required for searching specific items. |
| • Easy for distribution via internet, compact disk and digital versatile disk (DVD). |
| Conclusion |
| Computers are the effective tools for easy and effective access to relevant knowledge space across disjoined and exponentially growing research sources; it is becoming a crucial success factor for biomedical research. Computers can provide web -based knowledge space with search capabilities reaching beyond those of common search engines. One can envisage different approaches by using computers like highlighting the useful features of data set to ease exploratory methods that guide the search paths and discover unanticipated or unknown facts. Still with further investigation of applications of computing technology, we can reach to new heights in pharmaceutical research. |
References
- style="padding-left:25px; line-height:25px;" class="ref_width">
- Sean E (2006) Computer Application in Pharmaceutical Research and Development”, Wiley Interscience: A John Wiley and Sons Publication.
- Patil DJ (2008) Hospital and Clinical Pharmacy Published by NiraliPrakashan, Second Edition 10.1-10.5.
- Yadav S, Yadav M (2009) Computer assisted pharmacy services [CAPS] - A step towards pharmacy automation. Pharma buzz - A national pharma monthly magazine 4: 24-29.
- Jean-Pierre D, Jacques W (1997) Computer Aided Molecular Design: Theory and Application. Acedmic Press limited 1-10.
- Thomas PJ Catherine P (2006) Computer Assisted Drug Design: Methods and Applications Marcel Dekker INC19-52.
- Miller DD(2006) Remington: The Science and practice of Pharmacy. Lippincott Williams and Wilkins, 20th Indian edition 1: 477
- Yadhav AV, Yadhav BV (2008) Hospital and clinical pharmacy Second year diploma in pharmacy published by Niraliprakashan fifteenth edition 153-154.
- Richard F (2006) Information Technology for Pharmacist Published by Pharmaceutical Press 169-171
- Udayakumar M, Hemavathi K, Shanmugapriya P, Seenivasagam R (2013) Receptor-Based Pharmacophore Tool for Design and Development of Next-Generation Drugs International Int J Bioinform Res Appl 9: 487-516.
- Lexinwang (2001) computer-simulated pharmacology experiments for undergraduate pharmacy students: experience from an Australian university. Indian journal of pharmacology 33: 280-282.
- Stein L1 (2002) Creating a bioinformatics nation. Nature 417: 119-120.
- Goodford PJ (1985) A computational procedure for determining energetically favorable binding sites on biologically important molecules. J Med Chem28: 849-857.
- Bohm HJ (1992) The computer program LUDI: a new method for the de novo design of enzyme inhibitors. J Comput Aided Mol Des 6: 61-78.
- Seenivasagam R, Hemavathi K, Sivakumar G, Niranjan V (2013) Discovering novel carriers for oral insulin tablets: a pharmacoinformatics approach.Int J Bioinform Res Appl9: 184-206.
- Szarfman A, Tonning JM, Doraiswamy PM (2004) Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy 24: 1099-1104.
- Mallick M, Odedra D, Vidyarthi AS, Shankaracharya (2013) Meropenem: a potent drug against superbug as unveiled through bioinformatics approaches, Int J Bioinform Res Appl 9: 109-120.
- Etheredge LM (2007) A rapid-learning health system. Health Aff (Millwood) 26: w107-118.
- Gohlke H, Hendlich M, Klebe G (2000) Knowledge-based scoring function to predict protein-ligand interactions. J MolBiol 295: 337-356.
- Gohlke H, Hendlich M, Klebe G (2000) Predicting binding modes, binding affinities and ‘hot spots’ for protein-ligand complexes using a knowledge-based scoring function. Perspectives in Drug Discovery and Design 20: 115-144.
- Sotriffer CA, Gohlke H, Klebe G (2002) Docking into knowledge-based potential fields: a comparative evaluation of Drug Score. J Med Chem 45: 1967-1970.
- Hansch C, Maloney PP, Fujita T (1962) Correlation of biological activity of phenoxyacetic acids with Hammett subsistent constants and partition coefficients. Nature 194:178-180.
- Naidu S (2006)E-learning - A guidebook of Principles, Procedures and Practices, Published on behalf of the commonwealth educational media center for Asia by Dr. Usha V. Reddi, Director, CEMCA, New Delhi, 2ndrevised edition 1-3.
- FDA Science Forum: Advancing Public Health through Innovative Science.Washington, DC. April 27, 2005.
- Shaha YI, Paradkar AR., Dhayagude MG (2008) Introduction to Biostatics and Computer Science- For Pharmacy and Medical Student. NiraliPrakashan, Tenth Edition8.1 -8.5.
- Barnett ST, James JA (1995) Measuring the clinical development process. ApplClinTrials 4: 44-52.
- Szarfman A, Tonning JM, Doraiswamy PM (2004) Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy 24: 1099-1104.
- Reviewer Guidance Conducting a Clinical Safety Review of a New Product Application and Preparing a Report on the Review. U.S. Department of Health 13a and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER) February 2005.
- Gould AL (2003) Practical pharmacovigilance analysis strategies. Pharmacoepidemiol Drug Saf 12: 559-574.
- Bourquin J, Schmidli H, van Hoogevest P, Leuenberger H (1998) Comparison of artificial neural networks (ANN) with classical modelling technologies using different experimental designs and data from a galenical study on a solid dosage form. EurJ Pharm Sci6: 287-300.
- Rowe RC, Colbourn EA (2002) Applications of neural computing in formulation. Pharmaceutical Visions Spring Edition 4-7.
- Colbourn EA, Rowe RC (1996) Modeling and optimization of a tablet formulation using neural networks and genetic algorithms. Pharm Tech Eur8: 46-55.
- Rowe RC, Woolgar CG (1999) Neuro-fuzzy logic in tablet film coating formulation. Pharm SciTechnolo Today 2: 495-497.
- Barratt MD, Rodford RA (2001) The computational prediction of toxicity. CurrOpinChemBiol 5: 383-388.
- Norinder U (2005) Insilicomodelling of ADMET-a minireview of work from 2000 to 2004. SAR QSAR Environ Res 16: 1-11.
- Wolber G, Langer T (2005) LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J ChemInf Model 45: 160-169.
- Swaan PW, Szoka FC Jr, Oie S (1997) Molecular modeling of the intestinal bile acid carrier: a comparative molecular field analysis study. J Comput Aided Mol Des 11: 581-588.
- Langer T, Eder M, Hoffmann RD, Chiba P, Ecker GF (2004) Lead identification for modulators of multidrug resistance based on in silico screening with a pharmacophoric feature model. Arch Pharm (Weinheim) 337: 317-327.
- Mao Q, Unadkat JD (2005) Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J 7: E118-133.
- Ekins S, Johnston JS, Bahadduri P, D'Souza VM, Ray A, et al. (2005) In vitro and pharmacophore-based discovery of novel hPEPT1 inhibitors. Pharm Res 22: 512-517.
- Sheiner LB, Ludden TM (1992) Population pharmacokinetics/dynamics. Annu Rev PharmacolToxicol 32: 185-209.
- Sheiner L, Wakefield J (1999) Population modelling in drug development. Stat Methods Med Res 8: 183-193.
- Michael D(2014) Basics of research paper writing and publishing. Int J of Technology Enhanced Learning 6: 105-123.
- Fleming G (2014) Typing your research paper:Tips for working on the computer.
- Ambati V, Balkrishnan N, Reddy R, Pratha L, Jawahar CV (2006) The digital library of India project: Process, Policies and Architecture.
- Sood A, Chandrasekharan U (2004) Digital libraries in India - A baseline Study. A report commissioned by Educational Development Center (EDC).
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
Post your comment
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 603758
- [From(publication date):
February-2016 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 600401
- PDF downloads : 3357
