Recent Advances in the Chemotherapy of Breast Cancer
Received: 30-Jan-2018 / Accepted Date: 25-Mar-2018 / Published Date: 31-Mar-2018 DOI: 10.4172/2472-0429.1000126
Abstract
In the course of recent decades, the fundamental treatment of bosom tumor (early and progressed) has changed significantly. Chemoprevention is a recently introduced and quickly developing region of oncology that is distinguishing agents with a potentially preventive part in malignancy. For as far back as 40– 50 years, and since the disclosure and further restorative utilization of Tamoxifen, a particular estrogen receptor modulator, bosom disease treatment has turned to the advancement and accomplishment of custom fitted restorative treatment. Much still should be done in enhancing results, and particularly for the individuals who have propelled breast cancer, a testing zone for medicinal oncologists. Continuous global clinical trials are right now assessing new helpful methodologies and distinguishing particular natural subsets that could decide a patient's capacity to react to specific chemotherapeutic drugs.
Keywords: Breast cancer; Chemotherapeutic drugs; Breast cancer therapeutics; Endocrine-dependent breast cancer; HER2-positive Breast cancer; Metastatic breast cancer
Introduction
Chemotherapy is the utilization of pharmacologic products that hinder the advancement of intrusive disease either by obstructing the DNA harm that starts carcinogenesis or by capturing or switching the development of premalignant cells in which such harm has just happened. Late advances in our comprehension of the components of carcinogenesis have prompted the advancement of new medications that can repress tumor development. Approximately two million new cases are analyzed yearly. It likewise remains a main source of death with around 520,000 passing/year, as revealed by the WHO in a latest bosom growth actuality sheet of 2015. Restorative methodologies for bosom growth have changed over the span of late decades, and the utilization of fundamental treatment for right on time and propelled infection custom fitted to the every patient individually, holds the certification of passing on treatment to those in need and who could profit the most. In this report, rising helpful alternatives for patients with endocrine-subordinate bosom tumor, monoclonal antibodies for those with HER 2-positive infection, and fresher accessible foundational chemotherapeutic drugs will be discussed [1,2].
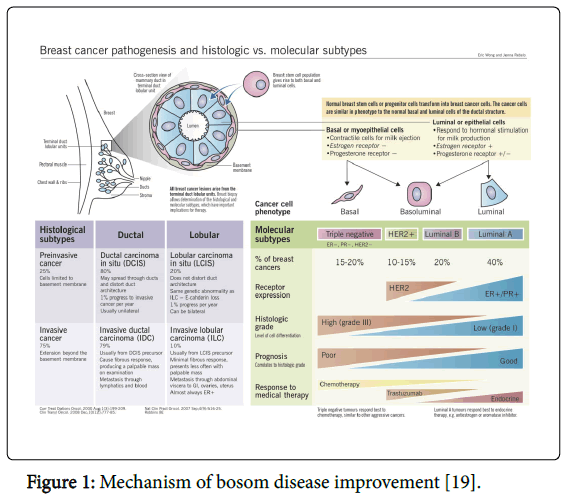
Mechanism of Breast Cancer
Up to 70 percent of all bosom growths, the tumor has receptors for the hormone estrogen. Today, these estrogen-receptor-positive malignancies can be dealt with moderately well. Since these tumors require estrogen for their development, the receptor is the objective of various medications that meddle with estrogen articulation, tie to the receptor or accelerate its degeneration. Be that as it may, around 33% of all patients does not respond to treatment or creates protection. So far it has not been conceivable to precisely anticipate who will react to this treatment in light of the fact that the basic sub-atomic systems are not yet seen completely (Figure 1) [3-18].
Cancer Detection Techniques
Oncotype DX test
Oncotype DX test report incorporates a Recurrence Score result (for patients with beginning time obtrusive bosom disease) or a DCIS Score result (for patients with non-intrusive bosom growth), which is a number in the vicinity of 0 and 100. The lower the Recurrence Score or DCIS Score, the lower the odds are that a lady's bosom growth will return; the higher the Recurrence or DCIS Score, the more noteworthy the odds that bosom disease will return. On account of beginning period obtrusive bosom tumor, the Oncotype DX test is the main genomic test that can likewise anticipate whether a lady will profit by chemotherapy. The lower the Recurrence Score result is, the more improbable a lady is to profit by chemotherapy, and the higher the Recurrence Score result, the more probable she is to profit by chemotherapy [20].
Prosigna Assay Techniques
CE stamping
The Prosigna bosom malignancy prognostic quality mark test, the nCounter Dx Prep Station and the nCounter Dx Digital Analyzer (the two sections of the nCounter Dx Analysis System) were CE set apart in October 2013 as Class I therapeutic gadgets under the In Vitro Diagnostic Medical Devices Directive 98/79/EC. The Prosigna measure is fabricated by NanoString Technologies.
Depiction
The Prosigna measure is an in vitro demonstrative device that utilizations data from quality articulation to ascertain the danger of ladies with HR positive beginning period bosom malignancy creating removed repeat 10 years from analysis. The examine measures the articulation profiles of qualities incorporated into the PAM50 quality mark, an arrangement of 50 qualities utilized for characterizing the 'inborn' subtypes of bosom disease (Parker et al. 2009), and in addition 8 housekeeping qualities (for standardization), 6 positive controls and 8 negative controls.
The Prosigna measure is performed on RNA disengaged from formalin settled paraffin installed (FFPE) bosom tumor tissue. A pathologist looks at the histology slides taken from the tumor biopsy, recognizing and denoting all regions containing bosom growth. A prepared professional at that point disconnects the RNA from these zones utilizing a RNA extraction pack. For the test to be done, tumor tissue must meet the accompanying criteria:
• Invasive ductal/not generally determined, intrusive lobular, or blended histopathological bosom malignancy write
• 10 μm thick tissue segments mounted on slides
• ≥ 4 mm2 tumor estimate
• ≥ 10% tumor cellularity (the extent of growth cells in an example)
• ≥ 125 ng RNA (12.5 ng/μL)
• tumour territory: ≥ 100 mm2 for 1 slide, or ≥ 20– 99 mm2 for 3 slides, or ≥ 4–19 mm2 for 6 slides.
Investigation is finished utilizing the nCounter Dx Analysis (a sidekick framework made by NanoString Technologies) [21].
Before surgery
Chemotherapy for bosom growth: Bosom growth chemotherapy is regularly used to treat patients with privately progressed or metastatic bosom disease. Your restorative oncologist may prescribe chemotherapy before you experience bosom disease surgery (neoadjuvant) or after surgery (adjuvant):
Neo-adjuvant (or essential fundamental) bosom malignancy chemotherapy: May be utilized before surgery to decrease the span of expansive bosom tumors and to annihilate growth cells. This sort of chemotherapy regularly makes bosom monitoring surgery conceivable. It additionally helps our disease specialists decide the adequacy of a specific regimen on the bosom tumor.
Adjuvant bosom growth chemotherapy: May be utilized after surgery or radiation treatment to dispose of any residual tumor cells that might not have been expelled amid bosom disease surgery and additionally radiation treatment. It might likewise keep the illness from spreading to different parts of the body.
A few cases of chemotherapy drugs used to treat bosom disease include: anastrozole (Arimidex®), bevacizumab (Avastin®), capecitabine (Xeloda®), cisplatin (Platinol®), cyclophosphamide (Cytoxan®), doxorubicin (Adriamycin®), doxorubicin liposomal infusion (Doxil®), exemestane (Aromasin®), fluorouracil (5-FU), gemcitabine (Gemzar®), ixabepilone (Ixempra®), letrozole (Femara®), paclitaxel (Taxol®) and trastuzumab (Herceptin®).
After surgery: After bosom malignancy surgery, our torment administration experts are accessible to help control your agony, and our mind-body advisors may give directing. Our recovery specialists may enable you to recapture quality and oversee issues with muscle fit, snugness or lymphedema. On the off chance that reproduction isn't performed quickly, a picture improvement master is accessible to enable you to discover approaches to look and can rest easy.
What's more, previously, amid and after your bosom tumor strategy, our dietitians and naturopathic clinicians are accessible to enable you to remain nutritiously sustained by giving eating routine and supplement proposals.
Targeted treatment for bosom tumor: Not withstanding chemotherapy drugs, we may suggest natural reaction modifiers, for example, Trastuzumab (Herceptin®), to treat bosom tumors that create excessively of, or overexpress, a protein called HER2. On the off chance that research center testing uncovers the HER2 quality in the growth cells, the drugs can enable close to down the HER2 quality, accordingly cutting the tumor cells off from their vitality supply [22].
As there is a continuous research for safest and efficacious drug for treating cancer, the year 2017 has seen the similar amount of research followed by approval and in the process of getting approval of many drugs. However, providing the complete information on them is out of the scope of this article. So, here in this article we attempt to provide the information on three drugs (Selection:Safety and Efficacy profile) Ribociclib, Palbociclib, Pertuzumab with their approval process, mechanisam of their actions, dose & side effects profile, contraindications along with combination therapy.
New treatment approval for certain breast cancers by FDA Ribociclib (Kisqali)
On 13th March, 2017, the U.S. FDA affirmed ribociclib (KISQALI, Pharmaceuticals Corp {Novartis}.), a cyclin-subordinate kinase 4/6 inhibitor, along with an aromatase inhibitor as primary endocrinebased treatment.
Randomized, twofold-blind, duplicate treatment-controlled, global clinical trial (MONALEESA-2), are based to endorsement of medication in post-menopausals with hormone receptor HR-positive, human epidermal development factor receptor HER2-negative progressed or metastatic bosom tumor who got no earlier treatment for advanced ailment. Randomized to takr either ribociclib in addition to letrozole (n=334) or duplicate treatment in addition to letrozole (n=334) of aggregate 668 patients. 600 mg of Ribociclib or duplicate treatment orally was given once every day for 21 continuous days, trailed by 7 days off, with letrozole 2.5 mg regulated orally once day by day for 28 days. Treatment proceeded until diseas development or unsatisfactory toxicity [6].
A pre-arranged interval viability examination showed a change in Progression free survival [PFS] with danger proportion of 0.556 (95% CI: 0.429, 0.720; p<0.0001). The evaluated average PFS had not been come to in the ribociclib-containing arm and was 15 months in the duplicate treatment containing arm. Objective reaction rate (ORR) in patients with quantifiable sickness was 52.7% (95% CI: 46.6, 58.9) in the ribociclib in addition to letrozole arm and 37.1% (95% CI: 31.1, 43.2) in the fake treatment in addition to letrozole arm. General survival information are immature [5].
Mechanism of action: CDKs 4 and 6 are compounds that have been appeared to advance cell division and increase in both ordinary and disease cells through multiplication. Numerous disease cells have indicated variations showing expansion of the CDK activity, prompting the inactivation of certain tumor suppressor genes [12].
At the point when utilized as a part of with different medications in combination, for example, an ALK or a MEK inhibitor, ribociclib has been appeared to have a synergistic impact, bringing about enhanced responses [19]. Again, this is likely a consequence of ‘’crosstalk’’ between signaling pathways. Essentially blocking one pathway in disease tumorigenesis can once in a while result in "tumor compensation", where the tumor makes up for the blocked signaling pathway by using different pathways to survive. By obstructing a few pathways immediately, it is suspected that the tumor is less ready to adjust, and a more noteworthy hostile to tumor reaction is regularly watched. Using ribociclib in blend with different specialists has been appeared to diminish the advancement of protection from these agents [13]. As it were, disease's improvement of medication resistance can be relieved with the expansion of ribociclib to the restorative administration.Refer Tab 1 for quick glance about drug.
Palbociclib (IBRANCE)
On March 31, 2017, granted consistent endorsement to palbociclib (IBRANCE®, Pfizer Inc.) by the U.S. FDA for the treatment of (HR) +ve, (HER2) –ve advanced or metastatic breast cancer along with an aromatase inhibitor as primary endocrine based treatment in postmenopausals [3,4].
Quickened endorsement in Feb 2015 was conceded by FDA, in blend with letrozole for the therapy of ER +ve, HER2-ve propelled bosom growth as starting endocrine based treatment in postmenopausal ladies. Standard endorsement in Feb 2016 was allowed by FDA, in blend with fulvestrant for the treatment of HR+ve, HER2-negative progressed or metastatic bosom malignancy in ladies with ailment movement receiving endocrine treatment [4].
From a global, randomized, fake treatment controlled, twofold visually impaired, clinical trial (PALOMA-2) endorsement is based that randomized 666 postmenopausal ladies (2:1) to palbociclib in addition to letrozole or fake treatment in addition to letrozole. Palbociclib 125 mg or fake treatment was controlled orally once every day for 21 sequential days, trailed by 7 days off. Letrozole 2.5 mg was managed orally once day by day. Treatment proceeded until illness movement or unsatisfactory danger. It was nearly 25 months in the palbociclib plus letrozole and 14.5 months in the duplicate treatment in addition to letrozole arm for movement free survival (PFS) (HR=0.576, 95% CI: 0.463, 0.718, p<0.0001). General survival information are juvenile.
Security information was assessed in 444 patients who got palbociclib in addition to letrozole. Low count of WBC was the most oftentimes revealed unfavorable response with an occurrence of 80% [4].
Mechanism of action: It is a particular inhibitor of the cyclin subordinate kinases CDK4 AND CDKG [9,10].
In the G1 period of the cell cycle, mammalian cells must pass a checkpoint known as the limitation guide, R, all together toward finish the cell cycle and partition. CDK4 and CDK6 complex with cyclin D to drive the phosphorylation of the retinoblastoma protein, Rb, which enables the cell to pass R and focus on division [10]. Regulation of at least one proteins associated with this checkpoint is lost in numerous diseases. Notwithstanding, by hindering CDK4/6 palbociclib guarantees that the cyclin D-CDK4/6 complex can't help in phosphorylating Rb. This keeps the cell from passing R and leaving G1, and thus from continuing through the cell cycle [11]. Refer Tab 1 for quick glance about drug.
Pertuzumab(PERJETA)
On 20th Dec, 2017, granted consistent endorsement (PERJETA, Genentech, Inc.) by the FDA for use in blend with trastuzumab and chemotherapy as adjuvant therapyt of patients with HER2 +ve early bosom growth at high danger of reference [7,8].
In view of information from APHINITY (NCT01358877) approval is done , a multicenter, randomized, twofold -blind, duplicate therapycontrolled trial in 4804 patients with HER2+ve early bosom malignancy who had their essential tumor excised prior to randomization. Patients were then randomized to receive pertuzumab or duplicate therapy, in mix with adjuvant trastuzumab and chemotherapy. Invasive disease-free survival (IDFS) is the principle viability result, characterized as the time from randomization to first event of ipsilateral neighborhood or provincial obtrusive bosom tumor repeat, far off repeat, contralateral obtrusive bosom growth, or passing from any cause [8].
The extent of IDFS occasions in the expectation to-treat populace was nearly 7% (n=170) in the pertuzumab arm and nearly 8.5% (n=210) for those accepting duplicate therapy (HR 0.82; 95% CI: 0.67, 1.00; p=0.047) after a average follow-up of 45 months,. High-hazard patients included such as those with HR-ve or those with node +ve bosom growth. In patients with HR-ve ailment the extent of IDFS events was about 8% (n=70) and 10.5% (n=91) in the pertuzumab and duplicate therapy arms, individually (HR 0.76, 95% CI 0.56, 1.04). For patients with node +ve ailment the extent of IDFS events was 9% (n=135) and 12% (n=180) in the pertuzumab and duplicate therapy arms, respectively (HR 0.77, 95% CI 0.62, 0.96). General survival data are immature [7].
Mechanism of action: When activated HER 2, sets off signal transduction through many pathways that empower cell expansion and cell development; if more expressed it can cause unpredicatble growth. It is due to mutation that outcomes in higher expression of HER2 in around 20-30% of bosom malignancy tumors [15].
In the same way as other receptors HER2 typically consolidates another protein keeping in mind the end goal to work (a procedure called dimerization); it can tie with a next HER2 receptor (going about as ahomodimer) and it can heterodimerize with an alternate receptor of the HER family. The strongest dimer for initiating signalling pathways is HER2/HER3 [14].
The epitope for pertuzumab is the area of HER2 where it bounds to HER3, and pertuzumab avoids the HER2/HER3 dimer from shaping, which pieces motioning by the dimer [15-17]. Trastuzumab is another monoclonal acting agent against HER2; its epitope is where HER2 bounds to another HER2 protein. These two monoclonal antibodies together avoid HER2 from functioning (Table 1) [17].
| Name of the Drug | Approved date | Indication and usage drug | Dosage and administration | Dosage form and strength | Adverse effects | Side effects | Combination therapy |
|---|---|---|---|---|---|---|---|
| Ribociclib (KISQALI) | March 13, 2017, by U.S. and FDA | Indicated for primary endocrine-based therapy for the therapy of postmenopausals (HER2)-ve bosom tumor. | Recommended starting dose: Three 200 mg tablets taken once daily for 21 days followed by off therapy for 7 days. | Tablets 600mg or placebo for 21days | fatigue, diarrhea, nausea, Neutropenia. | Hepatobiliary toxicity | Combination therapy with letrozole |
| Neutropenia | |||||||
| Palbociclib (IBRANCE) | March 31, 2017, by U.S. and FDA | indicated for the therapy of (HR)+ ve, (HER2)-ve bosom tumor. | Recommended starting dose: 125 mg taken followed by off therapy for 7 days. | Capsules: 125 mg once orally for 21 days | nausea, stomatitis, Neutropenia, infections, leukopenia,anemia, alopecia, vomiting, decreased appetite, diarrhea, thrombocytopenia, rash, asthenia, and pyrexia. | Neutropenia, Pulmonary Embolism | Combination therapy with fulvestrant. |
| Impairment of Male Fertility | |||||||
| Pertuzumab (PERJETA) | Decemner 20, 2017, by U.S. and FDA | indicated for the therapy of patients with HER2 +ve bosom tumor who have not taken before an anti-HER2 treatment. | For IV administration only. Do not give as an IV push or bolus. (2.3) · The primary dose is 840 mg administered as a 60-minute IV infuse, then next every 3 weeks thereafter by 420 mg given as a 30 to 60 min IV infusion. | 420 mg/14 ml single-use vial. | diarrhea, alopecia, fatigue, rash, neutropenia, nausea, and peripheral neuropathy | Embryo-fetal toxicity | PERJETA is a HER2 and combined with trastuzumab and docetaxel |
| Left Ventricular Dysfunction | |||||||
| Hypersensitivity |
Table 1: Brief information of drugs approved by FDA for breast cancer.
Conclusion
The upcoming of new medications by advents mainly focusing on particular significant targets has prompted extensive advance in the therapy of bosom tumor in the course of recent years. Challenges remain, for example, protection to systemic therapy, huge cost of medications, and constrained accessibility in numerous nations of proper ailment services.
We should keep on finding approaches to enhance our accessible innovation to give legitimate direction to those continuing their life with the disease, and for the people at danger of creating, and to grow new, more viable treatments to considerably enhance breast cancer patients' results around the globe. Tailoring medications to the every particular patient maintains the guarantee of directing them as they confront troublesome treatment choices with an end goal to enhance their long term results.
Acknowledgement
The authors are thankful to the Scientists who carried out this research and Journals for providing articles for providing information and also management of Vishwa Bharathi College of Pharmaceutical Sciences, Perecherla, Guntur, Andhra Pradesh, India for providing facilities to carry out this review article.
References
- https://communitymedicine4asses.wordpress.com/2015/02/25/who-factsheet-on-cancer-updated-on-25th-february-2015/
- Hong W, Sporn MB (1997) Recent advances in chemoprevention of cancer. American Association for the Advancement of Sci 278: 1073-1077.
- RichardS Finn (2016) Palbociclib and letrozole in advanced breast cancer. NJEM 375: 1925-1936.
- Tumer NS (2015) Palbociclib in Hormone receptor positive advanced breast cancer. N Engl J Med 373: 209-219
- Gabriel (2016) Ribocliclib as first line therapy for HR- positive advanced breast cancer. N Engl J Med 375: 1738-1748.
- FDA approves new treatment for certain advanced or metastatic breast cancers. Silver Spring, MD: US Food and Drug Administration. September 28, 2017.
- Minckwitz GV (2017) Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med 377: 122-131.
- Swain SM (2015) Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med 372: 724-734.
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, et al. (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. BCR 11: R77.
- Rocca A, Farolfi A, Bravaccini S, Schirone A, Amadori D (2014) Palbociclib (PD 0332991): targeting the cell cycle machinery in breast cancer.Expert Opin Pharmacother 15: 407-20.
- Hanxiao Yu, Shengnan Liu, Qian Yuan, Xun Mani, Sridhar Pestell, et al. (2017) Recent advances of highly selective CDK4/6 inhibitors in breast cancer.J of Hematology and Oncology10: 97.
- Samson, Kurt (2014) LEE011 CDK Inhibitor Showing Early Promise in Drug-Resistant Cancers.Oncology Times 36: 39-40.
- Kim S, Loo A, Chopra R, Caponigro G, Huang A, et al. (2014) Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6- Reactivating Rb in cancer. Molecular Cancer Therapeutics 12: PR02
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125409s109lbl.pdf
- Harbeck N (2013) HER2 Dimerization Inhibitor Pertuzumab - Mode of Action and Clinical Data in Breast Cancer.Breast care8: 49–55.
- Eric W (2012) Breast cancer pathogenesis and histologic vs. molecular subtypes. Pathophysiology Rev.
- http://www.mybreastcancertreatment.org/enUS/LearnAboutOncotypeDX/OncotypeDXScores
Citation: Navya V, Pavan S, Swami AP (2018) Recent Advances in the Chemotherapy of Breast Cancer . Adv Cancer Prev 3: 126. DOI: 10.4172/2472-0429.1000126
Copyright: © 2018 Navya V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 10429
- [From(publication date): 0-2018 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 9202
- PDF downloads: 1227