Review Article Open Access
Recurrent Cervical Cancer: How Can Radiology be Helpfull
| Dulce Antunes1* and Teresa Margarida Cunha2 | |
| 1Centro Hospitalar Lisboa Norte–Hospital St Maria, Lisbon, Portugal | |
| 2Instituto Português de Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal | |
| Corresponding Author : | Dulce Antunes Centro Hospitalar Lisboa Norte–Hospital St Maria, Lisbon, Portugal E-mail: dulceantu@gmail.com |
| Received May 06, 2013; Accepted August 12, 2013; Published August 19, 2013 | |
| Citation: Antunes D, Cunha TM (2013) Recurrent Cervical Cancer: How Can Radiology be Helpfull. OMICS J Radiology 2:138. doi: 10.4172/2167-7964.1000138 | |
| Copyright: © 2013 Antunes D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Uterine cervical carcinoma is one of the most common malignancies occurring in females and although the longer survival provided by the advances in early diagnosis and effective treatment, 30% of the patients develop persistent or recurrent disease. Recurrent disease is defined by local tumor re-growth or development of nodal or distant metastases at least 6 months after the lesion has regressed. Recurrent disease can occur centrally in the uterus or vaginal vault, laterally in the pelvic wall, can present as pelvic and extra-pelvic lymph node disease and as distant metastases. Although CT can be useful for surveillance MR is the most accurate imaging tool for characterization of pelvic recurrence. MR findings depend on previous therapeutics so the knowledge of imaging features of surgery and of the irradiated pelvis are crucial. Dynamic contrast-enhanced subtraction MR and diffusion weighted images give a valuable contribute in differential diagnosis of pelvic recurrence and inflammatory effects of radiation therapy but biopsy and serial imaging may be warranted. The authors review the spectrum of imaging findings of recurrent cervical carcinoma.
| Keywords |
| Uterine cervical carcinoma; Radiation therapy; CT scans; MR imaging |
| Introduction |
| Cervical carcinoma still represents a significant public health problem. Around 11,069 cases of invasive cervical cancer were diagnosed in the United States in 2008 and 3869 women died from the disease. Worldwide, cervical cancer has an even greater impact, with nearly 529,828 new cases and 275,128 deaths reported annually [1]. |
| Patients with early stage cervical cancer treated with surgery or radiotherapy are likely to have a good prognosis [2]. However, substantial treatment failure still occurs and about 30% of the patients treated for cervical carcinoma develop progressive or recurrent tumors. Patients who suffer recurrence have a dismal prognosis with a 1-year survival rate of between 15 and 20% [2]. |
| Recurrent cervical cancer is defined as local tumor re-growth or the development of nodal or distant metastases at least 6 months after the lesion has regressed. Residual disease is that which is evident within 6 months of primary treatment. The average rate of recurrence is that approximately two-thirds of cases recur within the first 2 years following initial treatment, and 90% of cases recur by 5 years [3]. |
| The risk of treatment failure depends on the initial staging and, consequently, on the chosen therapeutics. Risk factors for recurrence of cervical carcinoma include the histological type, size, depth of stromal invasion, and the nodal status at presentation. |
| Pelvic recurrence can locate centrally or laterally in the pelvis. Extra-pelvic recurrence most commonly involves para-aortic lymph nodes, lungs, liver and bone [4]. |
| Imaging Surveillance |
| The early detection of recurrent disease in the pelvis may pose a diagnostic challenge, because symptoms are nonspecific, physical examination of the irradiated pelvis is often limited and cytological examination of the irradiated cervix has a poor sensitivity [5]. For these reasons and given that the therapeutic options for recurrence of cervical carcinoma depend on the location and extent of the recurrence, imaging can be crucial for early diagnosis and adequate management [4]. |
| There are no formal recommendations based on a good level of evidence for the follow up of the patients treated for cervical carcinoma [6]. |
| Mabuchi et al. [7] showed that routine physical history, physical examination, and CT scans were the most common first abnormal tests leading to the 86% of the diagnosis of recurrent disease. CT is an effective diagnostic tool for detection of recurrent tumor [8], however has little use in differentiating recurrent tumor from posttherapeutic changes [9]. There is no consensus in the reviewed literature regarding the indication of pelvic MR for the follow-up of patients with medically treated cervical carcinoma and MR is usually performed when there is a clinical suspicion of recurrence. After surgery there is also no consensus for a systematic MR follow up with an exception for trachelectomy. Patients submitted to trachelectomy should be evaluated by MR at 6 months and 1 year given the higher recurrence rate comparing to patients who undergo brachytherapy or chemotherapy [10]. |
| Normal Post-Therapeutical Imaging Findings |
| Imaging evaluation of the treated cervical carcinoma depends on the knowledge of the normal post-therapeutical aspects after surgery and chemo-radiation therapy. Usually follow up MR is not indicated during the first 6 months after completion of radiation therapy due to limitations of MR in differentiating recurrent tumor from postoperative and post-radiation changes [9]. |
| The commonest surgical procedure is the Wertheim-Meigs surgery that comprises radical hysterectomy with resection of the upper third of the vagina, excision of parametrial and paravaginal tissue, including the sacro-uterine ligaments, and dissection of pelvic and para-aortic lymph nodes. |
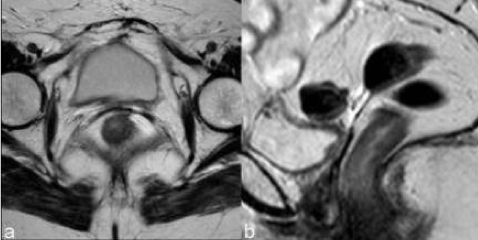
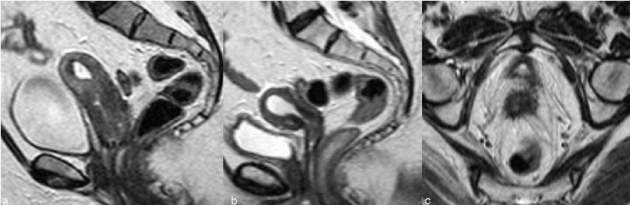
| The vaginal fornix is represented, both on CT and MR, by a soft-tissue linear structure [11] (Figure 1). MR has the advantages of multiplanar evaluation of the fornix and better definition of the vaginal wall. Normal vaginal vault has a strongly hypointense muscular wall in T2WI, with well-defined and regular contour. Frequently there is fibrotic scar tissue whose T2WI signal is slightly higher than the normal muscular tissue. |
| Laparoscopic vaginal radical trachelectomy is a fertilitypreserving alternative to radical hysterectomy or chemoradiation in young women with stage IA2 to IB cervical cancers [12]. The technique involves a proximal vaginotomy, cervical resection, and paracervical and paravaginal dissection. The cervix is resected, with preservation of the corpus uteri and an end-to-end anastomosis with the remaining vagina. Bilateral pelvic lymphadenectomy is also performed [13]. |
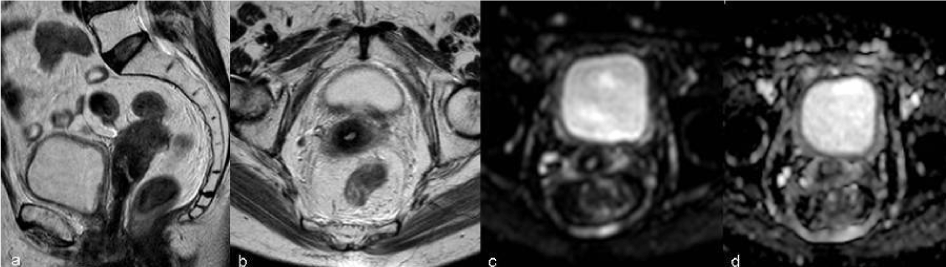
| The imaging findings of the uterus after trachelectomy include the end-to-end anastomosis of the uterine corpus to the vaginal vault (Figure 2), frequently with a posterior neofornix that can present with nodular configuration. These MR features should be known to avoid false positive diagnosis of recurrent tumor. |
| The para-vaginal dissection may cause vaginal wall thickening that is more prominent in the first 6 months and slowly diminishes till 1 year. Sometimes there is also loss of the low signal intensity on T2WI and this diffuse thickening can be mistaken for recurrent disease infiltrating the vaginal wall [13]. |
| In the evaluation of patients submitted to radiation therapy MR is more effective than CT [8]. In postmenopausal patients, radiation therapy does not result in any significant changes of the uterus at MR imaging. In premenopausal patients the junctional zone and outer myometrium become indistinct and, after 6 months, the endometrium becomes thin and hypointense [14]. |
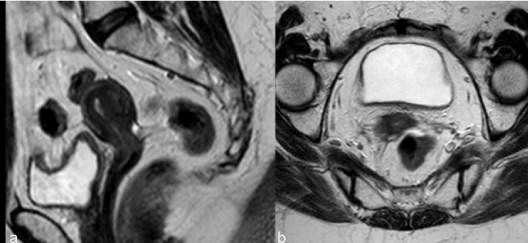
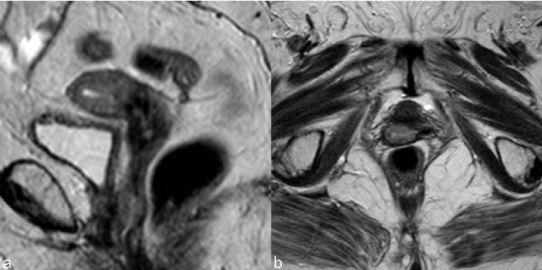
| Tumors treated with RT respond with a decrease in size and signal intensity on MR imaging (Figure 3). The response may be immediate (3-6 months) or, in larger tumors, delayed (6-9 months) [15]. Hricak et al. [14] reviewed the MR images of 69 patients submitted to radiotherapy to cervical cancer and reported that the reconstitution of the normal zonal anatomy of the cervix and homogeneous cervical low signal have high negative predictive value (97%) for recurrence. An early (2–3 months) and significant decrease in the signal intensity and volume of the tumor indicates a positive response to radiation therapy and a high probability of complete remission [16]. |
| Tumor Recurrence |
| The accuracy of MR for the diagnosis of recurrence after radiotherapy depends on the elapsed time since the ending of the therapeutics. In the first 6 months necrosis, inflammation and edema contribute to the higher signal intensity on T2WI making differential diagnosis with tumoral disease harder [17]. |
| It is consensual that dynamic contrast-enhanced subtraction MR imaging for the differentiation of benign from malignant lesions is advisable for recurrence detection. |
| Diffusion-weighted MR also gives a valuable contribute for the evaluation of the irradiated cervix [18]. Hyperintense signal on high b-value images associated with lower ADC values are suggestive of recurrent tumor. |
| Multiple studies found significant increases in lesion ADC values in patients treated with combined chemo-radiation therapy, however the post-therapeutical values might be inferior to normal cervical tissue (due to the presence of edema, hyaline degeneration and granulation tissue). Chen et al. [19] found that the difference of ADC values between normal cervical tissue and cervical area after therapy was statistically significant (P < 0.01) and reported that the optimal ADC threshold values for distinguishing between cervical area before and after therapy is 1.255×10-3 mm2/s (sensitivity of 95.5% and specificity of 100%) and between normal cervical tissue and cervical area after therapy is 1.525×10-3 mm2/s (sensitivity and specificity of 70% and 81.8%, respectively). |
| Weber et al. [20] found that the most helpful finding for differentiating recurrent cervical tumor from radiation fibrosis was the presence of a soft-tissue mass with higher signal intensity than that of adjacent pelvic sidewall muscle on T2WI [12] and Hricak et al. [14] documented that these findings carry a recurrence probability of 86%. However the recurrent tumor may show diffuse growth making differential with post-therapeutics scar more difficult [9]. |
| Recurrence exhibits an earlier and more pronounced enhancement compared with scar tissue, on condition that MR is not performed earlier than 6 months after the end of therapy [9]. |
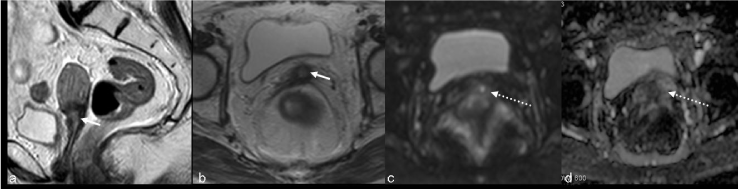
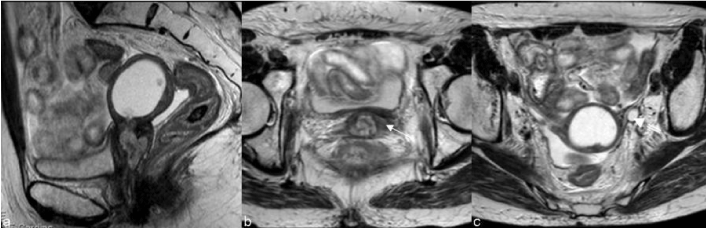
| Findings like widened endocervical canal and cervical diffuse high signal intensity are nonspecific and MR might not be conclusive (Figure 4). |
| The parametrium soft tissues may also undergo fibrotic changes and appear hypointense. This post-radiation imaging appearance may mimic parametrial invasion [21] thus becoming indistinguishable from the tumor. The intravenous administration of contrast material and DWI images can also help distinguishing between radiationinduced parametrial fibrosis and residual or recurrent disease [19]. |
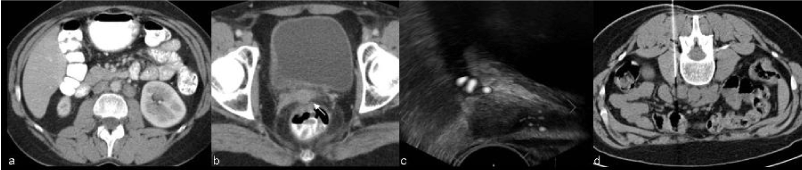
| When morphological and functional MR evaluation is inconclusive biopsies may be warranted (Figure 5). Roy et al. [22] showed transrectal ultrasound guided biopsy to be a feasible, safe and accurate method for establishing a histopathological diagnosis of recurrence and CT guided biopsy of lymph nodes and metastases is a current procedure (Figure 6). |
| In addition, serial or follow-up MR imaging is useful for distinguishing recurrent disease from radiation-induced fibrosis, as the latter is expected to remain stable or diminish over time [8]. |
| Most cervical tumors are 18F-fluorodeoxyglucose (FDG) avid, with exception to adenocarcinomas, which may reveal low FDG uptake. Revision of studies using FDG-PET/CT in the evaluation of recurrence/metastases in patients with uterine cancer revealed high diagnostic accuracy with 90-93% sensitivity, 81-100% specificity, and 87-96% accuracy [23]. It is especially useful for the assessment of peritoneal dissemination, lymph node metastases, local recurrence, and bone/muscle metastases and may have the greatest utility in situations in which tumor marker levels are rising and conventional imaging studies show negative or equivocal findings [3,23]. |
| Central Tumor Recurrence |
| About 30-45% of recurrences after radical hysterectomy are central-pelvic-type, generally situated above the vaginal cuff, between the bladder and the rectum (Figure 7). Five-year survival rates reported by literature for these central recurrences vary from 6% to 77% [16]. Patients with a central recurrence seem to have a better prognosis compared to those with recurrence of the pelvic wall [24]. |
| Central recurrence can affect the cervix, vagina, parametria and ovaries and grow to involve the bladder, rectum, or side wall. After radical hysterectomy most tumors show supra-vaginal recurrence (20%) at the vaginal stump (Figure 8) and at the recto-vaginal space [9]. Skip lesions in the vagina may be seen in recurrent cervical cancer, in which case lesions may be seen in the distal vagina (Figure 9). Patterns of involvement of the vagina follow the growth pattern of the primary cervical cancer (eg, as a mass- like lesion or as an infiltration along the vaginal wall) [25]. |
| Kim et al. [26] reported a higher incidence of central recurrence among patients treated by radiation therapy than by surgery. |
| The most common CT and MR imaging feature of recurrent tumor of the primary site is an irregular mass averaging 4-7 cm in diameter between the bladder and rectum [26]. Recurrent tumor may be recognized on CT scans obtained with intravenous contrast material, on which it may appear as a soft-tissue mass with diminished enhancement compared with that of normal cervical tissue [8]. On MR recurrence has high signal intensity on T2WI, similar to the corresponding primary tumor [8] (Figure 10). |
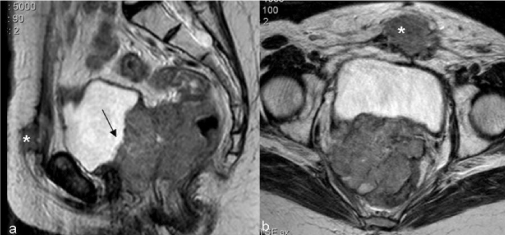
| Hydrometra, depicted by CT and MR as a symmetrically enlarged uterine corpus containing non-enhanced fluid [9] can be associated with central recurrence due to the obstruction of the internal os by the tumor. However it can also be due to radiogenic fibrosis and its appreciation depends on the definition of a tumoral mass (Figure 11). |
| Central pelvic recurrence with anterior extension may lead to ureteral obstruction by direct encasement of the ureter or by tumor infiltration of the bladder wall, which results in obstruction at the ureteral orifice [9]. |
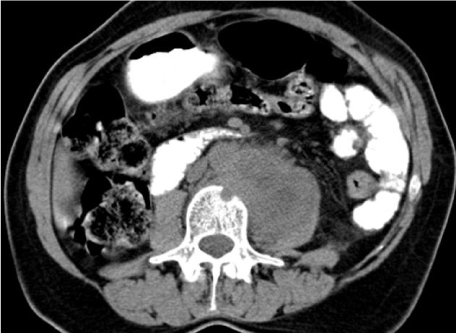
| Although the loss of the adipose cleavage plan of the tumoral mass with bladder and rectum rise the suspicion of visceral invasion the imaging diagnosis depends on the identification of luminal tumoral mass. The MR imaging findings of bladder invasion include nodularity and irregularity of the bladder wall, masses protruding into the bladder lumen, high signal intensity of the anterior aspect of the posterior bladder wall, and abnormal soft-tissue strands in the utero-vesical space [27]. Invasion of the rectum usually occurs at the recto-sigmoid junction and is detected by mass effect, spiculation, and luminal narrowing (Figure 12) [28]. |
| Pelvic Side Wall Recurrence |
| Pelvic side wall recurrence is characterized either by direct invasion of the mass to the pelvic side-wall by means of a linear strand of soft tissue, and involving loss of the fat planes, or by confluence of the solid mass and the muscle of the pelvic wall [27]. At times pelvic recurrences are identified as pelvic side wall masses that are not associated with a centrally located pelvic mass [28]. |
| Recurrent tumor of the pelvic sidewall may manifest with pain due to nerve infiltration or leg edema due to obliteration of the lymphatics. |
| The imaging criteria are the same used for the initial staging: the distance tumor–side wall is less than 3 mm; the piriformis and obturator internus muscles can be enlarged and demonstrate an enhancing soft-tissue mass; the iliac vessels can be encased and narrowed by tumor and direct extension and destruction of the pelvic wall [15]. |
| Kim et al. [26] reported that there is no significant difference in the incidence of pelvic side-wall extension between patients who undergo surgery and those treated by radiation therapy. |
| Lymph Node Recurrence |
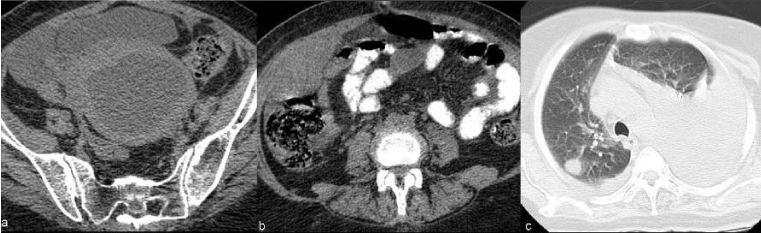
| In uterine cervical carcinoma lymphatic involvement has traditionally been separated into primary and secondary nodal groups. The first of these consists of the paracervical, parametrial, internal and external iliac, and obturator nodes, while those comprised by the second include the sacral, common iliac, inguinal and paraaortic [29]. In general, paracervical and parametrial lymph nodes are involved first, followed by the obturator nodes, the remaining external iliac nodes, and the internal iliac nodes. Extra-pelvic lymph nodes, including the supra-clavicular, para-aortic (Figure 13) , and inguinal, are common sites of distant metastases. The prevalence of recurrence at extra-pelvic lymph nodes is higher in patients treated with radiation therapy than in those who undergo surgery; this is probably because this tumor stage is generally more advanced. Multiple extra-pelvic and extra-abdominal nodal sites of recurrence, including the para-bronchial, supra-clavicular, and axillary nodes, have been reported but are less frequently involved than the primary and secondary groups [8]. |
| Yang et al. [18] compared the results of CT, MR and pathological evaluation of 949 lymph nodes in 43 women with biopsy proven cervical cancer and concluded that helical CT and MRI imaging showed similar accuracy (89.5% and 85.5%, respectively). |
| The findings of nodal metastases range from scattered, minimally enlarged lymph nodes to large, conglomerate nodal masses at CT and MR imaging [8]. Although enlarged lymph nodes may be hyperplastic and lymph nodes smaller than 1 cm may contain metastatic disease, nodes with a transverse diameter greater than 10 mm are usually considered malignant. Central necrosis within a lymph node is a strong predictor of metastatic disease (positive predictive value of 100%) [18,30]. Recent studies showed the potential of DW imaging to differentiate benign from malignant pelvic lymph nodes based on their underlying mean ADC value [31]. |
| Distant Recurrent Disease |
| After the pelvis and lymph nodes, the solid organs of the abdomen are the most frequent sites of involvement of recurrent cervical carcinoma [28]. |
| Liver metastases are present in one-third of patients and appear as solid masses with variable enhancement. Adrenal metastases are usually from cervical adenocarcinomas and are present in approximately 15% of patients. Peritoneal metastases appear as soft-tissue masses and ascites and are present in 5%–27% of cases in autopsy series (Figure 14) [15,28]. |
| Imachi et al. [29] documented pulmonary metastases in 6% of 817 patients with uterine cervical carcinoma and 81% of those had local recurrence or other distant metastatic lesions. Lymphangitic carcinomatosis is seen in less than 5% of patients and appears as diffuse interstitial lung disease [25]. Mediastinal or hilar adenopathy and pleural lesions or effusions are present in approximately onethird of patients with metastatic disease to the chest [15]. |
| The prevalence of osseous metastases (Figure 15) in the setting of recurrent cervical carcinoma ranges from 15% to 29%. The vertebral bodies are the most frequently involved bones, followed by the pelvis, ribs, and extremities. The most common mechanism of bone involvement is by direct extension of neoplasm from paraaortic nodes into the adjacent vertebral bodies (Figure 13). It can also occur by lymphatic or hematogenous spread or from direct extension of pelvic recurrence. Bone metastases may appear as destructive lesions associated with soft-tissue masses of variable size. The absence of a soft tissue mass, slow progression, blastic elements, and sharply defined borders on CT suggest radiation necrosis [30]. In some lesions gadolinium-enhanced fat-suppressed T1WI is useful for the diagnosis by the definition of foci of enhancement within the marrow space [28]. |
| Conclusion |
| Uterine cervical cancer recurrence is common (about two thirds within the first 2 years) and carries a dismal prognosis. |
| CT and MRI play key roles in identifying recurrent disease. Although both have reasonable sensitivities [3] it is generally accepted that MR is superior in distinction between tumor and post-radiotherapy inflammatory and fibrotic changes. Dynamic contrast-enhanced subtraction and diffusion weighted MR imaging are advisable. PET/CT has been considered a highly accurate imaging technique for restaging [23]. |
| Knowledge of the wide spectrum of imaging findings after surgery and radiotherapy is crucial for evaluation after cervical cancer therapy. Reconstitution of the normal zonal anatomy of the cervix and homogeneous cervical low signal have high negative predictive value (97%) for recurrence. The most specific sign of central recurrence is a hyperintense soft-tissue mass (T2WI) with earlier and pronounced enhancement. Imaging criteria of pelvic wall recurrence are the same used for the initial staging (distance tumor–side wall is less than 3 mm). Published studies show a certain degree of overlap between benign and malignant pelvic lymph nodes, similar accuracy for CT and MR and have not been able to define the negative predictive value of DW imaging [31]. Lung, liver and bone are the most common locations of distance recurrence [32]. |
References |
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
 |
 |
 |
 |
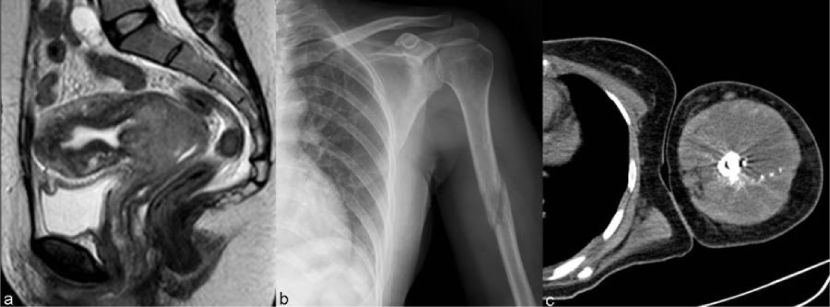 |
| Figure 11 | Figure 12 | Figure 13 | Figure 14 | Figure 15 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 16607
- [From(publication date):
September-2013 - Jul 30, 2025] - Breakdown by view type
- HTML page views : 11859
- PDF downloads : 4748
