Research Article Open Access
Ribavirin Transporter [Ent1] Polymorphism is a Pretreatment Predictor of Virologic Response: The Specific Role of Donor Liver Transporter
Valerio Giannelli1, Maurizio Simmaco2, Luana Lionetto2, Giovanna Gentile2, Michela Giusto1, Francesca Romana Ponziani3, Antonio Gasbarrini3, Ubaldo Visco-Comandini4, Adriano Pellicelli5, Stefano Ginanni Corradini1, Antonio Molinaro1, Elisa Biliotti6, Manuela Merli1* and Gloria Taliani6
1Department of Clinical Medicine, “Sapienza” University of Rome, Italy
2NESMOS Department, Advanced Molecular Diagnostic Unit, Sant’Andrea Hospital, Sapienza University of Rome, Italy
3Internal Medicine and Gastroenterology, Policlinico A Gemelli, Catholic University of Rome, Italy
4Infectious Disease and Hepatology, POIT San Camillo-INMI Lazzaro Spallanzani, Rome, Italy
5Epatologia, Ospedale San Camillo di Roma, Italy
6Tropical and Infectious Disease, Department of Clinical Medicine, “Sapienza” University of Rome, Italy
- Corresponding Author:
- Prof. Manuela Merli
Gastroenterology, Department of Clinical Medicine, “Sapienza” University of Rome, Via Filippo Turati, 33-37, 00185 Roma, Italy
Tel: 390649972001
E-mail: manuela.merli@uniroma1.it
Received Date: January 06, 2016; Accepted Date: September 20, 2016; Published Date: September 23, 2016
Citation: Giannelli V, Simmaco M, Lionetto L, Gentile G, Giusto M, et al. (2016) Ribavirin Transporter [Ent1] Polymorphism is a Pretreatment Predictor of Virologic Response: The Specific Role of Donor Liver Transporter. Clin Pharmacol Biopharm 5:163. doi: 10.4172/2167-065X.1000163
Copyright: © 2016 Giannelli V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
The genetic polymorphism of Equilibrative Nucleoside Transporter 1 [ENT1] is involved in ribavirin cellular uptake and it could positively enhance antiviral treatment response. The liver transplant setting offers the unique opportunity to selectively observe the effect(s) of the donor liver ENT1 gene on HCV treatment outcome. We aimed at studying donor polymorphism of ENT1 and HCV therapy outcome in transplanted patients. The role of ribavirin plasma concentration was evaluated. 39 patients after HCV recurrence were included. Genotyping of donor ENT1 and of IL-28B was performed in donor liver samples by RNA PCR. Allelic frequencies of liver ENT1 were: AA 43.6%; AG 28.2%; GG 28.2%. GG genotype was associated with rapid [RR=8; 95% CI 1.6-38; p=0.01] and sustained virological response [RR=9.5; 95% CI 1.6-53; p=0.01]. In multivariate analysis, GG genotype and a ribavirin plasma concentration >2.0 ng/mL at week 12 were independently associated with sustained virological response. In conclusion, the genetic polymorphism of ENT influences treatment response and a pre-treatment determination of its activity could help to predict treatment response in HCV patients.
Keywords
HCV; Ribavirin; ENT1; Liver transplant
Abbreviations
HCV: Hepatitis C Virus; ENT-1: Equilibrative Nucleoside Trasporter-1; RVR: Rapid Virological Response; EVR: Early Virological Response; SVR: Sustained Virological Response; IFN: Interferon; CNT: Concentrative Nucleoside Transporters; LT: Liver Transplant
Introduction
Hepatitis C virus [HCV] infection is the most common chronic liver disease and may lead to cirrhosis and end-stage liver failure [1]. Ribavirin represents a crucial component in the combined antiviral treatment for hepatitis C; in fact it lowers the relapse rate and the breakthrough episodes when compared with peg-IFN monotherapy [2]. Even the recent introduction of the protease inhibitors has not decreased the relevance of ribavirin since a lower SVR was reported when ribavirin was not administered or employed at low dose [3-5]. The antiviral activity of ribavirin occurs within the hepatocytes, thus an appropriate ribavirin uptake by these cells is considered an essential step for anti-viral ribavirin action [s] to occur [6,7].
The hepatocyte inflow of ribavirin is regulated by two subtypes of specific membrane nucleoside transporters. The major transporter is represented by the Equilibrative Nucleoside Transporters [ENT, with its known variants 1-4], encoded by SLC29 genes, which mediate facilitated bidirectional diffusion of nucleosides [7,8]. The other transporter family is represented by Concentrative Nucleoside Transporters [CNT, with its known variants 1-3], encoded by SLC28 genes, which can transport nucleosides against a concentration gradient [9].
ENT1 has been recently identified as the primary responsible for ribavirin cellular import [8,10]. In fact, the specific inhibition of ENT1 by mercaptopurine probes led to a significant reduction in ribavirin concentration within erythrocytes isolated from humans [11].
ENT1 is an integral membrane glycoprotein with 456 amino acid residues, located both in plasma and mitochondrial membranes [12].
The gene encoding the protein stays in the short arm of chromosome 6, precisely in the 6p21.1 band. Since ENT1 single nucleotide polymorphism [SNP] may influence the efficiency of ribavirin transport [13,14], the effects of ENT1 SNP on antiviral treatment outcome have been recently evaluated.
In a retrospective study conducted in 109 HIV-HCV co-infected patients, an association between the ENT1 SNP rs760370 and rapid virological response [RVR] to antiviral treatment for HCV was reported [15]. A similar result was also obtained in a study including 526 East Asian patients mono infected with HCV genotype 1b, in whom the ENT1 SNP rs6932345 was found to be an independent predictor of RVR and of treatment outcome [16].
Liver disease due to HCV is one of the main current indications for liver transplantation [LT] [17]. The recurrence of HCV after liver transplant is almost universal and has a negative impact on patient and graft survival [18]. HCV eradication was shown to improve survival; however antiviral treatment with the interferon-based therapy is still unsatisfactory being effective only in about one third of patients after LT [19].
Aim of the study was to analyze the effect of donor hepatic ENT1 polymorphism on treatment response after HCV recurrence in liver transplant patients. The role of ribavirin concentration in the early phase of therapy (12 weeks) was also examined.
Materials and Methods
Study population
Sixty-five liver transplant patients consecutively treated for HCV recurrence between June 2010 and February 2012 were considered for inclusion in the study. Shared criteria for antiviral treatment were: detectable serum HCV RNA; alanine amino-transferase [ALT] × 1.5 folds above the normal values; and histological evidence of hepatitis C recurrence with a fibrosis score between 1 and 5 according to the Ishak scoring system [20]. Exclusion criteria are described in Supplementary material. Treatment was based on peg-IFN-alpha-2b at standard dose [1.5 μg/kg/week] or Peg-IFN-alpha-2a [180 μg/week] plus weight-adjusted ribavirin [11 mg/kg/day] for 48 weeks. Growth factors [granulocyte colony-stimulating factor, Filgrastim; Amgen DompeĢ? Spa, Milan, Italy; and erythropoietin, Eprex; Janssen-Cilag, Titusville, NJ, USA] were used when needed to avoid dose reduction of both drugs.
Informed consents were obtained from subjects (recipients) who participated in this analysis. No donor living donors were included.
Virological analysis
HCV RNA testing was done by the quantitative real-time PCR assay HCV-Ampliprep TaqMan [sensitivity threshold of 15 IU/mL; Roche Diagnostics, Mannheim, Germany].
Serum HCV-RNA level was measured at baseline, and at weeks 4, 12, 24, 36 and 48 of therapy, as well as at week 24 following the completion of treatment.
Rapid Virological Response and Sustained Virological Response [SVR] were defined as the achievement of undetectable serum HCV-RNA level at week 4 and week 24 after treatment, respectively. The Early Virological Respose intended as the reduction of >2 log of HCV-RNA after 12 week of therapy was not considered a stopping rule for treatment.
DNA isolation and genotyping
Genomic DNA was isolated from donor liver tissue using the X-tractor Gene system [Corbett life Science, Australia] at the time of transplantation, before reperfusion.
The DNA polymorphisms for rs760370 for ENT1 gene [SLC29A1] and rs12979860 for Il28 beta gene, were studied.
Reference sequences for ENT1 genes and Il28B were obtained from NCBI GenBank database (http://www.ncbi.nlm.nih.gov/ ).
Genotyping of ENT1 and Il28 beta SNPs was performed by Pyrosequencing technology, using the Pyrosequencer PyroMark ID system [Qiagen]. Both the amplification and the sequencing primers of each SNP were obtained by the PSQ Assay Design software [Biotage AB and Biosystems, Uppsala, Sweden].
PCR primer pairs, sequencing primers for each SNP and PCR technique are reported in Supplementary materials 1 and 2. Pyrosequensing was performed by methods previously described by our center [21].
Ribavirin plasma concentration
Ribavirin plasma trough concentrations were measured at week 4, 12, 24, 36 and 48 of therapy. Blood samples were collected early in the morning before the intake of the first daily dose of ribavirin, which was, on average, 12 h after the last dose. Ribavirin blood concentration was quantified by methods previously described by Loustaud-Ratti et al. [22] and the HPLC mass spectrometry technique are described in Supplementary materials 3.
Statistical analysis
Results are presented as median and ranges. Dichotomous variables were examined by Fisher’s exact test, whereas continuous variables were examined by Wilcoxon and Mann-Whitney U tests for independent and paired samples, as appropriate. A ribavirin concentration greater than 2 ng/mL was considered adequate for treatment, according to what has been previously reported in the literature [15,23,24].
Univariate and multivariate logistic regression analyses were performed to assess factors independently associated with the achievement of virological response. A P value <0.05 was considered significant. Statistical analysis was done with SPSS [SPSS 19, SPSS Inc., Chicago, IL]. Graphs were done with GraphPad [GraphPad software, La Jolla, CA, USA].
Results
Thirty-nine LT patients undergoing antiviral treatment for hepatitis C recurrence were included in the study. Main baseline characteristics are shown in Table 1. 26 patients were excluded because liver biopsy was not performed preventing the ENT-1 polymorphism on-tissue determination. The main characteristics of the 39 treated patients are reported in Table 1.
| Variable | Value |
|---|---|
| Age*, years (range) | 62 (45-67) |
| Male sex, % | 88 |
| MELD* at LT (range) | 17 (12-22) |
| Donor age*, years (range) | 55 (19-68) |
| Diabetes, % | 33.3 |
| Tacrolimus/Cyclosporine, % | 68/32 |
| Fibrosis*, Ishak stage before treatment (range) | 2 (1-4) |
| HCV genotype 1, % | 84 |
| genotype 4, % | 5 |
| genotype 2/3, % | 10 |
| IL28B of donor liver | - |
| TT% | 15 |
| TC% | 32.8 |
| CC% | 52.2 |
| Basal HCV-RNA load* log10 (range) | 6.3 (4.5-7.6) |
| Basal ALT* (U/L) (range) | 101 (52-331) |
| *Values are expressed as median and ranges | |
Table 1: Characteristics of patients at baseline.
Median time from LT to antiviral treatment was 11.8 (range: 6-25) months. Median baseline HCV RNA levels were 6.3 log10 (range: 4.5-7.6). Liver biopsy showed mild to severe inflammatory activity (mean score 7 ± 2) and moderate fibrosis (1 patient stage 4; 11 patients stage 3; 17 patients stage 2, 10 patients stage 1). An episode of acute cellular rejection was recorded in the past history of 3 patients (7.7%). Renal function and the glomerular filtration rate were within normal limits in all patients.
ENT1 genotypeA
The distribution of the different genotypes according to SNPs at the ENT1 [SLC29A1] gene was as follows: rs760370 AA 43.6% (17/39 pts), AG 28.2% (11/39 pts), and GG 28.2% (11/39 pts). Patients’ characteristics according to their donor ENT1 genotype are shown in Table 2. No differences in the main predictors of response to the treatment were found among ENT1 genotypes.
| Variable | AA | AG | GG | p Value | |
|---|---|---|---|---|---|
| (17 patients) | (11 patients) | (11patients) | |||
| Recipient age*, years | 52 | 62 | 53 | 0.3 | |
| Â range | (45-64) | (49-63) | (45-64) | ||
| Donor age*, years | 47 | 46 | 42 | 0.1 | |
| Â range | (16-68) | (14-70) | (18-64) | ||
| Male sex, % | 92 | 82 | 90 | 0.4 | |
| Fibrosis at baseline, Ishak stage | 2 | 2 | 2 | 0.8 | |
| Â range | (1-3) | (1-4) | (1-4) | ||
| HCV genotype 1, % | 94 | 91 | 82 | 0.9 | |
| HCV RNA load at baseline*, log10 | 6.45 | 6.2 | 6.5 | 0.9 | |
| Range | (5.5-7.6) | (5.6-7.3) | (5.2-7.1) | ||
| ALT at baseline* (U/L) | 107 | 109 | 159 | 0.1 | |
| Range | (51-163) | (95-287) | (70-248) | ||
| IL28B of donor liver | |||||
| TT% | 45 | 64 | 50 | 0.2 | |
| TC/CC% | 55 | 36 | 50 | ||
*Values are expressed as median and ranges
Table 2: Characteristics of patients according to ENT 1 polymorphism (rs 760370).
Virological response and ENT1 polymorphism
Table 3 shows the association between predictors of response to the treatment and SVR by univariate analysis. Only ribavirin plasma concentration at week 12 and ENT1 genotype had a significant correlation to SVR. In detail, 15 out of the 39 patients attained RVR (38.5%) and 18/39 patients (46.2%) obtained a SVR. According to rs760370 genotype, RVR and SVR were achieved in 64% (7/11 pts.) and 73% (8/11 pts.) of the patients carrying the GG genotype, respectively, whereas they occurred in only 33% (13/39 pts.) and 36% (14/39 pts.) of the non GG patients, without any significant difference between AA or AG carriers. Among patients carrying both the HCV genotype 1/4 and the ENT-1 genotype AA or AG (35 pts.) the chance of SVR was even worse resulting in 30% of total. Thus, GG genotype was significantly associated with RVR [RR=8; 95%; CI 1.6-38; p=0.01] and SVR [RR=9.5; 95% CI 1.6-53; p=0.01].
| Variable | SVR % | O.R. (C.I) | p value |
|---|---|---|---|
| ENT1 (GG vs. AA/AG) | 73 vs. 36 | 4.8 (1.6-9.5) | 0.01 |
| Ribavirin plasma concentration           | 57 vs. 20 | 5.1 (1.0-25) | 0.04 |
| (=2 vs.<2 ng/mL) at week 12 | |||
| Donor IL28B (CC vs. TT/TC) | 50 vs. 33 | 3.7 (0.6-20) | 0.09 |
| Genotype 1 vs. non 1 | 41 vs. 69 | 0.4 (0.29-4.7) | 0.4 |
| Acute organ rejection | 50 vs. 62 | 0.4 (0.05-8) | 0.3 |
| (yes vs. no) | |||
| Diabetes | 40 vs. 50 | 0.7 (0.07-3.6) | 0.35 |
| (yes vs. no) | |||
| Immunosuppression | 50 vs. 42 | 0.7 (0.2-5) | 0.75 |
| (tacrolimus vs. cyclosporine) | |||
| Anaemia (<10 g/dL) | 59 vs. 35 | 1.7 (0.2-5) | 0.09 |
| (yes vs. no) | |||
| HCV-RNA viral load at baseline (log10) | SVR vs. Non SVR | - | |
| 6.47 vs. 6.33 | 0.1 |
Table 3: Predictors of SVR: results from univariate analysis.
Virological response and ribavirin concentration
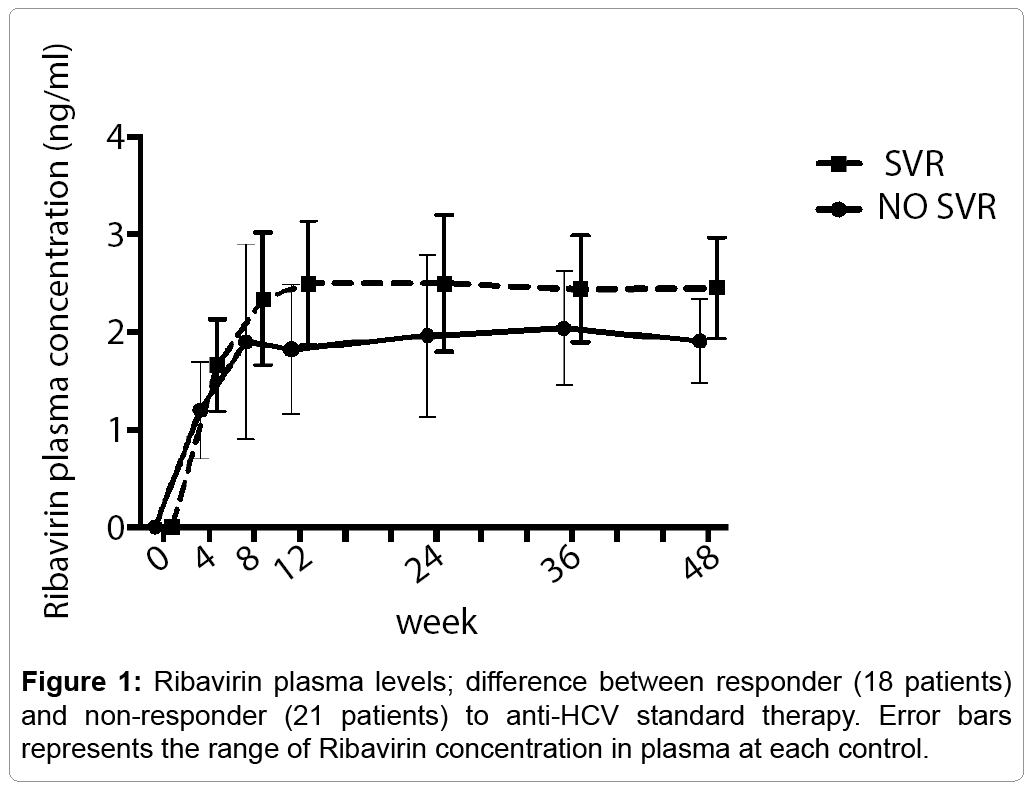
The median ribavirin trough concentration at week 12 was 2.2 (0.8-3.7) mg/mL. Patients who achieved SVR reached higher levels of plasma ribavirin in a shorter time compared to non-responders (Figure 1). In particular, ribavirin plasma levels at week 12 were 2.8 ng/ mL among responders vs. 1.6 ng/mL among non-responders (p=0.05; Figure 1). The ribavirin dose taken was about 11 mg/kg/day and 13% of patients (n. 5/39) were on erythropoietin treatment. The prevalence of severe anemia was independent with ENT1 SNPs (18% vs. 11%, GG vs. non GG genotypes respectively; p=0.3). At univariate analysis ribavirin dose reduction was not a predictor of SVR (p=0.08), probably due to the low number of patients involved.
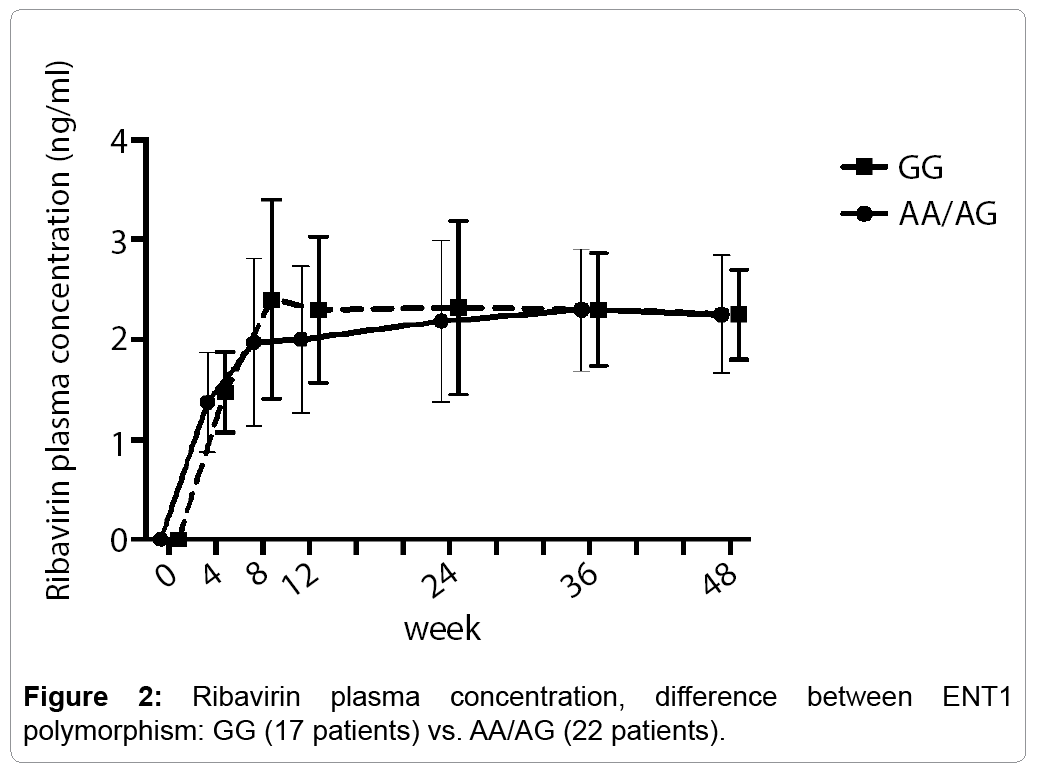
Interestingly, there were no significant differences in median ribavirin mean concentrations at any observed time point during treatment in patients carrying GG genotypes of liver ENT-1 compared to non-GG liver genotypes. In particular, at week 12 the values were 2.03 (1.0-2.2) and 2.3 (0.95-2.8) ng/mL, respectively; p=0.888 (Figure 2).
Multivariate analysis
By multivariate analysis (Table 4) only 2 factors resulted independently associated with the achievement of SVR: rs760370 GG genotype [OR, 7.8; 95% CI, 1.0-62; p=0.05] and plasma ribavirin trough concentrations ≥ 2 ng/mL at week 12 [OR, 7.4; 95% CI 1.2-45; p=0.03]. IL28B maintained only a positive trend for the achievement of SVR although it was not statistically significant (p=0.09).
| Variable | O.R.(C.I.) | p value |
|---|---|---|
| ENT1 GG genotype | 7.8 (1-12) |
0.05 |
| Ribavirin plasma concentration =2 ng/mL at week 12 | 7.4 (1.2-15) |
0.03 |
| Donor IL28B CC | 1.8 (0.03-2.4) |
0.09 |
| Genotype 1 | 0.1 (0.2-70) |
0.8 |
Table 4: Independent predictors of SVR: results from multivariate analysis.
Treatment related anemia
The pretreatment baseline Hb level was 13.1 g/dL (range= 10-14.8 g/dL) in women and 14.5 g/dL (range=10.6-17.6 g/dL) in men (P<0.01). Twenty-three patients (59%) developed anemia. Severe anemia occurred in 18 patients who required correction with erythropoietin; of these, 11 achieved a SVR, three patients relapsed and five patients were non-responders. In the univariate analysis the development of anemia (Hb level <10 g/dL) was not significantly associated with SVR (p=0.09). The occurrence of anemia was not different between patients carrying donor liver GG and AA/AG genotypes.
Discussion
It is well know that in LT patients, HCV clearance increases graft and patient survival by improving histology and decreasing the risk of cirrhosis or its complication [25]. The antiviral therapy in the setting of LT, the SVR is acheived by approximately one-third of patients treated with interferon-based theraphy [19,26,27]. Sofosbuvir and RBV is the first IFN-free combination that has been assessed in hepatitis C recurrence after LT [28]. Even with the new therapy for HCV hepatitis based on the direct antiviral agents, ribavirin has still a place to reduce viral relapse, especially in genotype 2 patients [28-30]. Interestingly, the present study suggests that, in the liver transplant setting, genetic polymorphism of the ribavirin liver transporter ENT1 is capable of influencing the outcome of antiviral therapy after HCV recurrence. Reasonably, this effect can be mediated by the influence that ENT1 genotype can exert on the inflow of ribavirin inside the hepatocytes [11,14]. Regarding pharmacokinetics and pharmacodynamics characteristics, host genetic features may have relevant implications in the ability to modulate the response to drugs and may be of great help in defining tailored treatments [15,16,31,32]. Recently, a growing importance has been attributed to ribavirin, the plasma level of which seems to be a significant predictor of treatment outcome [33]. Since ribavirin is hydrophilic and its activity takes place within the cells [34], it needs to be actively transported into the cells and ENT1 has been demonstrated to be the most efficient transporter of ribavirin into hepatoma cell lines [11] and cultured hepatocytes [13]. Thus, it is conceivable that the polymorphism of ENT1 could eventually affect antiviral treatment outcome by modulating the efficiency of ribavirin transport [11,14-16].
Transplanted patients are a very attractive population because liver ENT1 polymorphism is derived from the donor, while all other tissues express the recipient ENT1 polymorphism. Assuming that ENT1 is an important predictor of response to therapy, the transplanted liver is the best setting in which to evaluate this hypothesis without confounding factors related to the metabolism of ribavirin in other tissues. Donor liver samples were taken at the time of transplantation before blood reperfusion to avoid any DNA contamination from the recipient. Contrary to what have been already reported in literature, [23] we found that ribavirin steady state of plasma concentration was reached later than the fourth week of treatment, this was probably due to the schedule of ribavirin dose we used which considered a progressive increasing of the given daily dose during the first 2-3 weeks of treatment. In our study we confirm that LT patients who achieved SVR had a significantly higher ribavirin concentration at week 12 compared to non-responders [2.6 vs. 1.8 ng/mL; p<0.05] and that a ribavirin level higher than 2 ng/mL at week 12 is an independent predictor of SVR (Table 4).
Patients whose donor liver had GG ENT1 genotype had a 8-fold increase of SVR likelihood compared to non-GG patients (Table 4). It is not surprising that both high ribavirin levels and ENT1 genotype are independent predictors of SVR since ENT1 is an equilibrative transporter, which might modulate ribavirin distribution into cells. It has been already reported that liver ribavirin uptake in the early phase of ribavirin administration was 30 times higher in ENT1 wild compared to knockout mice [14]. And, the high serum levels of ribavirin reached during the early-phase of antiviral treatment are crucial to obtain a favorable virological response [24,33,35-37]. It may be hypothesized that the plasma ribavirin levels and intracellular concentration of the drug are in strict correlation. Indeed, ribavirin plasma levels during the first 12 weeks maintained a significant role in predicting treatment efficacy [38]. Thus, we hypothesize that the association of high ribavirin serum concentrations and favorable liver ENT1 genotype may have a synergic effect and lead to better intracellular bioavailability of the drug. The consequence of our observation may be that in patients with unfavorable ENT1 polymorphism, a higher ribavirin given dose could help to increase intra hepatocyte drug’s concentration.
Our study had some limitations due to the patient’s number which was not large enough to allow the analysis of the impact of other traditional baseline predictors of response such as IL28B genotype, viral load, HCV genotype and fibrosis.
In conclusion, the present study shows an association between GG genotype of liver ENT1 and increased SVR rate. This result contributes to the understanding of host genetic variants involved in ribavirin bioavailability and may help in individualizing ribavirin dosing for treatment of HCV recurrence after transplant.
Conflict of Interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Acknowledgements
The authors gratefully acknowledge the financial support by the Department of Clinical Medicine, Policlinico Umberto I, University of Rome “Sapienza”.
References
- Hajarizadeh B, Grebely J, Dore GJ(2013) Epidemiology and natural history of HCV infection.Nat Rev GastroenterolHepatol 10:553-562.
- Gane EJ, Lo SK, Riordan SM, Portmann BC, Lau JYN, et al. (1998)Randomized study comparing ribavirin and interferon alfamonotherapy for hepatitis C recurrence after liver transplantation. Hepatology27:1403-1407.
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie MD, Reddy KR, et al. (2011)Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med23:2405-2416.
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, et al. (2011) Boceprevir for previously treated chronic HCV genotype 1 infection. NEJM 364:1207-1217.
- Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol M, et al. (2009) Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. NEJM 360:1839-1850.
- Fukuchi Y, Furihata T, Hashizuma M, Likura M, Chiba K (2010) Characterization of ribavirin uptake systems in human hepatocytes. J Hepatol52: 486-492.
- Hoffmann WP, Hermann E, Sarrazin C, Zeuzem S (2008) Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int28: 1332-1343
- Pastor Anglada M, Cano Soldado P, Molina Arcas M, Lostao MP, Larráyoz I, et al. (2005) Cell entry and export of nucleoside analogues. Virus Res 107:151-164.
- Kong, W, Engel K, Wang J (2004) Mammalian nucleoside transporters. Curr Drug Metab 5: 63-84.
- Ibarra KD, Pfeiffer JK (2009) Reduced ribavirin antiviral efficacy via nucleoside transporter-mediated drug resistance. J Virol 83:4538-4547.
- Iikura M, Furihata T, Mizuguchi M (2012)ENT1, a Ribavirin Transporter, Plays a Pivotal Role in Antiviral Efficacy of Ribavirin in a Hepatitis C Virus Replication Cell System. Antimicrob Agents Chemother 56: 1407-1413.
- Yao SY, Ng AM, Cass CE, Baldwin SA, Young JD (2011)Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1). J BiolChem 16:286.
- Endres CJ, Moss AM, Ke B, Govindarajan R, Choi DS, et al. (2009) The role of the equilibrative nucleoside transporter 1 (ENT1) in transport and metabolism of ribavirin by human and wild-type or Ent1/-mouse erythroCytes. J PharmacolExpTher329:387-398.
- Ibarra KD, Jain MK, Pfeiffer JK (2011) Host-based ribavirin resistance influences hepatitis C virus replication and treatment response. J Virol 85:7273-7283.
- Morello J, Cuenca L, Soriano V, Medrano J, Vispo E, et al. (2010) Influence of a Single Nucleotide Polymorphism at the Main Ribavirin Transporter Gene on the Rapid Virological Response to Pegylated Interferon-Ribavirin Therapy in Patients with Chronic Hepatitis C Virus Infection. J Infect Dis 8:1185-1191.
- Tsubota A, Shimada N, Yoshizawa K, Furihata T, Agata R, et al. (2012) Contribution of ribavirin transporter gene polymorphism to treatment response in peginterferon plus ribavirin therapy for HCV genotype 1b patients. Liver Int 32:826-836.
- 17.Thuluvath PJ, Krok KL, Segev DL, Yoo HY (2007) Trends in post-liver transplant survival in patients with hepatitis C between 1991 and 2001 in the United States. Liver Transpl 13:719-724.
- Gane EJ (2008) The natural history of recurrent hepatitis C and what influences this. Liver Transpl 14: S36-S44.
- Berenguer M, Aguilera V, Rubín A, Ortiz C, Jimenez M, et al.(2012) Comparison of two non-contemporaneous HCV-liver transplant cohorts: strategies to improve the efficacy of antiviral therapy. J Hepatol 56:1310-1316.
- Ishak K, Baptista A, Bianchi L, Callea F, Groote JD, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696-699.
- Taliani G, Spaziante M, Biliotti E, Borro M, Palazzo D, et al. (2013) IL28B Gene Polymorphisms and US Liver Fatty Changes in Patients Who Spontaneously Cleared Hepatitis C Virus Infection. PLoS One 8:e67301
- Furusyo N, Murata M, Ogawa E, Toyoda K, Ihara T, et al. (2011) Ribavirin concentration in the later stages of 48 week pegylated interferon-alpha2b plus ribavirin therapy for chronic hepatitis C is useful for predicting virological response. J Antimicrob Chemother 66:1127-1139.
- Loustaud-Ratti V, Carrier P, Rousseau A, Maynard M, Babany G, et al. (2011) Pharmacological exposure to ribavirin: a key player in the complex network of factors implicated in virological response and anaemia in hepatitis C treatment. Dig Liver Dis 43:850-855.
- Loustaud-Ratti V, Alain S, Rousseau A, Hubert IF, Sauvage FL, et al. (2008)Ribavirin exposure after the first dose is predictive of sustained virological response in chronic hepatitis C. Hepatology47:1453-1461.
- Berenguer M, Palau A, Fernandez A (2006) Efficacy, predictors of response, and potential risk associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl12:1067-1076.
- Samuel D, Forns X, Berenguer M (2006) Report of the monothematic EASL conference on liver transplantation for viral hepatitis. J Hepatol 45:127-143.
- Giannelli V, Giusto M, Farcomeni A (2012) Treatment of hepatitis C recurrence is less successful in female than in male liver transplant recipients. TransplInt 25:448-454.
- Charlton M, Gane E, Manns M, Forns X (2013)Sofosbuvir and ribavirin for the treatment of established recurrent hepatitis C infectionafter liver transplantation: preliminary results of a prospective, multicenter study. Late breaking abstract (LB 2). Paper presented at: the 64thAnnual Meeting of the American Association for the Study of Liver Diseases; November 1-5;Washington, DC.
- Gane EJ, Stedman CA, Hyland RH, Hyland RH, Ding X,Svarovskaia E, et al. (2013) Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 368:34-44.
- Di Martino V, Richou C, Cervoni JP, Tapis JMS, Jesen DM, et al. (2011) Response-guided peg-interferon plus ribavirin treatment duration in chronic hepatitis C: meta-analyses of randomized, controlled trials and implications for the future. Hepatology 54:789-800.
- Doehring A, Hofmann WP, Schlecker C (2011) Role of nucleoside transporters SLC28A2/3 and SLC29A1/2 genetics in ribavirin therapy: protection against anemia in patients with chronic hepatitis C. Pharmacogenet Genomics 21:289-296.
- D'Avolio A, Cusato J, Calcagno A, Perri GD (2013)Estimating ribavirin plasma exposure: Genetics or therapeutic drug monitoring? J Hepatol 59:633-634.
- vanVlerken LG, van Oijen MG, van Erpecum KJ (2011) Ribavirin concentration is a more important predictor of sustained viral response than anemia in hepatitis C patients. Gastroenterology140: 1693-1694.
- Huber-Ruano I, Pastor-Anglada M (2009) Transport of nucleoside analogs across the plasma membrane: a clue to understanding drug-induced cytotoxicity. Curr Drug Metab 10:347-358.
- Merli M, Giannelli V, Gentili F, Giusto M, Simmaco M, et al. (2005) Ribavirin priming improves the virological response to antiviral treatment in transplanted patients with recurrent hepatitis C: a pilot study. AntivirTher 16:879-885.
- Lindahl K, Stahle L, Bruchfeld A, Schvarcz R(2005) High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology 41:275-279.
- Slavenburg S, Huntjens-Fleuren HW, Dofferhoff TS (2011) Ribavirin plasma concentration measurements in patients with hepatitis C: early ribavirin concentrations predict steady- state concentrations. Ther Drug Monit33:40-44.
- Jiménez Macías FM, Rodríguez-Novoa S, Alvárez Barco E, Bullon JM, Codejon OF, et al. (2013) Monitoring of factors related to plasma concentration of ribavirin could improve response to antiviral therapy in chronic hepatitis C genotype 1. J Hepatol 58: S341.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 11231
- [From(publication date):
November-2016 - Aug 20, 2025] - Breakdown by view type
- HTML page views : 10370
- PDF downloads : 861