Role of Advanced Glycation End Products (AGE) in Health and Disease: An Overview
Received: 01-Sep-2018 / Accepted Date: 27-Oct-2018 / Published Date: 05-Nov-2018 DOI: 10.4172/2168-9652.1000246
Abstract
Advanced Glycation End products (AGEs) are products derived from the reaction between reducing sugars and the aminoacids. The process of covalent linkage of a sugar with a protein or an amino acid is called as glycation. The AGEs are formed exogenously from animal derived foods which are rich in fat and protein. AGEs can be endogenously formed from hyperglycemic diet. The defense system of the body metabolizes the AGEs with the help of glyoxylase enzyme system utilizing glutathione and restricts AGEs formation. AGEs may accumulate in the body due to unhealthy dietary habits of an individual or due to uncontrolled diabetes. Thus, results in functional loss of long-lived proteins like collagen, skeletal and vascular smooth muscles. The cross-linking nature of AGEs impairs the function of proteins, damages the cell structure, produces Reactive Oxygen Species (ROS) and creates oxidative stress. In addition to this property of AGEs, its ability to bind with Receptors for Advanced Glycation End products (RAGE) leads to increased oxidative stress and activates inflammatory pathway in vascular endothelial cells. AGE-RAGE complex can in turn activate or inhibit the signaling pathways causing various clinical conditions like the Diabetes Mellitus (DM), Chronic Kidney Disease (CKD), neurodegenerative diseases, skin diseases, aging and cancer. Healthy living, good cooking and dietary habits can reverse the serious effects of AGEs.
Keywords: Advanced glycation end products; Receptors for advanced glycation end products; Glycation; Unhealthy dietary habits
Introduction
Advanced Glycation End products (AGEs) are a group of compounds/molecules produced in the human body as a result of a process called as glycation, where the reducing sugars react with a protein, or amino acid [1]. AGEs show deleterious effect on human tissues both by receptor and non-receptor mediated mechanisms. The receptors involved in AGEs metabolism include the soluble Receptor of AGE (sRAGE), and the endogenous secretory RAGE (esRAGE). Interaction of AGEs with the receptors (AGE-RAGE complex) has been associated with the stimulation of inflammatory cytokines and the synthesis of reactive oxygen species [2].
AGEs may be formed endogenously or exogenously. Foods high in protein and fat such as meat, cheese and egg yolk are a rich source of AGEs. The method of cooking food, that includes cooking at high temperatures may increase the formation of AGEs. Maillard reaction is the major source of AGE synthesis [3]. This reaction is catalyzed by transitional metals and is inhibited by ascorbic acid [4].
Intracellular sugars form AGEs faster than the exogenous glucose supplementation. The amadori product formed during the Maillard reaction undergoes oxidation, dehydration, and condensation to produce AGEs including the glyoxides like the Pentosidine and N-Carboxy Methyl Lysine (CML). It is also noted that when reactive intermediate products are formed after amadori re-arrangement, alpha dicarbonyls or oxoaldehydes are produced [5].
Normally, reactive dicarbonyl compounds (glyoxal, methyl glyoxal, 3 deoxyglucosone) are detoxified by an enzyme system called as Glyoxylase system. It is made of glyoxylase I and II enzymes, which break dicarbonyl compounds and inhibits formation of AGEs. The accumulation of these products creates an unstable environment inside the cell which is termed as carbonyl stress [6]. This unstable environment within a cell induces oxidative stress, cellular apoptosis and vascular damage [7]. The accumulation of such products, with their natural ability to cross link, coexist with carrier proteins like albumin, hemoglobin, lens crystals, and Low Density Lipoproteins (LDL) [8]. Although HbA1C is a glycated product, it is not recognized as a true AGE because it indicates a recent glycemia status (6-12 weeks), whereas advanced glycation reflects a process over a longer period [9].
Tobacco, food additives, and food colors constitute the major exogenous sources for AGE formation. Cigar smoking causes vascular disease but its relation to AGEs formation has recently been recognized. It has been observed that the tobacco smoke can rapidly react with proteins to form AGEs both in vitro and in vivo [10].
The aim of this paper is to re-emphasize the role of AGE's in human health and disease.
Review
Advanced Glycation End products (AGEs) are a group of heterogenous compounds/molecules produced as a result of glycation (addition of sugar to protein non-enzymatically with free amino groups on protein). Reaction that leads to the formation of AGEs is called as Maillard reaction.
Millard Reaction
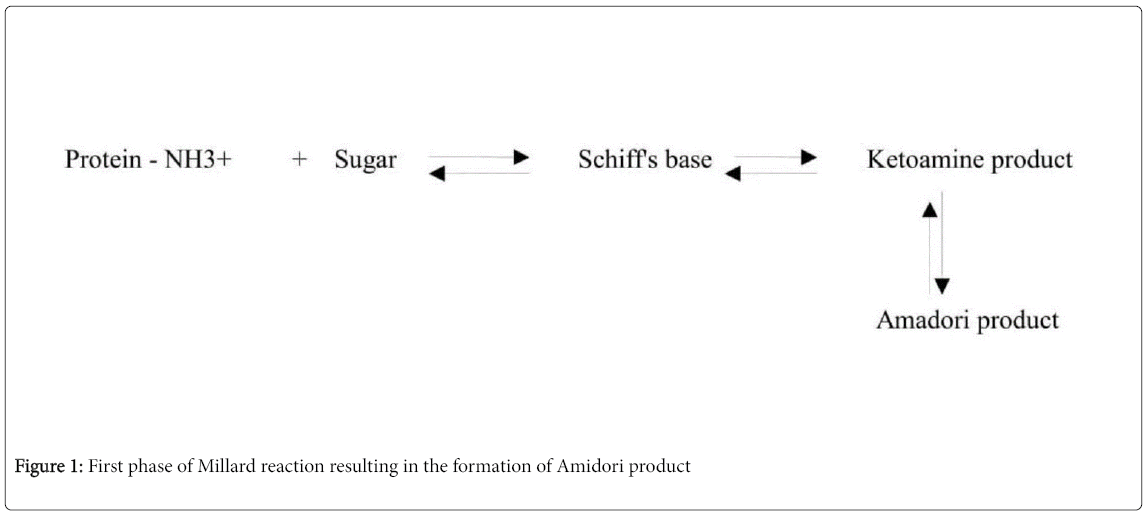
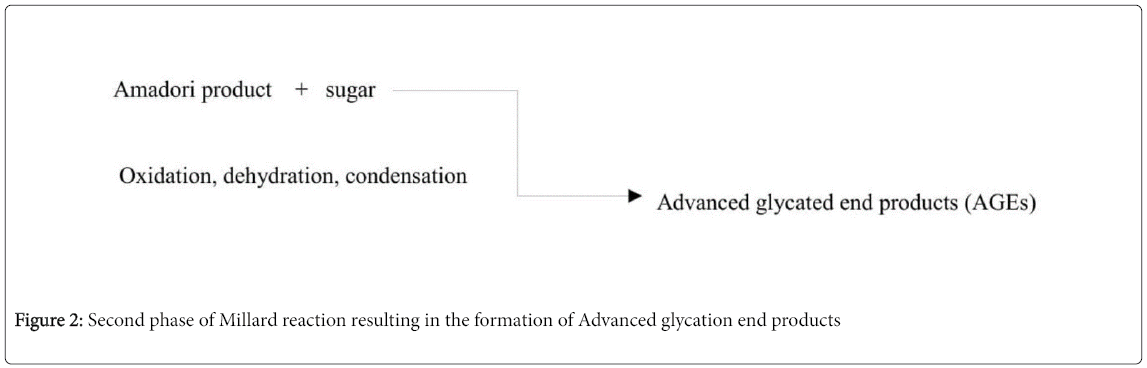
Millard reaction is divided into two phases. The first phase of the reaction proceeds until Amadori rearrangement and the second half involves AGEs formation via oxidation, dehydration and condensation.
During the first phase, the reducing sugar or glucose reacts with the free amino group of biological amines to form schiff's base, which later forms amadori product as shown in Figure 1.
Then second phase involves the degradation of amadori product into dicarbonyl compounds like glyoxal, methyl glyoxal, 3-deoxy glycosones etc via dehydration, oxidation, and reduction. The crosslinking nature of Amadori product (due to labile covalent bond) with other sugar groups irreversibly results in the formation of AGEs as shown in Figure 2 [11].
Apart from the Maillard reaction AGEs are also formed as intermediates or by-products of glucose auto-oxidation or lipid peroxidation or by polyol pathway [12]. AGEs formation is a natural phenomenon that occurs in all individuals. Chronic hyperglycemia, and stress may aggravate the formation of AGEs. CML, MG, Pentosidine are used as markers for AGE measurement. AGE can be measured either by Enzyme Linked Immuno-sorbent Assay (ELISA), High Performance Liquid Chromatography (HPLC) or mass spectrometry [13]. In India the use of AGEs by food industry has increased because of their ability to enhance taste. The disadvantage of AGEs use in food preparation is that their presence increases with increase in the temperature and sugar concentrations. Normally, the intracellular protective systems limit the accumulation of AGE derivatives. However, in high concentrations they may get accumulated. The accumulated AGEs in turn effects long lived proteins like collagen, hemoglobin, alkaline phosphatase, lysozyme, and elastin [14].
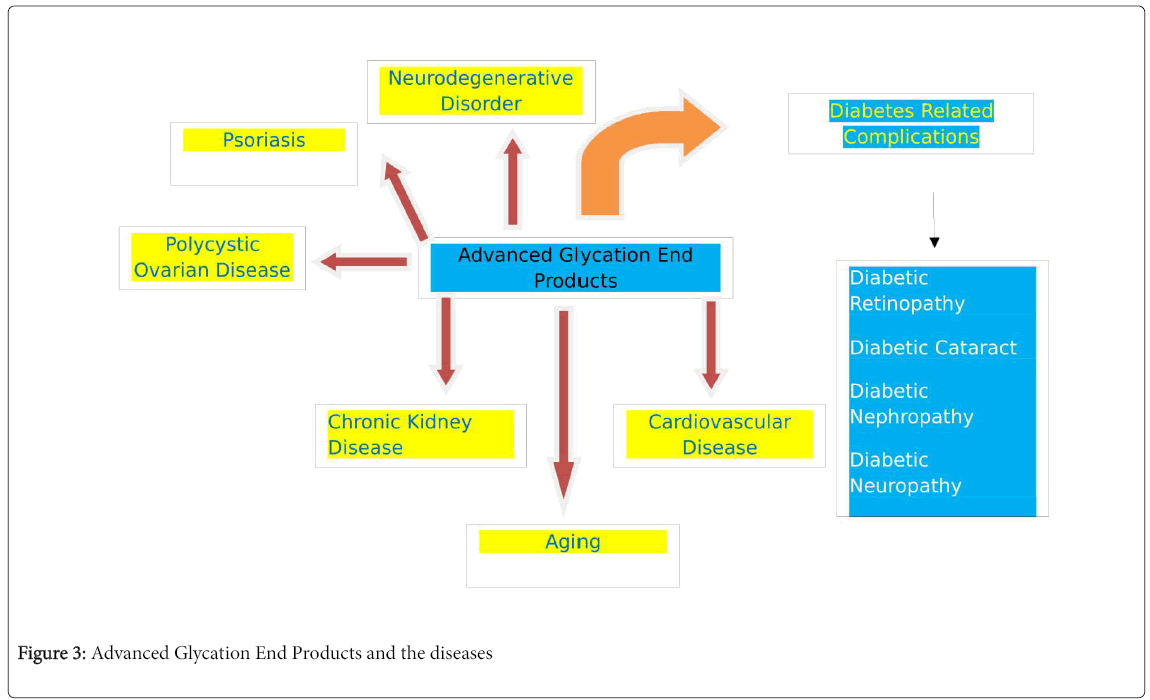
AGEs have the ability to modify proteins by their cross-linking nature. These modified proteins behave abnormally, damaging cell structure and function. Modified proteins decrease the elasticity of arteries, glomerulus and increase their permeability. Thus, decreases cell adhesion and inhibits angiogenesis. Modified proteins can bind to receptor for AGE (RAGE) on cells like macrophages or monocytes thereby inducing the production of Reactive Oxygen Species (ROS). As ROS are unstable due to unpaired electron in their outer shell, two ROS combine by cross linking and attain stability by further cross linking with AGEs [15]. Oxidative stress could be caused by an imbalance between ROS production and defensive mechanisms in the body. Thus AGE’s may be responsible for the development, progression and complications of several diseases as shown in Figure 3.
AGEs in Health and Disease
Diabetic retinopathy
The formation of AGE compounds and their role in the progression of diabetic retinopathy could be due to the death of various lens proteins and retinal cells of the eye as a result of AGEs accumulation [16]. The AGEs bind to its receptor RAGE in lens, which leads to the activation of a signaling pathway that causes oxidative stress, and release local hormones, cytokines and adhesive molecules [17]. The AGE–RAGE interaction causes vascular changes in pericytes (contractile cells of endothelium) leading to apoptosis of pericytes, which is an early sign of retinopathy [18]. The accumulation of AGEs in the retinal cells activates secretion of Interleukin 6 (IL-6) which induces angiogenesis by increasing expression of Vascular Endothelial Growth Factor (VEGF). Thus, AGEs may cause microvascular changes in the eye of diabetic patients.
Diabetic cataract
Glycation of eye lens protein is the major mechanism responsible for diabetic cataract formation. The AGE compounds accumulated around lens protein causes a conformational change in the structure of protein, decreasing protein–protein interactions and protein–water interaction. This reduces the transparency of the eye lens [19]. Experimental evidence revealed an increased AGEs formation around cataract lens and AGEs also have been associated with change of the color and opacity of the eye lens [20].
Diabetic nephropathy
It is the major cause of End Stage Renal Disease (ESRD). Even though genetic susceptibility is the risk factor, hyperglycemia is also linked with the pathogenesis of diabetic nephropathy [21]. The pathological link between hyperglycemia and development of diabetic nephropathy has been attributed to the formation of AGEs [22]. These AGEs and related products form cross-links with collagen, which could lead to structural and functional changes in the kidneys [23]. AGEs also induce production of inflammatory cytokines, chemokines, adhesive molecules, and growth factors which could be involved in the pathogenesis of diabetic nephropathy [24]. In vitro and in vivo studies indicates that AGEs via RAGE leads to overproduction of matrix proteins and inhibits its breakdown, initiating an oxidative stress pathway further damaging the organs involved [25].
Diabetic neuropathy
Polyneuropathy (damage of peripheral nerves) or mononeuropathy (damage of a nerve due to injury) is a common diabetic neuropathic complication. The glycation of cytoskeletal proteins, structural or functional changes of nerve fibers leads to diabetic neuropathy [26]. Experimental studies on diabetic rats showed decreased sensory motor conduction velocity, decreased nerve potential and also functions of sciatic nerve due to accumulated glycated proteins [27]. AGE accumulation may cause the loss of myelin sheath around neuron, further leading to vascular abnormalities [28].
Diabetic dermopathy
Increased skin lesions and delayed wound healing in diabetics is due to accumulated AGEs under the skin leading to changes in the physico-chemical structure of proteins within the skin leading to skin related disorders [29].
Diabetic macrovascular complications
The cross-linking nature of AGEs with matrix proteins in the vessel wall increases its rigidity, traps lipoproteins within arterial wall and disrupts its clearance [30]. AGE deposition in uncontrolled manner was also observed on atherosclerotic plaques and also in the radial artery of Chronic Renal Failure (CRF) patients with and without diabetes [31].
Neurodegenerative diseases
The AGEs accumulation in the brain has been noted to increase with the advance in age. The recent data has suggested that the accumulated AGEs in brain and other organs of the Central Nervous System (CNS) may be responsible for neurodegenerative disorders like the Alzheimers, Parkinson, Prionopathies and lateral amylotrophic sclerosis [32]. The accumulation of AGEs varies according to the pathology of the disease. Increased AGEs were noted in hippocampal region in Alzheimers, substantia nigra in Parkinsons, ventral spinal cord in amylotrophic lateral sclerosis patients. The measurement of AGEs, the methyl glyoxal derivative can be done by immunological, and chemical methods and further confirmed by proteomic analyses. Most interesting fact about AGEs is their accumulation at the target organ as well as along the path of the nerves. It is also evidenced that organs which never form AGE related compounds are more susceptible for its accumulation [33]. The methyl glyoxal derivatives (AGEs) obtained from diet has a capacity to penetrate the blood-brain barrier, enter into glial cells, and increase the synthesis of beta amyloids leading to plaque formation [34].
Cardiovascular diseases
AGEs accumulation has been observed both during diabetes and also in non-diabetic conditions. Smoking, consuming more amounts of deep fried and fatty foods and food cooked at high temperatures etc. could lead to increased AGEs in blood. The adverse effects of AGEs and related product is due to their cross-linking nature with matrix proteins, which decreases the flexibility and causes sequential dysfunction of the protein. The cross linking of AGEs with collagen, elastin, laminin in the myocardium leads to the rigidity and diastolic dysfunction of the heart [35]. Similarly, formation of AGE–RAGE complex in the myocardium induces fibrosis by enhancing the activity of Transformation Growth Factor β (TGF-β) (a super family of cytokines involved in cell functions). AGEs also may cause reduction in calcium concentrations by delaying the calcium re-uptake. AGEs may also delay re-polarisation of cardiac contractions, decrease myocardial contractility, and induce systolic and endothelial dysfunction [36].
Studies have also noted that AGE-soluble RAGE complex may cross link with LDL and result in decreased uptake by LDL receptors and its clearance, decrease macrophage LDL uptake and cause atherosclerosis [37]. Thus, AGE accumulation increases left ventricle end diastolic pressure, diastolic dysfunction, pulmonary congestion, dyspnoea, and systolic heart failure [38].
Chronic kidney diseases
The relationship between kidneys and AGEs could be via its clearance in kidneys. The AGEs, and related peptides and adducts (AGE compound linked to single aminoacid) are cleared by kidneys [39]. AGEs, due to their cross-linking nature can be taken up by epithelial cells of Proximal Convoluted Tubules (PCT) and then degraded by lysosomal system and are then excreted out through urine [40]. Increased AGEs cannot enter into Bowmans capsule and hence combine with receptors of endothelial matrix and forms RAGE. AGERAGE complex induces production of various cytokines like tumor necrosis factor β (TNF β), activates inflammatory response, and results in glomerulosclerosis [41]. AGE-RAGE complex also activates NAD(P)H oxidase, induces oxidative stress and cause decreased capacity of its excretion. AGEs accumulation in kidneys may lead to carbonyl stress, uremia and loss of its function.
Aging
Aging is a multifactorial process. The habits of an individual like smoking, increased consumption of non-vegetarian food, fried foods, food cooked in oil that is heated repeatedly at high temperatures contributes to formation of AGEs and related compounds [42]. Skin, due to its easy accessibility offers excellent opportunity for the process of glycation, especially to the long-lived proteins like collagen, skeletal and vascular smooth muscles. Normally, human body degrades the accumulated AGEs by activating glutathione dependent glyoxylase enzyme. During increased AGEs accumulation, the human defense system may weaken, thereby decreases the activity of glutathione dependent glyoxylase enzyme [43]. The glycation of long lived proteins resists its degradation by Matrix Metalloproteinase (MMP) inhibits its removal and replacement by newly synthesized proteins. Thus, impairs tissue permeability and turnover.
Psoriasis
It is a non-contagious, chronic skin condition that produces plaques of thickened scaling skin. The skin is sensitive to changes induced by AGEs and its accumulation causes increased production of free radicals, which increases oxidized LDL products in the skin [44]. AGEs also activate monocytes, macrophages, neutrophils and endothelial cells, which leads to chronic inflammation. In psoriasis, oxidative damage activates cytochrome C, which induces keratinocyte apoptosis and dysregulates keratinocytes, a possible mechanism involved in the pathogenesis of psoriasis. The AGE-RAGE complex regulates gene expression by regulating transcription factors like NF-KB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) and activator protein 1 (AP-1) mediated by tyrosine kinase protein [45].
Polycystic ovarian syndrome (PCOS)
PCOS is one of the most common endocrine disorders in reproductive aged women causing hyper androgenism and oligo or anovulation [46]. In PCOS women there is elevated AGEs, and AGEs exert their effect in association with its receptor RAGE. Elevated AGERAGE levels are observed in the ovarian tissue of women with PCOS, which can potentially alter steroidogenensis and folliculogenesis [47]. The oocyte maturation and ovulation require activation of Mitogen Activated Protein Kinase (MAPK) specifically ERK1 or 2 (Extracellular signal-Regulated Kinase 2) pathway [48]. The elevated AGEs interfere with the activation of ERK1/2 pathway in human ovarian granulose cells and thus can lead to altered genes involved in steroidogenesis in these cells [49,50].
Conclusion
The modern, and readily available or consumable food elevates the circulating AGEs in human. This may contribute to inflammation and could cause various diseases. The processing of food items at high temperatures, deep fried foods, food cooked in oil that is repeatedly heated at high temperatures, caramelization of food products to improve the taste of food items contribute to an increase in the activities of AGEs. Increased accumulation of AGEs in the body may be responsible for decreased activity of defensive glyoxylase enzyme system. The adverse effects of AGEs and related products could be due to their association with the receptor RAGE. AGE-RAGE complex activates inflammatory response, interferes with the transcriptional activation of related genes, inhibits normal response and decreases the defensive system of the body. The role of AGEs in various lifethreatening diseases is due to the ability of AGE-RAGE complex to control the activities of genes at their transcriptional level. This also can induce or inhibit normal or abnormal pathways leading to cancerous conditions. The processing of food at low temperatures, baking of the foods, steamed foods, increased consumption of fruits, green leafy vegetables, and physical exercise may help in improving the defensive system of body, which further degrades AGEs by activating glyoxylase enzyme system. In the Indian scenario, the traditional cooking methods although appear to be time taking, the cooking processes still keep food healthy as compared to the modern ways of cooking which use microwave ovens.
References
- Dayan C, WG (2010) Diabetes. In: Warell DA, Cox TM, Firth JD (editors). Oxford Textbook of medicine. Oxford University Press pp1987-2058
- Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE et al. (2009) RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20: 742-752.
- Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, et al. (2004) Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 104: 1287-1291.
- Mc Cance DR, Dyer DG, Dunn JA et al. (1993) Maillard reaction products and their relation to complications in insulin dependent diabetes melliteus. J Clin Invest 91: 2470-2478.
- Chappey O, Dosquet C, Wautier M-P, Wautier J-L (1997) Advanced glycation end products, oxidant stress and vascular lesions. Eur J Clin Invest 27: 97-108.
- Wells–Knecht KJ, Brinkmann E, Wells–Knecht MC, Litchfield JE, Ahmed MU et al. (1996) New biomarkers of maillard reaction dmage to proteins. Nephrol Dial Transplant 11(suppl 5): 41-47.
- Thornalley PJ, Westwood M, Lo TW, Mc Lellan AC (1999) Formation of glyoxal, methylglyoxal and 3-DG in the glycation of proteins. Biochem J 344: 109-116
- Okado A, Kawasaki Y, Husuike Y, Takahashi M, Teshima T et al. (1996) Induction of apoptic cell death by methylglyoxal and 3-deoxy glucasone in macrophage derived cell lines. Biochem Biophy Res Commun 225: 219-224
- Kennedy L (2010) Glycation of immunoglobulins and serum proteins In: Alberti KGMM, de Fronzo RA, Keen H, Zimmet P (eds). International text book of Diabetes mellitus (2nd Eds.) John Wiley, Chichester pp 985-1007
- Cerami C, Founds H, Nicholl I, Mitsuhashi T, et al. (1997) Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci 94: 13915-13920.
- John WG, Lamb EJ (1993) The maillard or browning reaction in diabetes. Eye 7: 230-237.
- Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015)Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5: 194-222.
- Anderson MM, Heinecke JW (2003) Production of N epsilon-(carboxy methyl) lysine is impaired in mice deficient in NADPH Oxidase. A role for phagocyte – derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes 52: 2137 -2143.
- C Ott, Jacobs K, Haucke E, Navarrete Santos A, Grune T, et al. (2014) Role of advanced glycation end products in cellular signaling. Redox Biology 2: 411-429.
- Phaniendra A, Jestadi DB, Periyasamy L (2014) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30: 11-26.
- Stitt AW, Curtis Tm (2011) Diabetes - related adduct formation and retinopathy. J Occul Biol Dis Infor 4: 10-18.
- Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, et al. (2002) RAGE blockade stabilizes established atherslerosis in diabetic apolipoprotein E null mice. Circulation 106: 2827-2835.
- Nakamura N, hasegawa G, Obayashi H, Yamazaki M, Ogata M, et al. (2003) Increased concentration of pentosidine, an advanced glycation end product, and retinopathy. Diabetes Res Clin Pract.61: 93-101.
- Beswick HT, Harding JJ (1987) Conformational changes induced in lens alpha and gamma crystallins by modification with glucose-6-phosphate implications for cataract. Biochem J 246: 761-769.
- Kumar MS, Reddy PY, Kumar PA, Suolia I, Reddy GB (2004) Effect of dicarbonyl -induced browning of alpha- crystalline chaperone-likw activity: Physiological significance and cavets of invitro aggregation assays. Biochem J 379: 273-282.
- Sheetz MJ, King GL (2002) Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288: 2579-2588.
- Peppa M, Uribarri J, Vlassara H (2004) The role of advanced gycation end products in the development of atherosclerosis. Curr Diab Rep 4: 31-36.
- Raabe HM, Hopner JH, Notbohm H, Sinnecker GH, Kruse K, et al. (1998) Biochemical and biophysical alterations of the 7S & NC1 doamin of collagen IV from human diabetic kidneys. Diabetologia 41: 1073-1079
- Skolnik Ey, Yang Z, Makita Z, Radoff S, Kristein M, et al. (1991) Human and rat mesangial cell receptors for glucose modified proteins : potential role in kidney tissue remodeling and diabetic nephropathy. J Exp Med 174: 931-939.
- Yang CW, Vlassara H, Peten EP, He CJ, Striker GE, et al. (1994) Advanced glycation end products upregulate gene expression found in diabetic glomerular disease. Proc Natl Acad Sci 91: 9436-9440.
- Boel E, Selmer J, Flodgard HJ, Jensen T (1995) Diabetic late complications: will aldolase reductase inhibitors of advanced glycosylation end product formation hold promise? J Diab Comp 9: 104-129.
- Poduslo JF, Curran GL (1992) Increased permeability across the blood nerve barrier of albumin glycated invitro and invivo from patients with diabetic polyneuropathy. Proc Natl Acad Sci 89: 2218-2222.
- Sugimoto K, Nishizawa Y, Horiuchi S, Yagihashi S (1997) Localization in human diabetic peripheral nerve of N (epsilon) -Carboxymethyllysine protein adducts, an advanced glycation end product. Diabetologia 40: 1380-1387.
- Â Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, et al. (2003) Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes 52: 2805-2813.
- Eble AS, Thorpe SR, Baynes JW (1983) Non-enzymatic glycosylation and glucose dependent cross-linking of proteins. J Biol Chem 256: 9406-9412.
- Yamada K, Miyahara Y, Hamaguchi K, Hatakeyama E, Tsuchida H, et al. (1994) Immuno histochemical study of human advanced glycation end products in chronic renal failure. Clin Nephrol 42: 354-361
- Cai W, Uribarri J, Zhu I, Chen X, Swamy S, et al. (2014) Oral glycotoxins are a modifiable cause of dementia and metabolic syndrome in mice and humans. Proc Natl Acad Sci USA 111: 4940-4945.
- Ilieva EV, Naudi A, Kichev A, Ferrer I, Pamplona R, et al. (2010) Depletion of oxidative and endoplasmic reticulum stress regulators in Pick disease. Free radic Biol Med 48: 1302-10.
- Donmez G, Wang D, Cohen DE, Guarente L (2010) SIRT1 suppresses β amyloid production by activating α secretase gene ADAM10. Cell 142: 320-332.
- Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, et al. (2002) Advanced glycation end product induced calcium handling impairment in mouse cardiac myocytes . J Mol Cell Cardiol 34: 1425-1421.
- Striker LJ, Striker GE (1996) Administration of AGEs in vivo induces extracellular matrix gene expression. Nephrol Dial Transplant11: 62-65.
- Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, et al. (1993) Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 143: 1699-1712.
- Witztum JL, Steinberg D (1991) Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88: 1785-1792.
- Parthenakis FI, Kanoupakis EM, Kochiadakis GE, Skalidis EI, Mezilis NE, et al. (2000) Left ventricular diastolic filling pattern predicts cardiopulmonary determinants of functional capacity in patients with congestive heart failure. Am Heart J 140: 338-344.
- Gugliucci A, Mehlhaff K, Kinugasa E, Ogata H, Hermo R, et al. (2007) Paraoxonase-1 concentrations in end stage renal disease patients increase after hemodialysis : Correlation with low molecular AGE adducts clearance. Clin Chim Acta 377:213-220
- Gugliucci A, Bendayan M (1996) Renal fate of circulating advanced glycated end products (AGE): evidence for reabsorption and catabolism of AGE peptides by renal proximal tubular cells. Diabetologia 39: 149-160.
- Yan HD, Li XZ, Xie JM, Li M (2007) Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK – 49F cells. Chin Med J 120: 787-793
- Verzijl N, De Groot J, Thorpe SR, Bank RA, Shaw JN, et al. (2000) Effects of collagen turnover on the accumulation of advanced glycation end prosucts. J Biol Chem 275: 39027-39031.
- Paul RG, Bailey AJ (1996) Glycation of collagen : the basis of its central role in the late complications of ageing and diabetes. Int J Biochem. Cell Biol 28: 1297-1310.
- Papagrigoraki A, Del Giglio M, Cosma C, Maurelli M, Girolomoni G, et al. (2017) Advanced glycation end products are increased in the skin and blood of patients with severe psoriasis. Acta Derm Venereol 97: 782-787.
- Gabr SA, A1-Ghadir AH (2012) Role of cellular oxidative stress and cytochrome C in the pathogenesis of psoriasis. Arch Dermatol Res 304: 451-457.
- Mezentsev AV, Bruskin SA, Sobolev AG, Sobolev VV, Piruzian ES (2013) Pharmaological control of recptor of Advanced glycation end product and its biological effects in psoriasis. Int J Biomed Sci 9: 112-122.
- Ehrmann DA (2005) Polycystic ovary syndrome. N Engl J Med 352: 1223-1236.
- Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E (2005) Increased levels of serum advanced glycation end products in women with polycystic ovary syndrome. Clin Endocrinol (oxf) 62: 37-43.
- Diamanti–Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A (2008) Increased serum advanced glycation end products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol (oxf) 69: 634-41.
Citation: Sabitha Vadakedath, Venkataramana Kandi (2018) Role of Advanced Glycation End Products (AGE) in Health and Disease: An Overview. Biochem Physiol 7: 246. DOI: 10.4172/2168-9652.1000246
Copyright: © 2018 Vadakedath S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8294
- [From(publication date): 0-2018 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 7101
- PDF downloads: 1193