Short Communication Open Access
Role of Focal Adhesions in Lamellipodia Dynamics
Yongwan Kim1 and Myeong Gu Yeo2*1Department of Hotel Culinary, Nambu University, Gwangju 506-706, Korea
2Department of Integrative Medical Sciences, Nambu University, Gwangju 506-706, Korea
- Corresponding Author:
- Myeong Gu Yeo
Department of Integrative Medical Sciences
Nambu University, 23 Chumdan Jungang-ro
Gwangsan-gu, Gwangju, 506-706, Korea
Tel: 82629700169
E-mail: mgy11@nambu.ac.kr
Received date:: January 30, 2015; Accepted date:: June 23, 2015; Published date:: June 29, 2015
Citation: Kim Y, Yeo MG (2015) Role of Focal Adhesions in Lamellipodia Dynamics. J Biotechnol Biomater 5:185. doi:10.4172/2155-952X.1000185
Copyright: © 2015 Kim Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Focal adhesions (FAs) are multi-protein structures containing integrin that serve as a focal point for the association between the extracellular matrix (ECM) and actin cytoskeleton. After cells adhere to the ECM, the cell membrane forms filopodia and lamellipodia. Cells deficient in FA complexes show reduced lamellipodia dynamics and this deficiency influences cell migration. Lamellipodia dynamics have distinguishable stages of lamellipodia protrusion, retraction, and persistence. Particularly, cells with decreasing or absent FA formation commonly show longer persistence time as analyzed using computer-assisted stroboscopic analysis. These results indicate that after cells adhere to the substratum, the reduced lamellipodia dynamics associated with defective FA formation influences cell motility.
Cell migration plays a central role in many physiological and pathological processes including embryogenesis, inflammatory response, wound healing, and metastasis. Cell migration is a distinctive, integrative, multistep process including formation of the cell adhesion, membrane protrusion at the front area, cell body contraction, and tail detachment [1]. Cell adhesion utilizes focal adhesions (FAs), fibrillary adhesions, and podosomes [2] to link the extracellular matrix (ECM) and various cytoplasmic proteins. After cells adhere to the ECM, the membrane begins filopodium formation and lamellipodium extension at the front edge of the cell. These are driven by actin polymerization and microtubule dynamics [3]. During the next steps of cell migration, the cell body moves forward in the migration direction and releases the cell-substrate adhesion at the cell rear [4]. At the cell front, the membrane begins as a flat cellular protrusion that is powered by actin polymerization and can be visualized by phase contrast microscopy as dark waves, which are called membrane ruffles [4,5]. Lamellipodia are sheet-like projections formed at the leading edge of many migrating cells, including fibroblasts, immune cells, neural crest cells, and melanoblasts [5,6]. This review will discuss the role of FA and its effect on lamellipodia dynamics in understanding adhesion-dependent cell migration. FAs are highly dynamic structures that form at sites of membrane contact with the ECM and associate with many cellular proteins known as FA complexes, including vinculin, focal adhesion kinase (FAK), Src family kinases (SFKs), paxillin, p130CAS (Crk-associate substrate), and Crk [2,7]. Deficiency of FA complexes in mouse embryo fibroblasts (MEFs) results in severe defects in cell spreading and culminates in embryonic death. For example, FAK-null MEFs show mesodermal defects in the late phase of gastrulation and have a delay in cell migration in vitro [8,9]. Deficiency of p130CAS causes severe defects in cell spreading [9,10]. In SYF cells (deficient for Src, Yes, and Fyn), MEFs show a decreased formation of FAs, which results in severe developmental defects, lethality, and delayed cell migration [11-13]. Additionally, Crknull mice die during a late stage of embryonic development [14] and present with a defect in cell-spreading in vitro [15]. These results imply that FA-associated proteins play primary roles in cell migration.
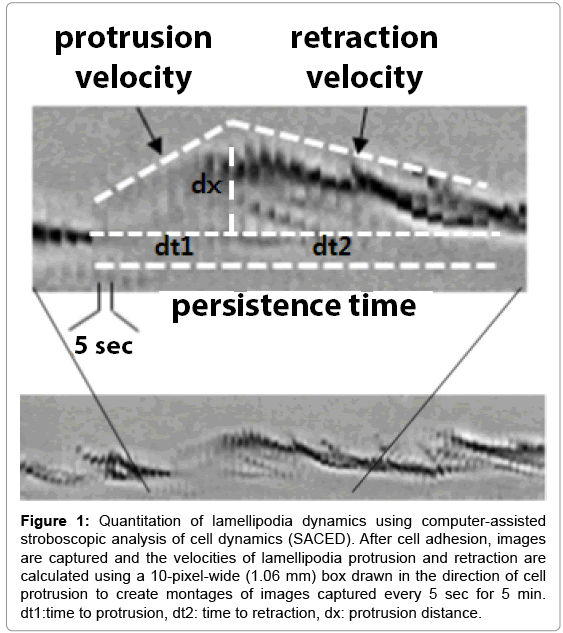
Lamellipodia and membrane ruffles form when cell adhesions fail or detach from the substrate and retract toward the cell body [4]. Membrane ruffles appear at the edge of cells moving in culture and disappear at the border between the lamella base and the main cell body [16,17]. To elucidate the mechanisms underlying cell motility, a quantitative analysis of lamella dynamics was introduced. This computer-assisted stroboscopic analysis of cell dynamics (SACED) is used for analyzing lamellipodia and ruffle formation after the cell adheres to the ECM [4,17, 18] (Figure 1). The SACED analysis of lamellipodia dynamics has distinctive stages including lamellipodia protrusion, persistence, and ruffle retraction. Membrane dynamics and ruffling involve many proteins including, α4β1 integrin [19], Rac1 [20], Arp2/3 [7], and others [21,22].
Figure 1: Quantitation of lamellipodia dynamics using computer-assisted stroboscopic analysis of cell dynamics (SACED). After cell adhesion, images are captured and the velocities of lamellipodia protrusion and retraction are calculated using a 10-pixel-wide (1.06 mm) box drawn in the direction of cell protrusion to create montages of images captured every 5 sec for 5 min. dt1:time to protrusion, dt2: time to retraction, dx: protrusion distance.
In the comparative studies on defective FA formation in MEFs, these MEFs commonly show longer lamellipodia persistence time than wild type (wt) MEFs. The SACED analysis of persistence time (sec; mean ± SD) of Crk-null MEFs is 40.30s ± 1.7 and wt Crk is 25.82s ± 0.66, SYF MEF is 90.81s ± 27.79 and wt Fyn (recovered Fyn cDNA in SYF MEF) [13] is 65.25s ± 38.46, and p130CAS-null MEF is 67.2 s ± 10.1083 and wt p130CAS is 36.5455s ± 6.3303 [13,15,18]. Furthermore, experiments in p130CAS-null MEFs show the lamellipodia protrusion and ruffle retraction velocity decreases compared with wt p130CAS MEFs [18]. The Fyn-null MEFs also show that the lamellipodia protrusion and ruffle retraction velocity decreases compared to the wt Fyn MEFs [13]. These SFK deficient MEFs commonly show less formation of FAs and reduced lamellipodia protrusion and retraction velocity, but membrane persistence times are prolonged compared to wt MEFs. Based on these results, FA-associated proteins modulate membrane dynamics and lamellipodia protrusion, ruffle retraction, and membrane persistence are involved in the cell spreading necessary for cell motility.
Overall, the findings summarized in this article suggest that lamellipodia dynamics control cell migration and membrane persistence time and thus are useful as one criterion of cell migration. However, to fully understand the precise mechanisms in lamellipodia dynamics, it will be necessary to examine the associated cellular factors during lamellipodia protrusion, retraction, and persistence.
Acknowledgement
This study was supported by research funds from Nambu University, 2014.
References
- Lauffenburger DA, Horwitz AF (1996)Cell migration: aphysicallyintegrated molecular process. Cell84: 359-369.
- Yeo MG, Song WK (2008) v-Crkregulates membrane dynamics and Rac activation. Celladhesion& migration 2: 174-176.
- Pollard TD, Borisy GG (2003) Cellular motilitydriven by assembly and disassembly of actin filaments. Cell112: 453-465.
- Borm B, Requardt RP, Herzog V, Kirfel G (2005) Membrane ruffles in cell migration: indicators of inefficient lamellipodiaadhesion and compartments of actin filament reorganization. Experimentalcell research302: 83-95.
- Krause M, Gautreau A (2014)Steeringcell migration: lamellipodiumdynamics and the regulation of directionalpersistence. Nature reviews Molecular cellbiology15: 577-590.
- Rorth P (2011)WhenceDirectionality: Guidance Mechanisms in Solitary and Collective Cell Migration. DevelopmentalCell20: 9-18.
- Craig SW, Chen H (2003)Lamellipodia protrusion: moving interactions of vinculin and Arp2/3. Current biology13: R236-R8.
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, et al. (1995)Mesodermaldefect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene11: 1989-1995.
- Carter N, Nakamoto T, Hirai H, Hunter T (2002) EphrinA1-induced cytoskeletalre-organizationrequires FAK and p130cas. Nat CellBiol4: 565-573.
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, et al. (1998) CAS/Crkcoupling serves as a “molecular switch” for induction of cell migration. The Journal of cellbiology140: 961-972.
- Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P (1999)Srcfamily kinases arerequired for integrin but not PDGFR signal transduction. The EMBO Journal18: 2459-2471.
- Yeo MG, Partridge MA, Ezratty EJ, QiongShen, Gregg GG, et al. (2006) Src SH2 arginine 175 is required for cellmotility: specific focal adhesion kinase targeting and focal adhesionassembly function. Molecular and cellular biology26: 4399-4409.
- Yeo MG, Oh HJ, Cho HS, Chun JS, Marcantonio EE, et al. (2011) Phosphorylation of Ser 21 in Fyn regulatesits kinase activity, focal adhesiontargeting, and is required for cell migration. Journal of Cellular Physiology2011; 226: 236-247.
- Park TJ, Boyd K, Curran T (2006)Cardiovascular and craniofacial defects in Crk-nullmice. Molecular and cellular biology26: 6272-6282.
- Oh H, Kim H, Shin B,Lee KH, Yeo MG, et al. (2013) Interaction of Crk with Myosin-1c Participates in Fibronectin-InducedCellSpreading. International journal of biological sciences9: 778-791.
- Abercrombie M, Joan E, Heaysman M, Pegrum SM (1970) The locomotion of fibroblasts in culture: II.“Ruffling”. Experimentalcell research60: 437-444.
- Hinz B, Alt W, Johnen C, Herzog V, Kaiser HW(1999) Quantifyinglamelladynamics of culturedcells by SACED, a new computer-assisted motion analysis. Experimentalcell research251: 234-243.
- Sung BH, Yeo MG, Oh HJ, Song WK (2008) v-Crkinduces Rac-dependent membrane ruffling and cell migration in CAS-deficientembryonicfibroblasts. Molecules and cells25: 131-137.
- Pinco KA, He W, Yang JT (2002) a4ß1 integrinregulateslamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Molecular biology of the cell13: 3203-3217.
- Allen WE, Jones GE, Pollard JW, Ridley AJ (1997) Rho, Rac and Cdc42 regulateactinorganization and celladhesion in macrophages. Journal of cell science110: 707-720.
- Legg JA, Bompard G, Dawson J,Morris HL, Andrew N, et al. (2007) N-WASP involvement in dorsal ruffle formation in mouse embryonicfibroblasts. Molecular biology of the cell18: 678-687.
- Rottner K, Behrendt B, Small JV, Wehland J (1999) VASP dynamicsduringlamellipodia protrusion. Nature CellBiology1: 321-322.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15388
- [From(publication date):
August-2015 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 10737
- PDF downloads : 4651