Roles of Nitricoxide Signaling Pathway in Atherosclerosis
Received: 12-Jan-2018 / Accepted Date: 28-Feb-2018 / Published Date: 05-Mar-2018
Abstract
Nitric oxide (NO) generated by endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) plays a critical role in the vasoprotective function of the endothelium under physiological conditions. However, under pathological conditions, eNOS and nNOS become dysfunctional, and inducible nitric oxide synthase (iNOS) is stimulated to produce excessive NO, which induce endothelial dysfunction. NO signaling pathways mainly include phosphatidylinositol 3-kinase (PI3K)/serine threonine protein kinase B(AKT)/eNOS pathway, nuclear factorkappa B(NF-κB)/iNOS pathway, extracellular regulated protein(ERK)1/2/nNOS pathway, etc. which are involved in the development and progression of atherosclerosis through affecting NO synthesis and its functions. Meanwhile, NO-soluble guanylyl Cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway is closely related to atherosclerosis, due to the ability of regulating the degree of vasodilatation. Elucidating NO signaling pathway associated with atherosclerosis is necessary for the improvement and treatment of atherosclerosis. This review summarized the relationships between the different NO signaling pathways and atherosclerosis in the present relative studies.
Keywords: Nitric oxide; Atherosclerosis; Signaling; Pathway
Introduction
Atherosclerosis is the underlying cause of most cardiovascular diseases, such as coronary artery disease (CAD), heart failure, stroke and so on. These diseases are the main cause of human mortality and disability in the world today [1]. NO was firstly proposed as an endothelium-derived relaxing factor by Furchgott and Ignarro in 1986, and it was also the first gaseous molecule accepted to be a signaling mediator in the organism [2]. Many studies have shown that NO produced under different conditions has an inhibitory or promoting effect on atherosclerosis. The role of NO in the pathogenesis of atherosclerosis has not yet been fully elucidated. In this paper, the progress in relationships between NO signaling pathways and atherosclerosis was reviewed.
Pathological Characteristics of Atherosclerosis and its Presumptive Pathogenesis
Atherosclerosis is an inflammatory disease that results in the development of atherosclerotic plaques and progressive stenosis of the coronary arteries [3]. Atherosclerosis starts at the arterial intima [4] where endothelial cells become activated, resulting in recruitment and accumulation of monocytes, which differentiate into macrophages [5]. Macrophages become foam cells through the uptake of oxidized low density lipoprotein (oxLDL). In addition, it has been reported that foam cells also derive from smooth muscle cells (SMCs) [6]. Mature SMCs that are present in the media of the no atherosclerotic vessel wall possess the potential to become activated during plaque development and trans-differentiate to macrophage-like cells with a Mox phenotype that reside within the lesion. The SMCs derived plaque macrophages have a clonal origin in the vascular media and can make up a major fraction of the intimal cells. Foam cells produce high levels of proinflammatory cytokines, chemokines and growth factors that induce SMCs proliferation and migration to the intima, leading to plaque growth. Due to intimal hyperplasia with macrophages and SMCs infiltration, vascular lumen becomes narrowed, causing the ischemic changes of the organ [7].
A variety of hypotheses have been developed to explain atherosclerosis, in which cholesterol deposition in arterial wall, viral or bacterial infection, response-to-injury, local arterial inflammation and autoimmune response were involved [8]. Although some progress has been made, the exact pathogenesis still remains to be clarified [9].
The current views on the pathogenesis of atherosclerosis are as follows: (1) Atherosclerosis has been considered as an inflammatory/ autoimmune disease, and its attack is initially caused by proinflammatory Th1 cells and cytokine immune responses. Now, the Th17/Th1 axis is thought to be shared by most sterile inflammatory diseases. The anti-inflammatory Th2 cells and cytokine immune response are initiated concomitantly with the former, the latter dampening their harmful reactions which culminate in full-blown atherosclerosis. Interleukin-33 (IL-33), as a novel member of the IL-1 cytokine superfamily, is a multifaceted mediator with both pro- and anti-inflammatory activities, and it participates in antiatherogenic response through mediating the Th1-to-Th2 switch of immune response [10]. The IL-1β induces the procoagulant activity, promotes monocyte and leukocyte adhesion to vascular endothelial cells, and accelerates the growth of SMCs in the development of atherothrombotic plaque [11]. Meanwhile, IL-1β drives the IL-6 signaling pathway, which promotes the development of inflammation. In CANTOS11, anti-inflammatory therapy targeting the IL-1β innate immunity pathway with canakinumab led to a significantly lower rate of recurrent cardiovascular events than placebo, independent of lipidlevel lowering. (2) DNA methylation in the genome plays an important role in the development of atherosclerosis. DNA methyltransferases is crucial in maintaining endothelial cell integrity, promoting SMCs proliferation, and inducing atherosclerosis formation in animal models. Some studies have shown that oxidative stress affected DNA methylation in the progression of atherosclerosis [12]. (3) Mitochondrial dysfunction and mitochondrial DNA (mtDNA) damage may be directly involved in atherosclerosis formation [13]. At least four mitochondrial genomic mutations, namely, A1555G in MT-RNR1 gene, C3256T in MT-TL1 gene, G12315A in MT-TL2 gene and G15059A in MT-CYB gene are related to lipofibrous plaques in human aortic intima [8]. (4) Endothelial cell metabolism is perturbed to promote atherogenesis when endothelial cells become dysfunctional5. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) is a major regulator of atherosclerosis-related cellular energy metabolism. It has been reported that PGC-1 was modulated at its transcriptional level by miR-19-3p, miR-221-3p and miR-222-3p targeting PGC1α in atherosclerosis, which contribute to endothelial cell dysfunction [14].
The Putative Role of NO in Atherosclerosis
NO is generated by a family of three nitric oxide synthase enzymes(NOS) isoforms, namely endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) [15]. Most of the NO in the vascular system is produced by eNOS [16]. Endothelium-derived NO (eNO) is a multifunctional signaling molecule that, as an effective endogenous vasodilator, inhibits the key process in vascular lesion formation. eNO reduces the production of reactive oxygen species(ROS) and lipid peroxidation [17]. In addition, eNO also has the effect of inhibiting platelet adhesion and aggregation, inhibiting adhesion molecule and chemokine expression, as well as reducing inflammatory cell infiltration and SMCs migration and proliferation [18].
The first evidence that nNOS played a role of vascular protection in atherosclerosis was from the work of Wilcox et al., which showed the correlation between the progression of plaque formation and nNOS mRNA [19]. And it is compatible with the studies showing accelerated neointimal formation and constrictive vascular remodeling in nNOS-knockout (nNOS-KO) mice in a carotid artery ligation model [20].
Vascular cells express iNOS, which produces large amounts of NO leading to vascular dysfunction in inflammatory conditions [16]. Zhao et al. proved that the high levels of NO produced by iNOS may interfere with the efficacy of the liverX receptor α(LXRα)-ATP-binding cassette transporter A1(ABCA1)–dependent cholesterol efflux and thereby promote oxLDL-mediated cholesterol accumulation in foam cells, thus leading to atherosclerosis progression [21]. The experimental results indicated that the anti-atherosclerosis effect of adiponectin is to reduce oxidative/nitrative stress by inhibiting iNOS, superoxide and peroxynitrite production [22]. Superoxide usually functions as a reducing agent, and the fate and effect of this radical depend on surrounding conditions. Under normal physiological conditions, cells generate small but significant amounts of superoxide. The superoxide dismutase catalyzed dismutation is the most important antioxidant systems in terms of superoxide anions [23]. Superoxide formation increases dramatically, and at the same time iNOS produces high concentrations of NO in atherosclerosis. Both radicals react in a diffusion-controlled fashion to yield the toxic and highly reactive nitrogen species peroxynitrite, which might account for numerous deleterious effects associated with nitro-oxidative stress [24]. In human atherosclerosis and in a mouse model of vascular remodeling (carotid artery ligation), Reventun et al. [25] found a significant correlation between increased levels of inducible nitric oxide and decreased integrin-linked kinase levels (ILK, plays a key role in controlling vasomotor tone and is decreased in atherosclerosis) comparing with iNOS knockout mice, and NO/iNOS promoted the dissociation of the complex ILK/heat shock protein 90/eNOS, leading to eNOS uncoupling. They provided the first molecular evidence about the negative effect of NO/iNOS on ILK stability by inducing internalization and molecular degradation of ILK in lysosomes. Inflammation of the vascular wall during atherosclerosis and vascular remodeling induces iNOS to release vast amounts of NO, promoting the development of atherosclerosis.
It can be seen that the NO/eNOS and NO/nNOS produced in physiological conditions have the effect of anti-atherosclerosis, and the NO/iNOS generated under pathological conditions will trigger or accelerate atherosclerosis.
NO Synthesis Pathway And Atherosclerosis
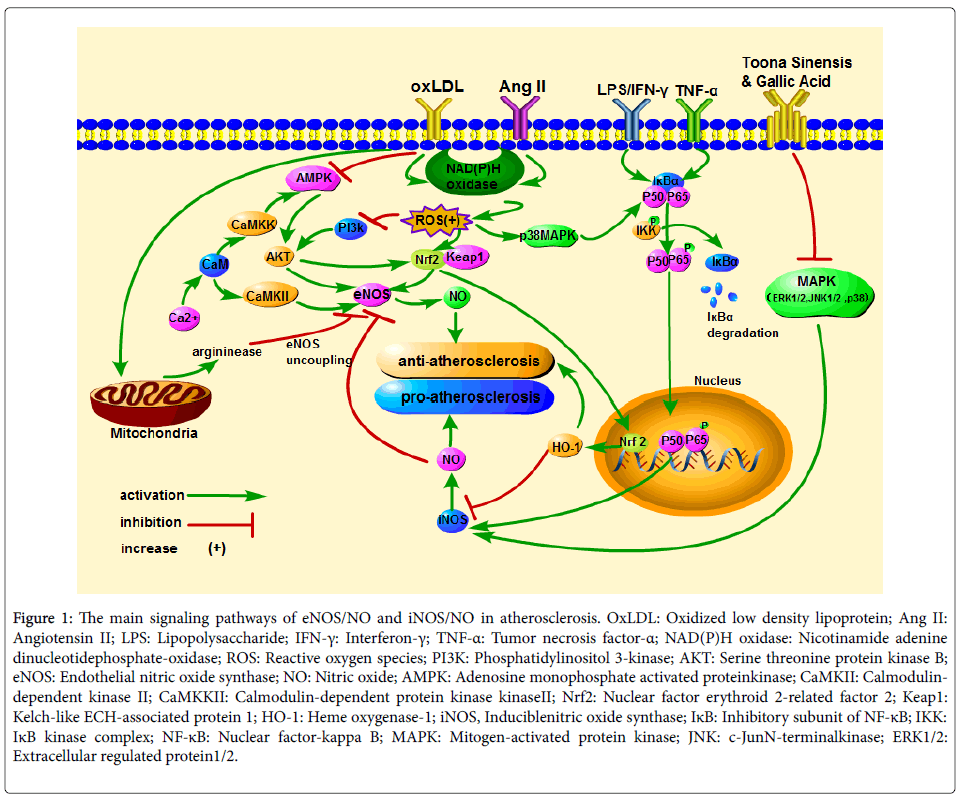
The main signaling cascades associated with NO in atherosclerosis are as follows (Figure 1): (1) Under pathological conditions, the activated nicotinamide adenine dinucleotide phosphate-oxidas e (NADPH oxidase) induces endothelial cells to produce a large amount of ROS, which blocks PI3K-mediated and adenosine monophosphate activated protein kinase(AMPK)-mediated phosphorylation of AKT, and then lead to a decrease in NO produced by eNOS. (2) Oxidative stress activates nuclear factor erythroid 2-related factor 2 (Nrf2), which can induce eNOS phosphorylation and enter the nucleus to trigger Heme oxygenase-1 (HO1) gene expression, and then inhibits the development of atherosclerosis. (3) eNOS is a Ca (2+)-dependent enzyme, and atherosclerosis is associated with the Ca(2+)/ calmodulin-dependent kinase II(CaMKII) or calmodulin-dependent protein kinase kinase(CaMKK)/AMPK/AKT/eNOS/NO pathway abnormality. (4) Under inflammatory conditions, NF-κB p50 and p65 transfer to the nucleus, and induce iNOS gene expression, then accelerate the process of atherosclerosis. Meanwhile, ROS promotes the above cascade reaction through activating p38 mitogen-activated protein kinase (MAPK). What’s more, the active MAPK (ERK1/2c-Jun N-terminal kinase (JNK)1/2 and p38) can increase the concentration of NO produced by iNOS directly.
Figure 1: The main signaling pathways of eNOS/NO and iNOS/NO in atherosclerosis. OxLDL: Oxidized low density lipoprotein; Ang II: Angiotensin II; LPS: Lipopolysaccharide; IFN-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α; NAD(P)H oxidase: Nicotinamide adenine dinucleotidephosphate-oxidase; ROS: Reactive oxygen species; PI3K: Phosphatidylinositol 3-kinase; AKT: Serine threonine protein kinase B; eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide; AMPK: Adenosine monophosphate activated proteinkinase; CaMKII: Calmodulindependent kinase II; CaMKKII: Calmodulin-dependent protein kinase kinaseII; Nrf2: Nuclear factor erythroid 2-related factor 2; Keap1: Kelch-like ECH-associated protein 1; HO-1: Heme oxygenase-1; iNOS, Induciblenitric oxide synthase; IκB: Inhibitory subunit of NF-κB; IKK: IκB kinase complex; NF-κB: Nuclear factor-kappa B; MAPK: Mitogen-activated protein kinase; JNK: c-JunN-terminalkinase; ERK1/2: Extracellular regulated protein1/2.
The eNOS/NO Signaling and Atherosclerosis
The PI3K/AKT/eNOS/NO pathway and atherosclerosis: OxLDL binding to lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) triggers the activation of NADPH oxidase, which results in the production of ROS and lipid peroxidation (Table 1) [26]. In addition, the PI3K/AKT / eNOS/NO pathway disordering induced by oxLDL leads to the reduced NO production, and these abilities of NO scavenging superoxide anions, preventing superoxide anion from forming its dismutation product and hydrogen peroxide are impaired, eventually induces endothelial cell damage [27]. PI3K, which is a heterodimeric enzyme, plays a critical role in proliferation and apoptosis, while its downstream serine-threonine kinase, AKT, transmits survival signals from growth factors [28]. The activation of AKT mediated by PI3K involves its phosphorylation on threonine 308 and serine 473, and the activated AKT promotes eNOS to produce NO [27]. Phosphocreatine (PCr) [27] and Myricitrin [26] have been proved to inhibit the apoptosis of human umbilical vein endothelial cells (HUVECs) induced by ox-LDL and reduce the formation of atherosclerotic plaques by activating PI3K/AKT/eNOS/NO pathway. The vascular endothelial dysfunction of high fat diet induced atherosclerosis rat model [29] and mouse model [30] was eased with drugs which upregulated the mRNA and protein expression of PI3K, AKT and increasing eNOS phosphorylation.
| Pretreatment | Animal | Cells | Signaling pathway | NO levels | Atherosclerosis | References |
|---|---|---|---|---|---|---|
| Myricitrin | atherosclerotic mouse model | ox-LDL-induced HUVECs | PI3K/AKT/eNOS(+); STAT3(+) | ↑ | ↓ | [26] |
| Phosphocreatine | - | ox-LDL-induced HUVECs | PI3K/AKT/eNOS (+) | ↑ | ↓ | [27] |
| Klotho | - | ox-LDL-induced HUVECs | PI3K/AKT/eNOS (+) | ↑ | ↓ | [28] |
| Wen-Xin Decoction | Wistar rats models of atherosclerosis |
- | PI3K/AKT/eNOS (+) | ↑ | ↓ | [29] |
| Salidroside | apoE(-/-) male mice with high-fat diet |
- | AMPK/PI3K/AKT/ eNOS (+) |
↑ | ↓ | [30] |
| Ethanol extract of Rumex acetosa L. |
phenylephrine- precontracted rat thoracic aorta |
HUVECs | PI3K/AKT/eNOS (+); Ca(2+)-eNOS-NO (+) |
↑ | ↓ | [31] |
| Progranulin | - | HUVECs | AKT/eNOS (+) | ↑ | ↓ | [32] |
| Osteocalcin | apoE (-/-) mice with high fat diet |
HUVECs | PI3K/AKT/eNOS (+) | ↑ | ↓ | [33] |
| Olmesartan | carotid atherosclerosis patients |
- | PI3K/AKT/eNOS (+) | ↑ | ↓ | [34] |
| Irisin | apoE(-/-) streptozotocin- induced diabetic mice |
HUVECs | AMPK-PI3K-AKT- eNOS (+) |
↑ | ↓ | [35] |
| TRAIL | diabetic rats | HUVECs | AKT/eNOS (+) | ↑ | ↓ | [36] |
| Vaspin | - | Ratbonemarrowderived endothelial progenitor cell with glucose treatment |
PI3K/AKT/eNOS (+) | ↑ | ↓ | [37] |
| EGCG | - | homocysteine- induced HUVECs | PI3K/AKT/eNOS (+) | ↑ | ↓ | [38] |
| Interleukin-1β | - | HUVECs | Ca(2 +) / CaMKII / eNOS (-) | ↓ | ↑ | [39] |
| β-carotene | - | HUVECs | Ca (2 +) / CaMKKII / eNOS (+) | ↑ | ↓ | [39] |
| Betulinic acid | - | HUVECs | Ca(2+)/CaMKII/ eNOS (+); Ca(2+)/CaMKK/ AMPK/eNOS (+) |
↑ | ↓ | [40] |
| CytochromeP450 enzymes(CYP) 2C8-derived epoxyeicosatrie-noic acids |
- | TNF-α-induced HUVECs | Nrf2/HO-1/eNOS (+) | ↑ | ↓ | [41] |
| Piceatannol | - | palmitic acid-stimulated HUVECs | Nrf2/HO-1/eNOS (+) | ↑ | ↓ | [42] |
| Prunella vulgaris | - | HUVECs with high glucose treatment | Nrf2/HO-1/eNOS (+) | ↑ | ↓ | [43] |
| Quercetin | - | ox-LDL-induced HUVECs | AMPK/NADPH oxidase/AKT/eNOS (+) | ↑ | ↓ | [44] |
| Ginkgo biloba extract | - | ox-LDL-induced HUVECs | AMPK / AKT /eNOS (+) | ↑ | ↓ | [45] |
| Vaspin | Sprague-Dawley rat | HAECs obtained from Lonza Inc. | STAT3/ DDAH / ADMA/eNOS (+) | ↑ | ↓ | [46] |
| Korean red ginseng water extract |
wild type mice;atherogenic model mice with high cholesterol diet |
- | OxLDL/arginase/ eNOS-uncoupling(-) |
↑ | ↓ | [47] |
| OxLDL | ApoE(-/-) mice | human aortic endothelial cells; murine aortic endothelial cells |
OxLDL/arginase/ eNOS-uncoupling(+) |
↓ | ↑ | [48] |
| OxLDL | ApoE(-/-) mice with Arg2(-/-);lectin-like OxLDL receptor-1 knockout mice |
- | OxLDL/arginase/ eNOS-uncoupling(-) |
↑ | ↓ | [48] |
| Tongxinluo | male Wistar Kyoto rats | - | ERK1/2-nNOS-NO(+) | ↑ | ↓ | [49] |
| Abbreviations: NO: Nitric oxide; eNOS: Endothelial Nitric oxide Synthase; nNOS: Neuronal Nitric Oxide Synthase; ox-LDL: Oxidized Low Density Lipoprotein; HUVECs: Human Umbilical Vein Endothelial Cells; PI3K: Phosphatidylinositol 3-kinase; AKT: Serine threonine Protein Kinase B; STAT3: Signal Transducer and Activator of Transcription 3; AMPK: Adenosine Monophosphate Activated protein Kinase; TRAIL: Tumor Necrosis Factor-related apoptosis-inducing ligand; EGCG: Epigallocatechin Gallate; CaMKII: Calmodulin-dependent Kinase II; CaMKKII: Calmodulin-dependent Protein Kinase II; TNF-α: Tumor Necrosis Factor-α; Nrf2: Nuclear Factor Erythroid 2-related factor 2; HO-1: Heme oxygenase-1; NADPH Oxidase: Nicotinamide Adenine Dinucleotide Phosphate-oxidase; DDAH: Dimethyl Arginine Dimethyl Amino Hydrolase; ADMA: Asymmetric Dimethyl Arginine; ERK1/2: Extracellular Regulated Protein1/2. | ||||||
Table 1: The role of different eNOS or nNOS/NO signaling in atherosclerosis.
The NOS-NO signaling of HUVECs treated with ethanol extract of Rumex acetosa L (ERA) [31], granule protein [32] and osteocalcin (OCN) [33] have been evaluated. The results showed that the phosphorylation of eNOS was induced through PI3k / AKT signaling in HUVECs treated with the former three, and the level of NO was significantly up-regulated, while the above effects could be blocked by PI3K inhibitors and AKT inhibitors [31-33]. In animal experiments, the isolated thoracic aortic vasodilatation induced by ERA was eliminated by L-NAME (NOS inhibitor) [31].
Gong et al. [34] selected 40 patients with carotid atherosclerosis. Patients were administrated olmesartan 20 mg/day for 3 months. The results showed that olmesartan could increase the number of circulating endothelial progenitor cells and the serum levels of eNOS and NO. Spearman rank correlation analysis showed there was no relationship between the promotion effects of olmesartan on endothelial progenitor cell mobilization and the clinical characteristics (e.g, gender, age, blood pressure, etc.) (P>0.05). They concluded that olmesartan effectively promoted the mobilization of endothelial progenitor cells and improved their function in patients with carotid atherosclerosis relying on the PI3K / AKT / eNOS / NO pathway [34].
Irtrexate [35], tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [36], and Vaspin [37] have been proved to activate the PI3K/AKT/eNOS/NO pathway in HUVECs, increase NO production, inhibit apoptosis, oxidative stress, NADPH oxidase production induced by high glucose, increase the expression of antioxidant enzyme, and finally improve diabetes-related atherosclerosis [35-37].
The Ca (2+)/CaMKII or CaMKK/AMPK/AKT/eNOS/NO pathway and atherosclerosis
eNOS is a Ca (2+) -dependent enzyme that is regulated by store-operated calcium entry (SOCE), and SOCE inhibitors impaire the activity of eNOS [50]. Yamagata et al. [39] found that IL-1β enhanced the expression of adhesion molecules, reduced NO production and induced atherosclerosis. The results showed that IL-1β reduced the expression of Ca (2+)/CaMKII, PI3K and eNOS in cultured human endothelial cells. In contrast, it increased the expression of intercellular adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1). It was found that IL-1β-induced atherosclerosis was associated with the Ca (2+)/CaMKII/eNOS/NO pathway abnormality.
Both AMPK and AKT are important regulators of eNOS activity, which promote NO secretion by increasing eNOS phosphorylation in human endothelial cells and vessels [44]. β-carotene and betulinic acid (BA) have the effect of preventing atherosclerosis, which is mediated by the Ca (2+)/CaMKII or CaMKK/AMPK/eNOS/NO pathway [39,40]. The research showed that the treatment of β-carotene [39] and BA [40] induced AMPK, eNOS phosphorylation and increased the level of NO in the cultivated human endothelial cells. They also increased the level of intracellular Ca (2+) and phosphorylation of Ca (2+)/CaMKIIα and Ca(2+)/CaMKKβ. At the same time, the treatment with AMPK inhibitor compound C, L-type Ca (2+) channel (LTCC) inhibitor, W7 (CaM antagonist), KN-93 (selective inhibitor of CaMKII) or STO 609 (selective inhibitor of CaMKK) reduced eNOS phosphorylation and the level of NO [39,40].
Studies have shown that oxLDL-induced endothelial oxidation involves the blockage of the AMPK/AKT/eNOS/NO pathway in atherosclerosis [44,45]. Hung et al. [44] pretreated HUVECs with or without quercetin and treated them with oxLDL. The results showed that quercetin protected against the decrease of AMPK activity and reduced oxLDL-activated NOX2 and NOX4. However, silent AMPK weakened quercetin’s protective function of anti-oxidation. At the same time, oxLDL inhibited AKT/eNOS/NO pathway, impaired mitochondrial function and enhanced the formation of ROS. Ou et al. [45] pretreating HUVECs with or without Ginkgo biloba extract (GbE) and treated them with oxLDL. Western blot results showed that GbE inhibited membrane translocation of NADPH oxidase subunits p47 (phox) and Rac-1, and attenuated the AMPK dephosphorylation and the increase of subsequently activated protein kinase C (PKC)-induced membrane subunits gp91 and p22 (phox) protein expression. AMPK- α(1)-specific small interfering RNA-transfected cells that had been exposed to GbE followed by oxLDL revealed the increased levels of PKC and p47(phox). In addition, exposuring to oxLDL resulted in the reduced AMPK-mediated AKT/eNO signaling [45].
The Nrf2/HO-1/eNOS/NO pathway and atherosclerosis
Nrf2 is a redox-sensitive master regulatory transcription factor which usually binds to Kelch-like ECH-associated protein (Keap)-1 in the cytoplasm. Nrf2 plays an important role in ensuring sustained release of NO and protecting endothelial cells from apoptosis [41]. HO1 is an antioxidant and anti-inflammatory inducible enzyme. Nrf2 is activated by oxidative stress and then enters the nucleus, where it binds to AUrich elements in the HO1 promoter to trigger HO1 gene expression [42]. Tumor necrosis factor-α(TNF-α) incubation significantly reduced Nrf2 expression in the nuclear fraction and inhibited Ser-1177 eNOS phosphorylation as well as HO-1 induction in HUVECs, causing endothelial cell apoptosis [41]. Meanwhile, other experiment showed that aqueous extract from Prunella vulgaris (APV) induced the activation of HO-1 and eNOS and enhanced the production of NO by activating the PI3K/AKT-mediated Nrf2 pathway and inhibiting Keap1 degradation, thus inhibited the diabetes-related atherosclerosis [43].
The STAT3/DDAH/ADMA/eNOS/NO pathway and atherosclerosis
Signal transducer and activator of transcription 3(STAT3) is a key transcription factor that regulates cell survival, inflammation and angiogenesis. ApoE-/- mouse atherosclerosis model [26], ox-LDL-induced HUVECs [26] showed that drug therapy activated the STAT3 signaling to relieve endothelial cell apoptosis and the early atherosclerotic plaque formation.
Asymmetric dimethylarginine (ADMA) is an endogenous competitive inhibitor of eNOS. And ADMA derives from methylation of arginine residues in proteins and is metabolized to citrulline and dimethylamine by the enzyme dimethylarginine dimethylaminohydrolase (DDAH) [46]. Jung et al. [46] proposed that vaspin affected the activity of DDAH II promoter, activated DDAH II gene expression and reduced the level of ADMA by mediating STAT3 signaling in the aorta model isolated from Sprague-Dawley (SD), finally increased STAT3 phosphorylation and eNOS activity in STAT3- dependent way and played the role of anti-atherosclerosis.
The OxLDL/arginase/eNOS-uncoupling/NO pathway and atherosclerosis
Hypercholesterolemia reduces the bioavailability of NO by affecting the function and activity of eNOS. OxLDL stimulates NADPH oxidase in the vessel wall, which mediates the oxidation of eNOS cofactor tetrahydrobiopterin (BH4), resulting in uncoupling of eNOS [51]. Arginase (a key enzyme in the urea cycle) directly competes with eNOS for the common substrate L-arginine, inhibits the activity of eNOS and affects the production of NO, leading to endothelial dysfunction. Recent studies have supported that arginase (including enzyme I and enzyme II) causes eNOS uncoupling, and the expression and activity of arginase are upregulated in atherosclerosis [47,52,53]. Pandey et al. [48] found that oxLDL triggered argininease II transfering from mitochondria to cytoplasm in human aortic endothelial cells and rat aortic intima, accompanied by an increase in arginase activity and eNOS uncoupling. Mitochondrial processing peptidase inhibitors or LOX-1-knockdown reduced the decrease in endothelial-specific NO production and the increase in superoxide production induced by ox-LDL [48].
The iNOS/NO Signaling and Atherosclerosis
The NF-κB-iNOS-NO pathway and atherosclerosis
NF-κB represents the transcription factor family, including p50 and p65 that are important in the regulation of inflammatory responses. NF-κB p50 and p65 transfer to the nucleus, and bring out a variety of gene transcription participating in diverse cellular processes (including inflammation, proliferation, apoptosis and cellular senescence) (Table 2) [54]. Activation of the NF-κB signaling has been found in macrophages, SMCs and endothelial cells in human atherosclerotic lesions, but has not been found in healthy blood vessels [32]. NF-κB regulates iNOS gene: iNOS catalyzes NO generation, and NO is a molecule that plays a role in inflammation and immune response [55]. Vascular inflammation induced by pathogens or cytokines is accompanied by the production of peroxynitrite, which is an effective vasotoxic molecule formed by the reaction of NO and superoxide [56]. The activation of NF-κB-iNOS-NO pathway accelerates the development of atherosclerosis.
| Pretreatment | Cells | Signaling pathways | NO levels | Atherosclerosis | References |
|---|---|---|---|---|---|
| Tormentic acid | H2O2-induced RVSMCs | IKK/IκBα/NF-κB/iNOS(-) | ↓ | ↓ | [54] |
| Progranulin | LPS-induced HUVECs | IKK/IκBα/NF-κB/iNOS(-) | ↓ | ↓ | [32] |
| Artemisinin | RVSMCs co-incubated with TNF-α |
IKK/IκBα/NF-κB/iNOS(-) | ↓ | ↓ | [55] |
| Andrographolide | TNF-α-stimulated HUVECs | JNK-AKT-NF-κBp65-iNOS(-) | ↓ | ↓ | [56] |
| hydrophilic α-tocopherol derivative(PMC) |
LPS/IFN-γ-stimulated VSMCs |
ROS-PP2A-NF-κBp65- iNOS(-) |
↓ | ↓ | [57] |
| Cyanidin-3- Glucoside (C3G) |
Ang II-stimulated human endothelial cells |
IKK/IκBα/NF-κB/iNOS(-) | ↓ | ↓ | [58] |
| atorvastatin and C3G | Human aortic smooth muscle cells exposed to Ang II | IKK/IκBα/NF-κB/iNOS(-) | ↓ | ↓ | [59] |
| GPx1 knockout | primary aortic endothelial cells isolated from GPx1 KO mice | IKK/IκBα/NF-κB/iNOS(+); MAPK(P38, ERK and JNK)-iNOS(+) |
↑ | ↑ | [60] |
| Honokiol | palmitic acid-stimulated HUVECs | IKK/IκBα/NF-κB/iNOS(-) | ↓ | ↓ | [61] |
| Resokaempferol | LPS-stimulated RAW264.7 macrophages |
JAK2 / STAT3-iNOS(-); NF-κB-iNOS(-); JNK/p38 MAPK-iNOS(-) |
↓ | ↓ | [62] |
| Prunella vulgaris ethanolextract | TNF-α-stimulated human aortic smooth muscle cells | p38MAPK/ERK-iNOS(-) | ↓ | ↓ | [63] |
| Toona sinensis and gallic acid | LPS-treated rat aortic smooth muscle cells | NF-κB/iNOS(-); MAPK (ERK1/2, JNK1/2, and p38)-iNOS(-) |
↓ | ↓ | [64] |
| Abbreviations: iNOS: Induciblenitric Oxide Synthase; H2O2: Hydrogen Peroxide; RVSMCs: Rat Vascular Smooth Muscle Cells; IκB: Inhibitory Subunit of NF-κB; IKK: IκB Kinase Complex; NF-κB: Nuclear Factor-kappa B; LPS: Lipopolysaccharide; JNK: c-JunN-terminal Kinase; IFN-γ: Interferon-γ; VSMCs: Vascular Smooth Muscle cells; ROS: Reactive Oxygen Species; PP2A: Protein Phosphatase 2A; Ang II: Angiotensin II; MAPK: Mitogen-activated Protein Kinase; JAK2: Janus Kinase 2. | |||||
Table 2: The roles of different iNOS/NO signaling in atherosclerosis.
The IKK/IκBα/NF-κB/iNOS/NO pathway
The most common form of NF-κB is the p65/p50 heterodimer in most cell types, and the NF-κB signaling is controlled by the IκB(inhibitory subunit of NF-κB) kinase(IKK) complex, which is composed of IKKα, IKKβ, IKKγ and the downstream substrate IκBα (the main type of IκB) [55]. Activated IKK phosphorylates IκBα due to stimulation, resulting in IκBα degradation, enhancing NF-κB nuclear translocation and subsequent transcriptional activation [55].
Angiotensin II(Ang II) [56], hydrogen peroxide(H2O2) [52], TNF-α [53] activate NADH/NADPH oxidase, and induce the ROS-activated NF-kB signaling. ROS has been assumed to be the second messenger of NF-kB activation [56]. The increased production of ROS activated the NF-kB signaling in Ang II-induced human endothelial cells [56,57] H2O2-induced rat vascular smooth muscle cells (RVSMCs) [52] and TNF-α-induced RVSMCs [53]. Pretreating the above models with drugs could attenuate the formation of NO by iNOS and atherosclerotic plaques by inhibiting the degradation of IkBα inhibitors and down-regulating the expression of NF-κB subfamily (P50 and p65) [52,56,57]. In diabetic-related atherosclerosis, glutathione peroxidase-1 (GPx1) is a key antioxidant enzyme [58]. The IkBα degradation was prolonged in the primary aortic endothelial cells (PAECs) isolated from GPx1-KO mice, and the activation of NF-κB signaling promoted the development of atherosclerosis [58].
In the model of HUVECs damage induced by palmitic acid (PA), Honokiol reduced iNOS expression and NO production by inhibiting IκB phosphorylation and NF-κB activation in IKK/IκB/NF-κB pathway, thereby reduced the PA-induced inflammatory response and repaired endothelial dysfunction [59].
The IKK-IκBα-independent-NF-κB-iNOS-NO pathway
The combination of lipopolysaccharide (LPS) with interferon (IFN)- γ [55] and TNF-α [54] induced VSMCs to produce inflammatory responses that promoted the progression of atherosclerosis. The pathway involved in above reaction is the IKK-IκBα-independent-NF- κB-iNOS-NO pathway.
The treatment with 2,2,5,7,8-pentamethyl-6-hydroxychromane (PMC) [55] suppressed the action of LPS and IFN-γ in VSMCs. PMC significantly inhibited the translocation and phosphorylation of p65, protein phosphatase 2A(PP2A) inactivation and ROS formation, and decreased the expression of iNOS, but it had no effect on IκBα, IKK. PP2A-selective inhibitor okadaic acid and PP2A siRNA transfection significantly reversed the role of PMC on inhibiting iNOS expression, NF-κB-promoter activity and p65 phosphorylation. Immunoprecipitation analysis of LPS/IFN-γ-stimulated VSMCs extracts showed that p65 colocalized with PP2A, and PP2A induced p65 phosphorylation. It indicated that LPS/IFN-γ stimulated activation of ROS-PP2A-NF-κB p65-iNOS-NO pathway to promote atherosclerosis [55].
Andrographolide [54] inhibited the action of TNF-α in VSMCs. Andrographolide reduced the TNF-α-induced phosphorylation of JNK, AKT and p65, thereby inhibited iNOS from producing NO. But Andrographolide affected neither IκBα degradation nor p38MAPK or extracellular signal-regulated kinase (ERK) 1/2 phosphorylation. The activity of NF-κB in the VSMCs inhibited by Andrographolide was mediated by JNK-AKT-p65 pathway [54].
The JAK2/STAT3-iNOS-NO pathway and atherosclerosis
The STAT3 activation in endothelial cell and immune cell may exacerbate inflammation in the advanced stage of atherosclerosis [26]. Excessive or prolonged production of inflammatory mediators can lead to atherosclerosis. The productions of iNOS, NO, cyclooxygenase-2 (COX-2) were increased in the LPS stimulated RAW264.7 macrophages, and the medication inhibited nuclear translocation of signal transducer and STAT3 activation, reduced LPS-mediated phosphorylation of Janus kinase (JAK) 2 and STAT3 at serine 727 and tyrosine 705 sites, thereby reduced LPS-mediated inflammatory responses of macrophage [60].
The MAPK (ERK1/2, JNK1/2 and p38) iNOS-NO pathway and atherosclerosis
Inhibiting the production of NO could reduce TNF-α-induced inflammation of human aortic smooth muscle cells (HASMC) through inhibiting p38 MAPK / ERK signaling [61].
In LPS-induced rat aortic smooth muscle cell inflammatory model, Yang et al. [64] found that pretreatment with non-cytotoxic concentrations of Toona sinensis (TS) and gallic acid (GA) (anti-atherosclerosis effect) inhibited inflammatory NO production by down regulating iNOS expression, and further investigation showed that the inhibition of iNOS/NO signaling was associated with the decreased phosphorylation of MAPK (ERK1/2, JNK1/2 and p38). Yu et al. [62] found that Resokaempferol (RES) (anti-inflammatory effect) reduced NO, iNOS production in LPS-induced RAW264.7 macrophages and inhibited LPS-induced activation of JNK/p38 MAPK signaling. The study from Sharma et al. [60] confirmed the enhancement of TNF-α- induced phosphorylation of P38, ERK and JNK in PAECs isolated from GPx1 KO mice. It is concluded that the MAPK signaling associated with inflammatory response promotes the development of atherosclerosis.
The Nrf2/HO-1/iNOS/NO pathway and atherosclerosis
Some studies have shown that Ang II-treated human endothelial cells [56] and HASMCs [57] had the increased oxidative stress. Enhancing the production of superoxide dismutase (SOD) and the expression of HO-1 through Nrf2 signaling decreased ROS production induced by Ang II, thereby inhibited the ROS-NF-κB-iNOS-NO pathway and relieved Ang II-induced vascular endothelial dysfunction [56,57]. The effective Nrf2/anti-oxidative response element (ARE) activation inhibits LPS-induced NF-kB nuclear translocation and DNA binding activity, and further prevents iNOS expression and NO production in liver [65]. Nrf2-KO mice showed the significantly elevated iNOS expression [66]. To sum up, Nrf2 signaling reduces the expression of iNOS and the excessive production of NO to relieve the development of atherosclerosis.
The nNOS/NO Signaling and Atherosclerosis
The ERK1 / 2-nNOS-NO pathway and atherosclerosis
NO synthesis by vascular nNOS can regulate vascular tone and have the effect of anti-atherosclerosis. Guan et al. [49] divided 24 male Wistar Kyoto rats into two treatment groups (n=12): vehicle and Tongxinluo (TXL) (400 mg·kg·d). After 2 weeks of treatment, adventitia injury was induced by placing the silicone collar around the left carotid artery for 2 weeks, which leaded to chronic vasoconstriction and vascular hypersensitivity to serotonin, and the above reaction was attenuated by TXL treatment. TXL improved carotid blood flow and normalized the vascular hypersensitivity to serotonin. The expression of nNOS and ERK 1/2 phosphorylation was increased in both collared carotid and VSMCs with the TXL treament, and the above effect was abolished by ERK kinase inhibitor PD98059, indicating that TXL increased the expression of nNOS mediated by ERK 1/2 phosphorylation (Table 1) [64].
The NO-sGC-cGMP Pathway and Atherosclerosis
NO regulates the degree of VSMCs contraction by stimulating soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP). In addition, NO also mediates the augmentation of coronary artery contraction in hypoxia state, which depends on sGC but is independent of cGMP production [2]. Sun et al. [31] have demonstrated that ethanol extract Rumex acetosa L. (ERA)- induced vasorelaxation was removed by ODQ (as GC inhibitor) in the isolated phenylephrine pre-contracted rat thoracic aorta, and concluded that the vasodilatation medicated by NO was regulated by the muscle NO-sGC-cGMP pathway [31]. In addition, ROS promotes the development of atherosclerosis by oxidizing NO receptor sGC to impair NO function [65]. GUCY1A3 was identified as a risk gene for coronary artery disease and myocardial infarction. It promotes the formation of atherosclerosis by promoting the transformation of SMCs phenotype from the contracted state to the synthetic state through encoding the α1 subtype of sGC. Preventing this conversion by inhibiting sGC activity may provide a new therapeutic goal for atherosclerosis [67].
Summary
The appropriate amount of NO synthesized by eNOS and nNOS plays the anti-atherosclerosis role, but the excessive amount of NO synthesized by iNOS promotes the occurrence and development of atherosclerosis. Under the pathological conditions, such as lipid infiltration, oxidative stress and inflammatory reaction, the activity of eNOS and nNOS decreased, whereas the activity of iNOS increased, which involved a series of signaling pathways to promote the formation of atherosclerosis, and among them, the main signaling pathways are the PI3K/AKT/eNOS/NO pathway, the Ca(2+)/ CaMKII or CaMKK/ AMPK/ AKT/ eNOS/ NO pathway, the Nrf2/HO-1/ eNOS/NO pathway, and the IKK/IκBα/NF-κB/iNOS/NO pathway. The enhancers or inhibitors of signaling molecules in the above NO pathway and avoiding harmful stimulation on the NO pathways will contribute to the treatment of atherosclerosis. However, the mechanism of NO signaling pathways associated with atherosclerosis is not yet fully elucidated. Further research is helpful to better understand and interfere atherosclerosis with NO signaling pathway related anti-atherosclerosis drugs.
Acknowledgements
This work was supported by National Natural Science Foundation of China (№ 81660751, 81660151, 81260504); Key Research and Development Program of Jiangxi Province of China (No 20161BBG70067and Jiangxi Provincial Natural Science Foundation of China (No 20171BAB205085).
References
- Zheng TP, Yang F, Gao Y, Baskota A, Chen T, et al. (2014) Increased plasma DPP4 activities predict new-onset atherosclerosis in association with its proinflammatory effects in Chinese over a four year period: A prospective study. Atherosclerosis 235: 619-624.
- Zhao Y, Vanhoutte PM, Leung SW (2015) Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci 129: 83-94.
- Khan R, Spagnoli V, Tardif JC, L'Allier PL (2015) Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 240: 497-509.
- Le BM, Caligiuri G, Nicoletti A (2015) Once Upon a Time: The Adaptive Immune Response in Atherosclerosis-a Fairy Tale No More. Mol Med 21: S13-S18.
- Pircher A, Treps L, Bodrug N, Carmeliet P (2016) Endothelial cell metabolism: A novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis 253: 247-257.
- Zheng XX, Zhou T, Wang XA, Tong XH, Ding JW (2015) Histone deacetylases and atherosclerosis. Atherosclerosis 240: 355-366.
- Winkel LC, Hoogendoorn A, Xing R, Wentzel JJ, Van der Heiden K, et al. (2015) Animal models of surgically manipulated flow velocities to study shear stress-induced atherosclerosis, Atherosclerosis 241: 100-110.
- Sobenin IA, Sazonova MA, Postnov AY, Bobryshev YV, Orekhov AN (2013) Changes of mitochondria in atherosclerosis: possible determinant in the pathogenesis of the disease, Atherosclerosis 227: 283-288.
- Fu R, Zhang Y, Guo Y, Zhang Y, Xu Y, et al. (2014) Digital gene expression analysis of the pathogenesis and therapeutic mechanisms of ligustrazine and puerarin in rat atherosclerosis, Gene 552: 75-80.
- Kunes P, Mandak J, Holubcova Z, Kolackova M, Krejsek J (2014) Actual position of interleukin(IL)-33 in atherosclerosis and heart failure: Great Expectations or En attendant Godot? Perfusion 30: 339-436.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, et al. (2017) Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine 377: 1119.
- Hai Z, Zuo W (2016) Aberrant DNA methylation in the pathogenesis of atherosclerosis. Clin Chim Acta 456: 69-74.
- Sobenin IA, Zhelankin AV, Mitrofanov KY, Sinyov VV, Sazonova MA, et al. (2015) Mutations of mitochondrial DNA in atherosclerosis and atherosclerosis-related diseases. Current Pharmaceutical Design 21: 1158-1163.
- Xue Y, Wei Z, Ding H, Wang Q, Zhou Z, et al. (2015) MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis 241: 671-681.
- Roe ND, Ren J (2012) Nitric oxide synthase uncoupling: a therapeutic target in cardiovascular diseases. Vascul Pharmacol 57: 168-172.
- Herranz B, Marquez S, Guijarro B, Aracil E, Aicart-Ramos C, et al. (2012) Integrin-Linked Kinase Regulates Vasomotor Function by Preventing Endothelial Nitric Oxide Synthase Uncoupling: role in Atherosclerosis. Circ Res 110: 439-449.
- Sukhovershin RA, Yepuri G, Ghebremariam YT (2015) Endothelium-Derived Nitric Oxide as an Antiatherogenic Mechanism: Implications for Therapy. Methodist Debakey Cardiovasc J 11: 166-171.
- Saini V, Bhatnagar MK, Bhattacharjee J (2012) Endothelial nitric oxide synthase Glu298Asp (G894T) gene polymorphism in coronary artery disease patients with type 2 diabetes mellitus. Diabetes Metab Syndr 6: 106-109.
- Costa ED, Rezende BA, Cortes SF, Lemos VS (2016) Neuronal Nitric Oxide Synthase in Vascular Physiology and Diseases. Front Physiol 7: 206.
- Li H, Horke S, Förstermann U (2014) Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208-219.
- Zhao JF, Shyue SK, Lin SJ, Wei J, Lee TS (2014) Excess nitric oxide impairs LXR(α)-ABCA1-dependent cholesterol efflux in macrophage foam cells. J Cell Physiol 229: 117.
- Cai X, LI X, Li L, Huang XZ, Liu YS, et al. (2015) Adiponectin reduces carotid atherosclerotic plaque formation in ApoE−/− mice: Roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol Med Rep 11: 1715-1721.
- Zou MH (2007) Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins Other Lipid Mediat 82: 119-127.
- Daiber A, Daub S, Bachschmid M, Schildknecht S, Oelze M, et al. (2013) Protein Tyrosine Nitration and Thiol Oxidation by Peroxynitrite-Strategies to Prevent These Oxidative Modifications. Int J Mol Sci 14: 7542-7570.
- Reventun P, Alique M, Cuadrado I, Márquez S, Toro R, et al. (2017) iNOS-Derived Nitric Oxide Induces Integrin-Linked Kinase Endocytic Lysosome-Mediated Degradation in the Vascular Endothelium. Arterioscler Thromb Vasc Biol 37: 1272-1281.
- Qin M, Luo Y, Meng XB, Wang M, Wang HW, et al. (2015) Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol 70: 23-34.
- Ahsan, A, Han, G, Pan, J, Liu S, Padhiar AA, et al. (2015) Phosphocreatine protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Apoptosis 20: 1563-1576.
- Yao Y, Wang Y, Zhang Y, Liu C (2017) Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis 16: 77.
- Li T, Li D, Xu H, Zhang H, Tang D, et al. (2016) Wen-Xin Decoction ameliorates vascular endothelium dysfunction via the PI3K/AKT/eNOS pathway in experimental atherosclerosis in rats. BMC Complement Altern Med 16: 27.
- Xing SS, Yang XY, Zheng T, Li WJ, Wu D, et al. (2015) Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascul Pharmacol 72: 189-189.
- Sun YY, Su XH, Jin JY, Zhou ZQ, Sun SS, et al. (2015) Rumex acetosa L. induces vasorelaxation in rat aorta via activation of PI3-kinase/Akt- AND Ca(2+)-eNOS-NO signaling in endothelial cells. J Physiol Pharmacol 66: 907.
- Hwang HJ, Jung TW, Hong HC, Choi HY, Seo JA, et al. (2013) Progranulin Protects Vascular Endothelium against Atherosclerotic Inflammatory Reaction via Akt/eNOS and Nuclear Factor-κB Pathways. Plos One 8: e76679.
- Dou J, Li H, Ma X, Zhang M, Fang Q, et al. (2014) Osteocalcin attenuates high fat diet-induced impairment of endothelium-dependent relaxation through Akt/eNOS-dependent pathway, Cardiovasc Diabetol 13: 1-12.
- Gong X, Shao L, Fu YM, Zou Y (2015) Effects of Olmesartan on Endothelial Progenitor Cell Mobilization and Function in Carotid Atherosclerosis. Med Sci Monit 21: 1189-1193.
- Lu J, Xiang G, Liu M, Mei W, Xiang L, et al. (2015) Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 243: 438-448.
- Liu M, Xiang G, Lu J, Xiang L, Dong J, et al. (2014) TRAIL protects against endothelium injury in diabetes via Akt-eNOS signaling. Atherosclerosis 237: 718-724.
- Sun N, Wang H, Wang L (2015) Vaspin alleviates dysfunction of endothelial progenitor cells induced by high glucose via PI3K/Akt/eNOS pathway. Int J Clin Exp Pathol 8: 482-489.
- Liu, S, Sun, Z, Chu P, Li H, Ahsan A, et al. (2017) EGCG protects against homocysteine-induced human umbilical vein endothelial cells apoptosis by modulating mitochondrial-dependent apoptotic signaling and PI3K/Akt/eNOS signaling pathways. Apoptosis 22: 672-680.
- Yamagata K, Tanaka N, Matsufuji H, Chino M (2012) β-carotene reverses the IL-1β-mediated reduction in paraoxonase-1 expression via induction of the CaMKKII pathway in human endothelial cells. Microvasc Res 84: 297-305.
- Sun WJ, Choi CY, Hwang YP, Kim HG, Kim SJ, et al. (2016) Betulinic Acid Increases eNOS Phosphorylation and NO Synthesis via the Calcium-Signaling Pathway. J Agric Food Chem 64: 785-791.
- Â Liu WJ, Wang T, Wang B, Liu XT, He XW, et al. (2015) CYP2C8-derived epoxyeicosatrienoic acids decrease oxidative stress-induced endothelial apoptosis in development of atherosclerosis: Role of Nrf2 activation. Journal of Huazhong University of Science and Technology [Medical Sciences]
- Jeong SO, Son Y, Lee JH, Cheong YK, Park SH, et al. (2015) Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep 12: 937-944.
- Hwang SM, Lee YJ, Yoon JJ, Lee SM, Kim JS, et al. (2012) Prunella vulgaris Suppresses HG-Induced Vascular Inflammation via Nrf2/HO-1/eNOS Activation. Int J Mol Sci 13: 1258-1268.
- Hung CH, Chan SH, Chu PM, Tsai KL (2015) Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol Nutr Food Res 59: 1905-1917.
- Â Ou HC, Hsieh YL, Yang NC, Tsai KL, Chen KL, et al. (2013) Ginkgo biloba extract attenuates oxLDL-induced endothelial dysfunction via an AMPK-dependent mechanism. J Appl Physiol 114: 274-285.
- Jung CH, Lee WJ, Hwang JY, Lee MJ, Seol SM, et al. (2012) Vaspin increases nitric oxide bioavailability through the reduction of asymmetric dimethylarginine in vascular endothelial cells. Plos One 7: e52346.
- Shin W, Yoon J, Oh GT, Ryoo S (2013) Korean red ginseng inhibits arginase and contributes to endotheliumdependent vasorelaxation through endothelial nitric oxide synthase coupling. J Ginseng Res 37: 64-73.
- Pandey D, Bhunia A, Oh YJ, Chang F, Bergman Y, et al. (2014) OxLDL triggers retrograde translocation of arginase2 in aortic endothelial cells via ROCK and mitochondrial processing peptidase. Circ Res 115: 450-459
- Guan Q, Liu M, Liu R, Zhang H, Pang X, et al. (2015) Tongxinluo Induces nNOS Expression Through ERK Activation: Possible Contribution to the Effects of Tongxinluo to Attenuate Vasoconstriction. J Cardiovasc Pharmacol 66: 9-15.
- Wang LY, Zhang JH, Yu J, Yang J, Deng MY, et al. (2015) Reduction of Store-Operated Ca(2+) Entry Correlates with Endothelial Progenitor Cell Dysfunction in Atherosclerotic Mice. Stem Cells Dev 24: 1582-1590.
- Li H, Förstermann U (2013) Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol 13: 161-167.
- Steppan J, Nyhan D, Berkowitz DE (2013) Development of Novel Arginase Inhibitors for Therapy of Endothelial Dysfunction. Front Immunol 4: 278.
- Yan Z, Ming XF (2013) Arginase: The Emerging Therapeutic Target for Vascular Oxidative Stress and Inflammation. Front Immunol 4: 149.
- Wang YL, Sun GY, Zhang Y, He JJ, Zheng S, et al. (2016) Tormentic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-κB signaling pathway. Mol Med Rep 14: 3559-3564.
- Cao Q, Jiang Y, Shi J, Xu C, Liu X, et al. (2015) Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-α in vascular smooth muscle cells through nuclear factor kappa B pathway. J Surg Res 194: 667.
- Chen YY, Hsu MJ, Hsieh CY, Lee LW, Chen ZC, et al. (2014) Andrographolide inhibits nuclear factor-κB activation through JNK-Akt-p65 signaling cascade in tumor necrosis factor-α-stimulated vascular smooth muscle cells. Scientific World Journal 2014: 130381.
- Hsieh CY, Hsiao G, Hsu MJ, Wang YH, Sheu JR, et al. (2014) PMC, a potent hydrophilic α-tocopherol derivative, inhibits NF-κB activation via PP2A but not IκBα-dependent signals in vascular smooth muscle cells. J Cell Mol Med 18: 1278-1289.
- Sivasinprasasn S, Pantan R, Thummayot S, Tocharus J, Suksamrarn A, et al. (2016) Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells. Chem Biol Interact 260: 67-74.
- Pantan R, Tocharus J, Suksamrarn A, Tocharus C (2016) Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angiotensin II-induced inflammation in vascular smooth muscle cells. Exp Cell Res 342: 104-112.
- Sharma A, Yuen D, Huet O, Pickering R, Stefanovic N, et al. (2016) Lack of glutathione peroxidase-1 facilitates a pro-inflammatory and activated vascular endothelium. Vascul Pharmacol 79: 32-42.
- Qiu L, Xu R, Wang S, Li S, Sheng H, et al. (2015) Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IκB/NF-κB, in atherosclerotic cell model. Exp Mol Med 47: e171.
- Yu Q, Zeng K, Ma X, Song F, Jiang Y, et al. (2016) Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int Immunopharmacol 38: 104-114.
- Park SH, Koo HJ, Sung YY, Kim HK (2013) The protective effect of Prunella vulgaris ethanol extract against vascular inflammation in TNF-α-stimulated human aortic smooth muscle cells. BMB Rep 46: 352-357.
- Yang HL, Huang PJ, Liu YR, Senthil KJ, Hsu LS, et al. (2014) Toona sinensis inhibits LPS-induced inflammation and migration in vascular smooth muscle cells via suppression of reactive oxygen species and NF-κB signaling pathway. Oxidative Medicine & Cellular Longevity.
- Aboonabi A, Singh I (2015) Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2–ARE as an indicator and modulator of redox. Biomed Pharmacother 72: 30-36.
- Ritchie RH, Drummond GR, Sobey CG, De Silva TM, Kemp-Harper BK, et al. (2017) The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol Res 116: 57-69.
- Segura-Puimedon M, Mergia E, Al-Hasani J, Aherrahrou R, Stoelting S, et al. (2016) Proatherosclerotic Effect of the α1-Subunit of Soluble Guanylyl Cyclase by Promoting Smooth Muscle Phenotypic Switching. Am J Pathol 186: 2220-2231.
Citation: Wang XF, Ye ZX, Chen JY, He SJ, Chang J, et al. (2018) Roles of Nitricoxide Signaling Pathway in Atherosclerosis. Atheroscler Open Access 3: 120.
Copyright: © 2018 Wang XF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 7478
- [From(publication date): 0-2018 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 6404
- PDF downloads: 1074