Significance of Expression of Cancer Stem Cell Markers CD133
Received: 12-Oct-2018 / Accepted Date: 30-Oct-2018 / Published Date: 09-Nov-2018 DOI: 10.4172/2476-2024.1000146
Abstract
Pancreatic adenocarcinoma of ductal origin (PDAC) is considered one of the most aggressive malignancies and also one of the most chief causes of deaths related to cancer. It has a very poor prognosis and high recurrence rate. We investigated the expression of CSC markers (CD133 and nestin) in different grades of pancreatic intraepithelial neoplasms (PanINs) and PDAC and correlate the expression levels of these markers with clinicopathological data with the aid of Ki67 expression. Our findings showed that both cancer stem markers (CSC) are related to the PanINto- PDAC sequence. Both markers may contribute in proliferation index, tumor differentiation and infiltration. So they may also be useful for developing new therapeutic modalities for PDAC.
Keywords: Cancer stem cell markers; Pancreatic adenocarcinoma; Pancreatic intraepithelial neoplasms; Nestin; CD133
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive tumor types and one of the main leading causes of cancer related death. It has only 5% five-year survival rate [1]. Most of the cases usually diagnosed at a very advanced stage. This poor prognosis may be related to late discovery and unsuccessful treatment strategies. This is why it is obligatory to explore its biological behavior trying to early diagnose and get better prediction for the tumor [2].
Recent studies have shown that a few cells acquire stem cell-like characters in various cancers, and such cells have been called cancer stem cells (CSC). CSCs have similar features including pluripotency and the ability to self-renew. CSCs comprise a small number of cancer cells and possess high tumorigenic potential in vivo . In the cancer mass, two types of cells are present: CSCs and proliferative cells, which are differentiated from CSCs and have a short lifetime. CSCs remain in the G0 phase of the cell cycle and are less responsive to radiation and chemotherapy than proliferating cells. The resistance of CSCs to radiotherapy is a native feature, caused by differential activation of the DNA damage checkpoint response, limited initiation of reactive oxygen species, or activation of the Notch-1 pathway. The acquisition of migratory properties is an essential requirement for CSCs to become metastatic, and the term migrating cancer stem cells has been proposed [3]. Several researchers recently paid attention to the therapeutic approach targeting CSCs as a new strategy for cancer treatment.
Classifications of cells with malignant potential have been isolated from many tumors depending on the expression of definite surface markers. Evidence supports the cancer stem cell theory, according to that CSCs may be accountable for tumor initiation, metastasis, recurrence and resistance to treatment [2].
Irrespective of the debate about the origin in PDAC, increasing evidence suggests that cells with CSC features entirely drive pancreatic tumorigenesis in humans [4].
Multiple evidence have shown that CSC in PDAC can be detected by specific markers, including CD24, CD44, CD133, CXCR4, epithelial cell adhesion molecule (EpCAM; epithelial-specific antigen), nestin and combinations of these markers [5-8]. However, definitive CSC markers for PDAC and their clinical relevance are still controversial.
CSC exhibit resistance to standard cytotoxic drugs due to many causes as it may remain in a quiescent state. Also, the group of CD133+CXCR4+ CSCs is related to tumor metastasis in pancreatic cancer. Therefore, the isolation and characterization of CSC from PDAC suggested this type of cells is to be blamed for the failure of classical therapy in pancreatic tumors [4].
There is substantial evidence that PDAC does not arise de novo , rather progresses through multistep model involving non-invasive precursor lesions known as pancreatic intraepithelial neoplasias (PanINs), and culminating in invasive cancer [9]. However, the relationship between CSCs and PanIN lesions remains unclear.
CD133 is one of the most frequently used among stem cell markers. CD133 has been engaged in different tumors. In addition, its value in different cancer types has been widely studied. CD133 is a transmembrane glycoprotein and is basically expressed in a subset of stem cells in the hematopoietic system as well as in tumours of brain, prostate, colon, and the genitourinary system [4]. The expression of CD133 has been studied in PDAC, however, the relationship between its expression and prognosis is still controversial.
Nestin was primarily described as a marker for neural stem cell. Nestin, an exocrine progenitor cell markers in pancreas, has significant roles in invasion, and metastasis of PDAC cells. It is recently incorporated in pancreatic intraepithelial neoplasias (PanINs), which are putative precursors of PDAC [7]. However, the clinical significance of nestin expression and relationship to other stem cell markers such as CD133 in PDAS is also debatable and is in need to more clarification.
In this study, we analyzed the expression of CSC markers (CD133 and nestin) in PanINs and PDAC and correlate the expression of these markers with clinicopathological data with the aid of Ki67 expression.
Materials and Methods
Patients and tissues selection
Tissues from 100 patients with pancreatic tumors were obtained from pathology department, Tanta University between 2010 and 2018. No one received preoperative chemotherapy or radiotherapy. The patients were 60 males and 40 females, with a median age of 68 years (range=55-87 years). Tumor grade and stages were determined according to the WHO classification and the TNM staging system of the Union for International Cancer Control (UICC) [10]. The normal tissues (N=10) were taken from patients who were operated for ectopic spleen. Patients gave informed consent for the use of the pancreatic tissues.
Immunohistochemistry study and the used antibodies
Paraffin-embedded sections (4 μm) were subjected to immunohistochemistry (IHC). After deparaffinization, antigen retrieval was done (except for nestin) at 120°C for 15 min in sodium citrate buffer solution (pH 6.0). Endogenous peroxidase activity was blocked by incubation for 30 min with 0.3% hydrogen peroxide in methanol. Sections were then incubated with each antibody in phosphate-buffered saline (PBS) overnight at 4°C. Antibodies were detected using diaminobenzidine tetrahydrochloride as the substrate. The sections were then counterstained with Mayer’s hematoxylin. Negative control sections were prepared by omitting the primary antibody.
The following markers were used; mouse monoclonal anti-nestin antibody (from R&D Systems Inc. Westerville, OH, USA, 1:750); rabbit polyclonal anti-CD133 (from Abcam plc. Cambridge, UK, 1:300); mouse monoclonal anti-Ki 67 antibody (from Dako Corp. Santa Barbara, CA, USA).
Evaluation of nestin and CD133 expression
Reactions in the capillary endothelial cell and peripheral nerve for nestin were used as internal positive controls. Because of the morphological heterogeneity of the tumor, semi-quantitative summation method was used.
The intensity of CD133 staining was scored on a scale of 0 to 3, in which 0=negative staining, 1=weakly positive, 2=moderately positive, and 3=strongly positive staining.
The staining intensity of the internal control was considered as moderately positive staining. The extent of staining was scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%) according to the percentages of the positivity in relation to the total carcinoma area. The sum of the intensity together with the extent score was used as the final staining score (0-7) for each antibodies. Tumors having a mean final staining score >3 by each antibody summation were considered high expression while staining score ˂3 were considered low expression [4].
Nuclear staining of Ki-67 (proliferation index) was quantified as percentage of positive nuclear staining of all tumor cell nuclei in five visual fields and semi quantitatively given the scores of +, ++ and +++ if the positivity is (0-25%, 25-50% and more than 50% respectively) [11].
Statistical analysis
The data between different groups were compared using the Student’s t-test. Chi-square test and Fisher’s exact test were used to analyze the correlation between marker expression and clinicopathological features. Data between multiple groups were compared using one-way ANOVA, P<0.05 was considered significant. SPSS version 16 software (version 16.0 for Windows; SPSS INC., Chicago, IL) was used.
Results
Clinicopathological data of the studied cases:
The study included 10 normal pancreatic tissue obtained from gastrectomy specimens. Besides 30 PIN cases which were graded into PIN1, 2 and 3 and 70 PDAC cases which were classified into conventional type, adenosquamous carcinoma and anaplastic carcinoma (Table 1). Most of the tumors were in the head of pancreas (64%) and mostly of moderately differentiated (53%) or poorly differentiated (44.3%). The stage was variable with most of the tumours shown perineural (60%), lymphovascular invasion (63%) and liver metastasis (73%). The details of the site, size, grade, stages, perineural, lymphovascular and liver metastasis were shown in (Table 2).
| Studied cases | |
| Normal control cases (n=10) | |
| Pan IN cases (n=30) | PIN-1 (n=8) |
| PIN-2 (n=10) | |
| PIN-3 (n=12) | |
| Carcinoma cases (n=70) | Conventional adenocarcinoma (n=45) |
| Adenosquamous carcinoma (n=8) | |
| Anaplastic carcinoma (n=17) | |
Table 1: Histopathological classification of the studied cases.
| Carcinoma cases | Site | Size | Grade | Stage | Spread | ||||||||||
| Head | Body | Tail | ≤ 2cm | ˃2cm | well | moderate | poorly | I | II | III | IV | Perineural | Lymphovascular | liver | |
| Conventionl adenocarcinoma (n=45) | 30 | 8 | 7 | 28 | 17 | 2 | 35 | 8 | 16 | 17 | 3 | 9 | 27 | 25 | 9 |
| Adenosqumous carcinoma(n=8) | 5 | 2 | 1 | 3 | 5 | 0 | 2 | 6 | 1 | 6 | 1 | 0 | 5 | 7 | 0 |
| Anaplastic carcinoma(n=17) | 10 | 4 | 3 | 6 | 11 | 0 | 0 | 17 | 0 | 2 | 12 | 3 | 10 | 12 | 3 |
Table 2: Clinicopathological data of the studied cases.
Immunohistochemistry of nestin in normal, PanINs and PDAC
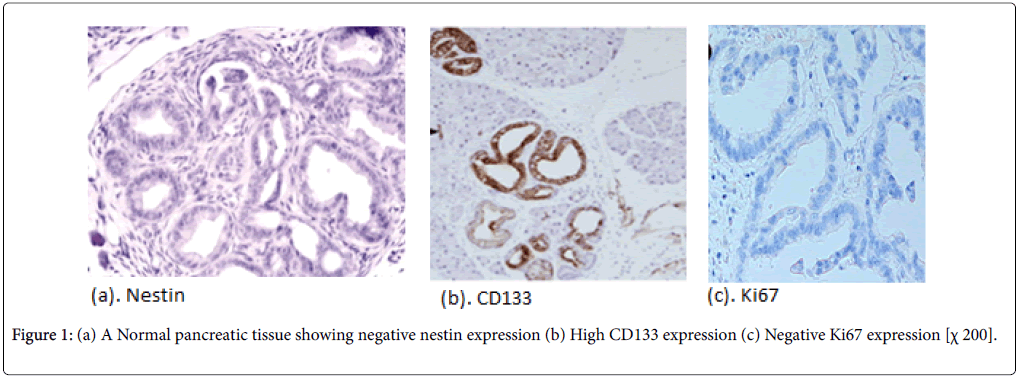
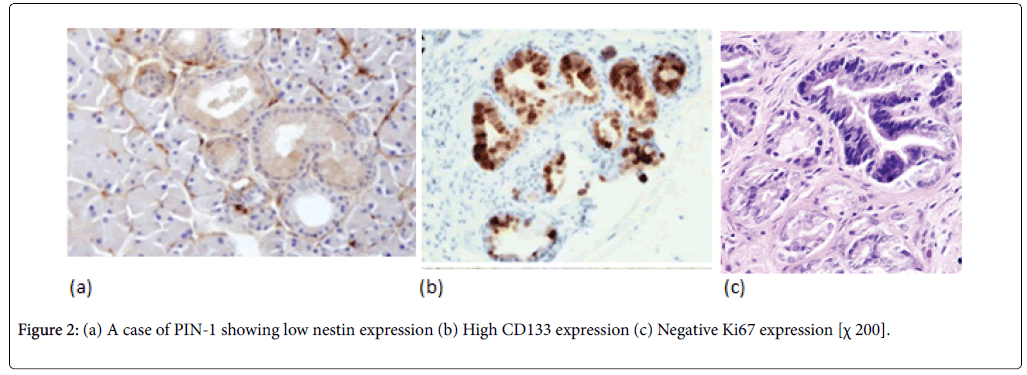
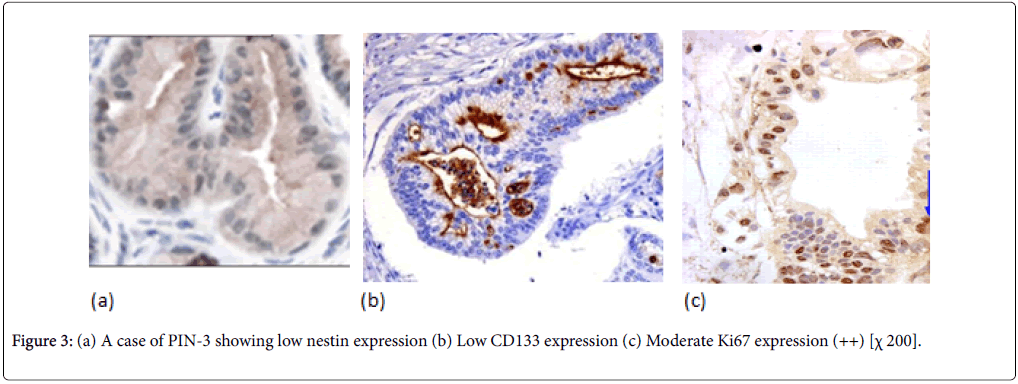
Normal pancreatic tissues were negatively stained for nestin, Nestin expression was recognized in capillary endothelial cells within the islets of the pancreas, while acinar and ductal, cell expressions were not noted (Figure 1). The expression score of nestin showed tendencies to increase with the increase in the grade of PINs. Almost all the PIN-3 lesions showed low positive expressions of nestin in ductal cells (Figures 2 and 3) (Table 3).
| Studied cases | Nestin expression | CD133 expression | Ki expression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | - | + | ++ | +++ | ||
| Normal control cases (n=10) | 10 | 0 | 0 | 10 | 10 | 0 | 0 | 0 | |
| Pan IN cases (n=30) | PIN-1 (n=8) | 8 | 0 | 0 | 8 | 2 | 6 | 0 | 0 |
| PIN-2 (n=10) | 10 | 0 | 4 | 6 | 0 | 2 | 8 | 0 | |
| PIN-3 (n=12) | 11 | 1 | 10 | 2 | 0 | 0 | 12 | 2 | |
| Carcinoma cases (n=70) | Conventionl adenocarcinoma (n=45) | 12 | 33 | 20 | 25 | 0 | 0 | 24 | 21 |
| Adenosqumous carcinoma (n=8) | 2 | 6 | 2 | 6 | 0 | 0 | 5 | 3 | |
| Anaplastic carcinoma (n=17) | 0 | 17 | 17 | 0 | 0 | 0 | 0 | 17 | |
Table 3: Immunohistochemistry of nestin, CD133 and Ki67 in normal, PanINs and PDAC.
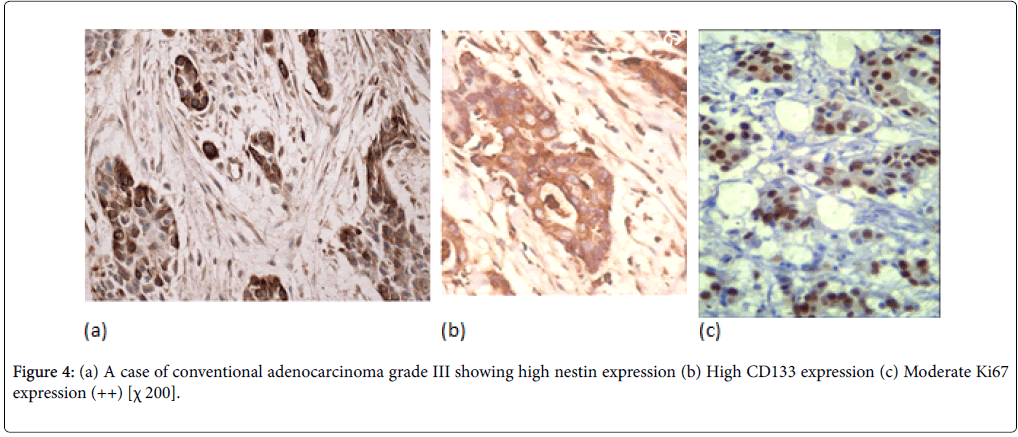
PDAC showed expressions of nestin in cytoplasm. In PDAC, nestin positivity was seen in all cases of PDAC with variable intensity (Figures 4 and 5). PDAC showed significantly higher positivity score for nestin compared to normal, PIN tissues.
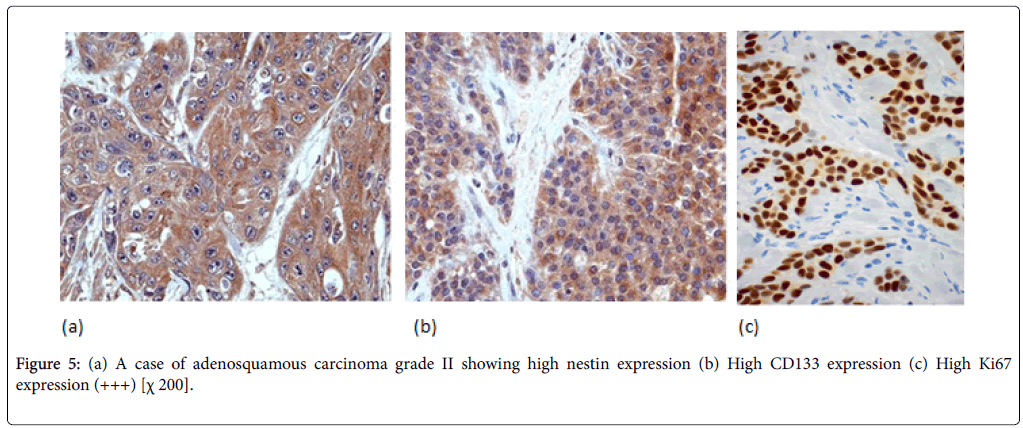
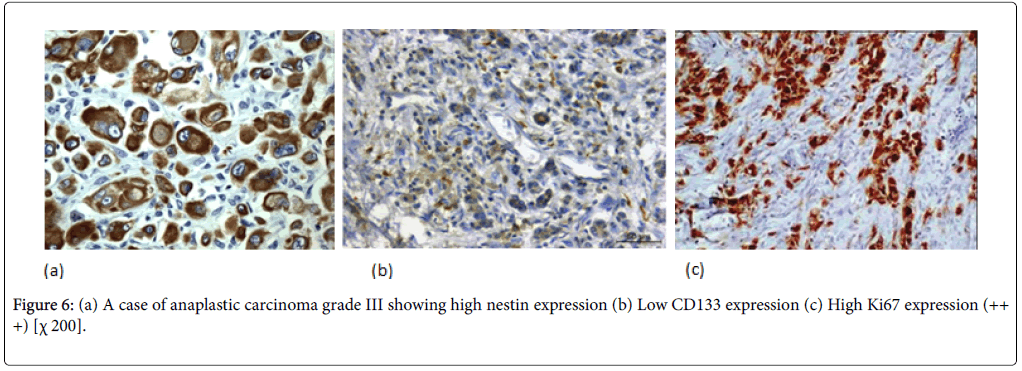
Nestin expression was seen to be high in 80% of the PDAC cases. Also Nestin expression was identified in the nerve bundle with tumor invasion and in the peritumoral small capillary vessels. Interestingly, this stromal nestin positivity had large bulky immunoreactive foci together with the tumor glands positivity. This peritumoral/stromal nestin expression was seen in 54% of the PDAC cases. In anaplastic carcinoma, nestin was expressed in all the cases and predominantly expressed in the anaplastic component (Figure 6).
Immunohistochemistry of CD133 in normal, PanINs and PDA
Different patterns of CD133expression in normal pancreas, PanINs and in PDAC have been recognized, ranging from cytoplasmic staining in up to 19% of the tumor cells to a prominent apical membrane staining of cells within ductal structures with only occasional cytoplasmic expression.
In normal pancreatic tissue, CD133 is expressed in the centroacinar epithelium and terminal ductal cells (Figure 1b). The expression levels of CD133 showed tendencies to decrease according to the malignancy grade of PINs (Figure 2b). Almost all PIN-3 lesions showed low expression of CD133 in ductal cells and occasionally completely negative (Figure 3b). PDAC showed expressions of CD133 in cell membranes of cancer cells. CD133 showed highest positivity in normal epithelial duct if compared with PDAC and PIN cases.
In PDAC cases, 31 cases (44%) showed high CD133 expression. We observed two distinctive CD133 staining patterns. In only 10 cases, CD133 showed apical membranous staining pattern in the glandular epithelial cells and this was observed in well and moderate differentiated cases. However, all the remaining positive cases especially cases of poorly differentiated PDAC showed apparent perinuclear/cytoplasmic positivity (Figure 4b). Moreover, in poorly differentiated PDAC, cytoplasmic globules expressing CD133 were observed with mucus had been retained in the cytoplasm. In adenosquamous carcinoma, CD133 was expressed principally in the adenocarcinoma component (Figure 5b). Anaplastic carcinoma without ductal arrangement completely lacked cytoplasmic CD133 positivity (Figure 6b).
Immunohistochemistry of Ki67 in normal, PanINs and PDAC
To grade the PanIN lesions and the carcinoma cases selected in the present study, we used the proliferative index together with histopathological picture trying to correlate it with the other parameters. We analyzed Ki 67 expression which was seen to be increased along with the PanIN grades, and PDAC showed significantly higher proliferative activity than normal and PanIN tissues (Figures 1c, 2c, 3c, 4c, 5c and 6c).
Correlation study between the studied markers and the clinicopathological features in PDAC
Both Nestin and CD133 expression were correlated positively with the large sized tumours, high grade, advanced stage, perneural, lymphovascular invasion and liver metastasis with statistically significant results but it wasn't related to the site of the tumor (Table 4).
| Carcinoma cases | Site | Size | Grade | Stage | Spread | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head (n=45) | Body (n=14) | Tail (n=11) | ≤ 2cm (n=37) | ˃2cm (n=33) | Well (n=2) | Moderate (37) | Poorly (n=31) | I (n=17) | II (n=25) | III (16) | IV (n=12) | Perineural (n=42) | Lymphovascular (n=44) | Liver (n=12) | ||
| Nestin | Low (n=14) | 6 | 7 | 1 | 11 | 3 | 2 | 12 | 0 | 7 | 7 | 0 | 0 | 3 | 4 | 0 |
| High (n=56) | 39 | 7 | 10 | 26 | 30 | 0 | 25 | 31 | 10 | 18 | 16 | 12 | 39 | 40 | 12 | |
| P value | 0.4 | 0.03 | 0.009 | 0.01 | 0.02 | 0.01 | 0.001 | |||||||||
| CD133 | Low (n=39) | 25 | 4 | 10 | 12 | 27 | 0 | 14 | 25 | 2 | 19 | 13 | 5 | 5 | 9 | 0 |
| High (n=31) | 20 | 10 | 1 | 25 | 6 | 2 | 23 | 6 | 15 | 6 | 3 | 7 | 37 | 35 | 12 | |
| P value | 0.24 | 0.02 | 0.05 | 0.004 | 0.02 | 0.01 | 0.001 | |||||||||
Table 4: Correlation study between the studied markers and the clinicopathological features in PDAC.
Correlation study between nestin and CD133 in the studied cases
Correlation study between the both markers showed that both markers are statistically significantly correlated inversely to each other’s in both studied PIN cases and the carcinoma cases and also through the normal tissue. Beside the correlation between both markers in both groups of PIN and carcinoma showed that the relation was statistically significant also (Table 5).
| CD133 expressions | Nestin expression | P value | ||||
| Studied cases | Low | High | ||||
| Normal control | Low | 0 | 10 | 0.001 | ||
| High | 10 | 0 | ||||
| PIN | PIN -1 | Low | 0 | 8 | 0.02 | |
| High | 8 | 0 | ||||
| PIN-2 | Low | 4 | 0 | |||
| High | 6 | 0 | ||||
| PIN-3 | Low | 10 | 0 | |||
| High | 1 | 1 | ||||
| P value | 0.008 | |||||
| Carcinoma | Conventional | Low | 12 | 8 | 0.007 | |
| High | 0 | 25 | ||||
| adenosquamous | Low | 2 | 0 | |||
| High | 0 | 6 | ||||
| Anaplastic | Low | 0 | 17 | |||
| High | 0 | 0 | ||||
| P value | 0.03 | |||||
Table 5: Correlation study between the two studied markers in the studied cases.
Discussion
Many data that support the hypothesis that pancreatic ductal adenocarcinoma does not arise de novo , but rather progresses through a multistep model composed of noninvasive precursor lesions known as pancreatic intraepithelial neoplasias (PanINs), culminating in invasive cancer.
Various pathologic factors, including tumor size, resection margin status, lymph node status, and histologic grade, affect the outcomes of patients with PDAC who undergo resection [12]. Despite adjuvant treatment most patients who undergo curative resection for PDAC eventually develop recurrence within 2 years. The prognosis for patients with recurrent PDAC is extremely poor and resection for recurrent disease in general does not prolong survival [11]. Recognition of pancreatic CSCs is important for developing new lines of therapy and elucidating the putative origin cell of PDAC [7].
Nestin plays a role in cytoskeleton linking and coordination of cell dynamics. The protein is phosphorylated by cdc2 kinase and/or cyclin dependent kinase and modulates mitosis. The expression of nestin has been reported to be correlated to poor prognosis in several tumors. According to Shoko et al. nestin expression increased with progression through the PanIN-to-PDAC sequence. It showed significantly higher expression starting from PanIN-1 [7]. They results also indicate that nestin is expressed at different stages of PDAC, and that its expression is linked to the proliferative index of PanIN and PDAC.
Recently, in previous study, it was found that nestin plays a significant role in pancreatic cancer cell migration, invasion and metastasis by selectively affecting the expression of actin and cell adhesion molecules [4]. They proved that high nestin expression groups had a positive correlation with histological types and the stage. This was similar to the present study.
The role of nestin in pancreatic epithelial neoplasms may be used as a potential target for a targeted therapy correlated with tumor metastasis, nerve invasion, and one of the CSCs markers in some tumor types [13].
Hermann et al. demonstrated that CD133 CSCs are accountable for the metastatic properties of PDAC [11]. These cells were mainly found at the invasive front. Maeda et al. observed cytoplasmic CD133 staining in up to 15% of tumor cells, while Welsch et al. described that the prominent expression pattern of CD133 was an apical membrane staining [14,15]. According to Thilo et al. cytoplasmic CD133 staining was much rarer and the prominent expression pattern of CD133 was an apical membrane staining towards the lumen of inflammatory (reactive), non-neoplastic cells in ductal arrangement, which was absent in tumor cells. These inflammatorily altered ductal structures are typically found in the vicinity of PDAC cells, and sometimes it is difficult to distinguish them from tumor cells [16].
The apical CD133 enrichment at ductal structures has been described by other groups in the normal pancreas, but also in PDAC cells that lacked apical CD133 positivity in the Thilo et al. study [16]. The expression of CD133 along the apical and intraluminal margins was observed in well and moderately differentiated tumors with a varying degree. This finding was similar with previous reports, as they found that CD133 has a role in regulating the formation of lumina and ducts [4]. Immervoll et al. described that cytoplasmic CD133 was seen in less than 1% of the malignant cells. This result could be adopted with the localization CD133 was influencing cell polarity, forming lumina, and growth pattern of tumor cells [17]. However, in this study, we observed 10 cases with apical membranous staining pattern in well and moderate differentiated tumours. However, all the remaining positive cases especially cases of poorly differentiated PDAC mainly showed apparent perinuclear/cytoplasmic positivity. The divergent staining pattern theoretically can result from varying technical methods (e.g., antigen retrieval and detection methods) used in the different studies, or from different antibodies against the CD133 molecule. Also it has been argued that CD133 probably is not an exclusive CSC marker in PDAC [18]. Further researches should include mRNA expression pattern together with protein expression to clarify the implication of CD133 in PDAC.
CD133 expression was closely linked to tumor grades, size, and node metastasis. Previous works also have proved that CD133 was closely related with lymph node metastasis [2]. In Hou, et al. study, the 5-year survival rate for CD133-positive patients was lower than that for CD133-negative patients. CD133 expression levels were significantly higher in tumor cells than in nontumor cells [19]. The results of our study support the hypothesis that the function of the node metastasis might be reliant on CD133. In addition, we found this marker to be inversely related to tumor differentiation, in line with studies reporting both the presence of CD133 in different types of stem cells, and its down-regulated expression in differentiated cells.
Some of the CSC markers were related to vascular invasion or histological grade; CD133 was positively correlated with vascular invasion. These data indicate that these markers may be crucial for metastasis and differentiation of PDACs, as previously reported [20]. Our study clearly showed the same results.
In the current study, correlation between nestin and CD133 expression showed that CD133 decreased with progression through the PanIN-to-PDAC sequence and this was opposite to nestin. These results indicate that each CSC marker is expressed at different stages of PDAC carcinogenesis, and that the expressions of CSC markers may relate to the proliferative activity of PanIN and PDAC. These data indicate that these markers may be vital for metastasis or differentiation of PDACs.
In conclusion, our findings show that CSC marker expressions are associated with carcinogenesis via the PanIN-to-PDAC sequence. Furthermore, CSC markers may contribute to proliferation index, differentiation, invasiveness, and even histological typing of PDAC. Consequently, analysis of expression level and localization of CSC markers in each step of PDAC progression may prove useful for developing new detection and therapeutic modalities for PDAC. However, the use of a single CSCs marker as a predictor of prognosis remains controversial, suggesting that the combination of markers especially nestin and CD133 may be more useful.
Conflict of Interests
The author declares that there is no conflict regarding the publication of this paper.
References
- Liang JJ, Kimchi ET, Staveley-O’Carroll KF, Tan D (2009) Diagnostic and prognostic biomarkers in pancreatic carcinoma. Int J Clin Exp Pathol 2: 1-10.
- Li X, Zhao H, Gu J, Zheng L (2015) Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis. Int J Clin Exp Pathol 8: 12084-12092.
- Matsuda Y, Kure S, Ishiwata T (2013) Nestin and other putative cancer stem cell markers in pancreatic cancer. Med Mol Morphol 45: 59-65.
- Kim HS, Yoo SY, Kim KT, Park JT, Kim HJ, et al. (2012) Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. Int J Clin Exp Pathol 5: 754-761.
- Ikenaga N, Ohuchida K, Mizumoto K, et al. (2010) Characterization of CD24 expression in intraductal papillary mucinous neoplasms and ductal carcinoma of the pancreas. Hum Pathol 41: 1466-1474.
- Lee HJ, You DD, Choi DW (2011) Significance of CD133 as a cancer stem cell markers focusing on the tumorigenicity of pancreatic cancer cell lines. J Korean Surg Soc 81: 263-270.
- Kure S, Matsuda Y, Hagio M, Ueda J, Naito Z, et al. (2012) Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int J Oncol 41: 1314-1324.
- Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17: 313-319.
- Hruban RH, Adsay NV, Albores-Saavedra J (2001) Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 25:579-586.
- Hartwig W, MW Büchler (2014) Pancreatic Cancer: Current Options for Diagnosis, Staging and Therapeutic Management. Gastrointest Tumors 1: 41-52.
- Hermann PC, Huber SL, Herrler T (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Stem Cell 1: 313-323.
- Winter JM, Cameron JL, Campbell KA (2006) 1423 Pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10: 1199-1210.
- Lenz J, Karasek P, Jarkovsky J, Muckova K, Dite P, et al. (2011) Clinicopathological correlations of nestin expression in surgically resectable pancreatic cancer including an analysis of perineural invasion. J Gastrointestin Liver Dis 20: 389-396.
- Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, et al. (2008) CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer 98: 1389-1397.
- Welsch T, Keleg S, Bergmann F, Degrate L, Bauer S, et al. (2009) Comparative analysis of tumorbiology and CD133 positivity in primary and recurrent pancreatic ductal adenocarcinoma. Clin Exp Metastasis 26: 701-711.
- Welsch T, Keleg S, Bergmann F, Degrate L (2009) Comparative analysis of tumorbiology and CD133 positivity in primary and recurrent pancreatic ductal adenocarcinoma. Clin Exp Metastasis 26: 700.
- Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A (2013) Expression of the “stem cell marker†CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer 8: 48.
- Lardon J, Corbeil D, Huttner WB et al. (2008) Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas 36: e1-e6.
- Hou YC, Chao YJ, Tung HL, Wang HC, Shan YS (2014) Coexpression of CD44-Positive/CD133-Positive Cancer Stem Cells and CD204-Positive Tumor-Associated Macrophages Is a Predictor of Survival in Pancreatic Ductal Adenocarcinoma. Cancer 120: 2766-2777.
- Li C, Wu JJ, Hynes M (2011) c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141: 2218-2227.
Citation: Alshenay A (2018) Significance of Expression of Cancer Stem Cell Markers CD133. Diagn Pathol Open 3: 146. DOI: 10.4172/2476-2024.1000146
Copyright: © 2018 Alshenay A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4336
- [From(publication date): 0-2018 - Oct 17, 2025]
- Breakdown by view type
- HTML page views: 3441
- PDF downloads: 895