Structural Analysis and Physiological Properties of Exopolysaccharides Secreted by a Newly Isolated Pseudomonas stutzeri Bl58 under Alkaline Conditions
Received: 17-Sep-2016 / Accepted Date: 24-Oct-2016 / Published Date: 31-Oct-2016 DOI: 10.4172/2155-952X.1000250
Abstract
We have identified an ethanol-assimilating bacterial strain of Pseudomonas stutzeri that produces copious amounts of an exopolysaccharide when cultured in 5% ethanol under the alkaline condition of pH 10. The objective of this study was to evaluate the potential industrial applications of this exopolysaccharide designated BL58 by characterization of its chemical, rheological and physiological properties. The polymer was purified from the 72 h culture broth with periodic feeding of ethanol and the chemical structure of the polymer was determined by NMR spectroscopy, GC-mass spectrometry and alditol-acetate analysis of methylated hydrolyzates. The analytical data showed the presence of glucose, mannose and 3-O-carboxyethyl rhamnose and suggested that the main chain consists primarily of 1,4-linked glucose and mannose units with unique branching of 3-O-[(R)carboxyethyl] rhamnose at the 3 position of the glucose unit, a structure not previously reported. The polysaccharide has an average molecular weight of 1,800 kDa and the rheological behavior of the aqueous solution from high viscosity sol to viscoelastic gel depended on temperature and pH. Although no overt cytotoxicity was observed, the polymer displayed anti-proliferative activity when tested with leukemia, liver carcinoma and normal pulmonary cells, indicating potential pharmaceutical applications.
Keywords: Pseudomonas stutzeri; Exopolysaccharides; 3-O-carboxyethyl rhamnose; Rheological characterization; Acute toxicity; Anti- proliferating activity; Leukemia cells; Liver carcinoma cells
249748Highlights
• The isolated alkaliphilic soil bacterium produced a novel exopolysaccharide from ethanol.
• The main chain of the exopolysaccharide consists primarily of 1,4-linked glucose and mannose units and has branches of 3-O-carboxyethyl rhamnose at the 3 position of the glucose unit.
Introduction
Synthetic polymers, such as plastics and elastics, are important materials in our lives and have contributed to the development of novel industrial technologies in many fields, such as medicine [1-3], food [4,5] and electronics [6,7]. Over time, many functional polymers have been developed for a wide range of purposes, but environmental problems have resulted from the large scale distribution and disposal of synthetic polymers that are not biodegradable [8]. To avoid environmental problems, some biodegradable polyester produced by microorganisms are used in the medical and food industries [9-11]. These polyesters show many unique physical and chemical properties and therefore, are expected to be useful for application in other fields.
Carbohydrate polymers produced by plants, such as starch, cellulose and agar, are also biodegradable polymers [12-14] and many novel derivatives have been developed [15-19]. Xanthan gum, dextrin, alginate, curdlan, scleroglucan gellan and pullulan are biodegradable polymers produced by microorganism [20-23]. In particular, xanthan gum is a representative biodegradable polymer that has been used as a food stabilizer, thickener and gelation agent all over the world [24,25].
We have discovered an ethanol-assimilating bacterium forming a large swollen colony on agar plates containing 5% ethanol as sole carbon source under alkaline conditions at pH 10. The culture broth inoculated with the isolated strain changed from a viscous sol to the viscoelastic gel during aerobic cultivation, indicating that the strain produced exopolysaccharides and secreted it from cells. The present study was conducted to determine the chemical structure of the exopolysaccharides and to characterize some physical and physiological properties in anticipation of possible applications in cosmetics and pharmaceuticals.
Materials and Methods
Isolation of exopolysaccharides-producing bacteria
A bacterial strain, which produces an exopolysaccharide, was isolated from soil samples by enrichment culture using the minimal medium containing 5% ethanol, 0.15% NH4HCO3, 0.15% (NH4)2SO4, 0.15% NH4NO3, 0.15% KH2PO4, 0.07% K2HPO4, 0.03% MgSO4•7H2O, 0.001% CaCl4•2H2O, 0.001% Zn SO4•7H2O, 0.001% FeSO4•7H2O, 0.0001% MnSO4•7H2O and 0.03% yeast extract, pH 10.0. For screening, 5 ml of the above medium in test tubes were used and the cultivation was done at 30°C for 5 to 10 days with shaking. The broths that exhibited viscosity and gelation were selected and bacterial colonies were isolated by a streak culture on the minimal agar plate. Large and swollen colonies were inoculated into the minimal medium and cultivated at 30°C with shaking. After cultivation for 5 days, bacteria producing polysaccharides were selected based on the amount of a total sugar by phenol-sulfate method [26]. The exopolycarbohydrate producing strain BL58 was isolated using these methods, and the identification of the strain as Pseudomonas stutzeri was carried out by the National Collection of Industrial and Marine Bacteria, Ltd, Scotland.
Batch fermentation with jar-fermenter
For the production of the BL58 polymer, the nitrogen source of the minimal medium was exchanged from inorganic nitrogen salts to 0.5% peptone. Inoculum (50 ml) of strain BL58 were grown for 48 h with vigorous shaking at 30°C using the medium supplemented with 3% ethanol as a sole source of carbon. These cultures were inoculated into a 3 l fermenter (New Brunswick Bioflo/Celligen 115, USA) containing 1 l of fresh medium supplemented with periodic addition of 1% ethanol every 24 h. The fermenter temperature was controlled at 30°C, and the flow rate of air was at 1.0 l min-1 with stirring rate of 500 rpm. Sampling was collected at every 24 h and the turbidity of growth (at 610 nm), viscosity, pH and a total sugar of samples were measured.
Purification of polysaccharides
One liter of the 72 h cultured broth from the jar-fermenter was diluted with 5 l of distilled water, and centrifuged at 7,000 rpm for 15 min. For adsorption of metabolites and nutrients in cultured broth, 20 g of activated charcoal was added to the supernatant, and the suspension was stirred gently at room temperature for 3 h. The activated charcoal was removed by centrifugation at 7,000 rmp for 15 min and then 50 g of diatomaceous earth (Hyflo super-cell) was added to the supernatant to adsorb proteins derived from cells. After filtration with filter paper, the odorless and colorless supernatant, containing BL58 polymer, was collected, dialyzed, and concentrated with ultrafiltration (UF) using a 10,000 Da cut-off polysulfone membrane module (microza UF SLP-1053, ASAHI KASEI CHEMICALS Co. Ltd.) against distilled water overnight at 4°C. Finally, the volume of polymer solution was concentrated to 2 l and lyophilized, as purified BL58 polymer.
For the structure analysis, the lyophilized polymer was dissolved in an aliquot of distilled water and applied to a Toyopearl HW-65 column (2 × 91 cm), equilibrated with distilled water and chromatographed. The total sugar of each fraction was analyzed by the phenol-sulfate method. The eluates around the void volume corresponding to a molecular size of more than 1,800 kDa were pooled and lyophilized.
Structural characterization of BL58 polymer
Molecular mass determination: The average molecular mass of the polysaccharides was determined by HPLC on a Shodex Asahipack G3000PW (7.5 mm × 300 mm) GPC column. The Molecular weights of the polysaccharides were calculated using various saccharides as standards; these included polysaccharides blue dextran (average mol. Wt.: 2,000 kDa), dextran high (average mol. Wt.: 170-200 kDa), dextran low (average mo. Wt.: 50-70 kDa), tetrasaccharide maltotetraose, trisaccharide maltotriose, disaccharide maltose, and monosaccharide glucose. The carrier was degassed water, the flow rate at 75ºC was 0.5 ml min-1 and the eluate was measured with a RI detector.
General procedures: Concentration of the polymer was determined by the phenol-sulfate method by using glucose as a standard. Viscosity of the culture medium diluted 1:5 was measured by rotary viscometer (DV-1+, Viscometer, Brook Field Co. Ltd.) at 30ºC and the values were expressed in “cp per 1 rpm for 30 s”.
Hydrolysis of the BL58 polymer: To a solution of the BL58 polymer (1 (w/v)%), the same volume of 4 N trifluoroacetic acid solution was added and hydrolysis was carried out at 100ºC for 4 h. The hydrolysate was lyophilized and dissolved in distilled water. The components of hydrolysates were analyzed qualitatively with TLC silica gel 60 plates (Merk) by using ethyl acetate/acetic acid/water (2/1/1 (v/v/v)) as a developing solvent. The developed products were detected by heating at 105ºC after spraying with 50% sulfuric acid. Fine crystal cellulose TLC plates (Funacel SF) were also used with phenol/ammonia/water (160/1/40 (v/v/v)) as a developing solvent and detection was carried out by heating at 105ºC after spraying with diphenylamine-aniline solution. HPLC was used for the quantitative analysis of hydrolysates on a Shodex KS801 (8 mm × 300 mm) as the separation column and H2O as the eluent (column temperature, 70ºC; flow rate, 0.5 ml min-1; detection, RI).
Alditol-acetate analysis of the hydrolysates of the BL58 polymer: Purified BL58 polymer (3 mg) was hydrolyzed with 2 N trifluoroacetic acid solution for 12 h at 100ºC and the hydrolysate was lyophilized. The hydrolysate was dissolved in 150 μl of water and reduced with 100 mg of sodium borohydride for 12 h at 30ºC. After 12 h, excess sodium borohydride was dischanged with acetic acid, and the reaction mixture was concentrated under reduced pressure. The residues were dissolved in 1 ml of methanol/acetate (20/1 (v/v)), and concentrated under reduced pressure twice. To the residues, 1.5 ml of acetic anhydride was added and incubated at 80ºC for 12 h. The alditol-acetates were extracted with dichloromethane and dried by evaporation. The alditolacetates were redissolved in dichloromethane and analyzed by GC-MS with a Perkin Elmer Q-MASS910 using a DB17 capillary column and helium as the carrier gas. The column was initially heated at 120ºC for 2 min and the temperature was increased by 10ºC min-1 to 190ºC, followed by a further increase by 2ºC min-1 to 210ºC and finally by 10ºC min-1 to 280ºC where it was maintained for 2 min. The injection temperature was 280ºC.
Methylation analysis of BL58 polymer: The purified exopolysaccharides were methylated by the method of Hakomori [27]. The BL58 polymer or its partial hydrolysate (10 mg) was dried under reduced pressure for 24 h. Two mL of DMSO was added to the dried material, and a homogeneous suspension was obtained by ultrasonication (1 min, 5 times) under ice-cooled conditions. To the suspension, methyl sulfinyl carbanion (0.5 ml) was added dropwise under a nitrogen atmosphere and the reaction mixture was stirred for 4 h at room temperature. Methyl iodide (1 ml) was added and the mixture was stirred for another 1.5 h under ice-cooled conditions. After excess methyl iodide was removed by dialysis against deionized water, the reaction mixture was dialyzed against distilled water for 48 h. The dialyzed sample was concentrated to dryness under reduced pressure, and used as the methylated derivative. To the dried residue of the methylated BL58 polymer, 0.3 ml of 90% formic acid was added and the solution was heated at 100ºC. After 12 h, distilled water was added and concentration was repeated under reduced pressure to remove the residual formic acid. To the dried material, 0.2 ml of distilled water and 0.2 ml of 4 N trifuloroacetic acid was added and the methylated polymer was completely hydrolyzed at 100ºC for 7 h. To remove excess trifluoroacetic acid, the reaction mixture was repeatedly concentrated under reduced pressure by the addition of methanol-water to give dried material. The dried material was dissolved in distilled water and one or two drops of 2 N aqueous ammonium solutions was added for neutralization. Sodium borodeuteride (50 mg) was added to the solution and stirred for 15 h at room temperature. To stop the reaction, Amberlite IR120 (H+) was added to the reaction mixture until hydrogen gas generation ceased. The reaction mixture was filtered and the filtrate was evaporated with methanol to dryness. The residue was acetylated with 0.2 ml of acetic anhydride and 0.2 ml of pyridine. The acetylated residue was dissolved in 0.1 ml of chloroform and was applied for GCMS analysis under the same conditions as the alditol-acetate analysis.
NMR spectroscopy: 1H-NMR data for the BL58 polymer was recorded on a JEOL JNM-EPC500 FT-NMR spectrometer in 0.6% D2O solution (w/v) at 60ºC. Spectra were externally referenced to 3-trimethylsilylpropanoate. 1H-NMR, 13C-NMR, H-H COSY, HMQC, HMBC, DEPT and NOESY data for compound X were recorded on the same spectrometer in D2O solution at room temperature. In all cases, chemical shifts are reported in ppm relative to sodium 3-(trimethylsilyl)- 1-propane-sulfonate, which was used as an internal standard.
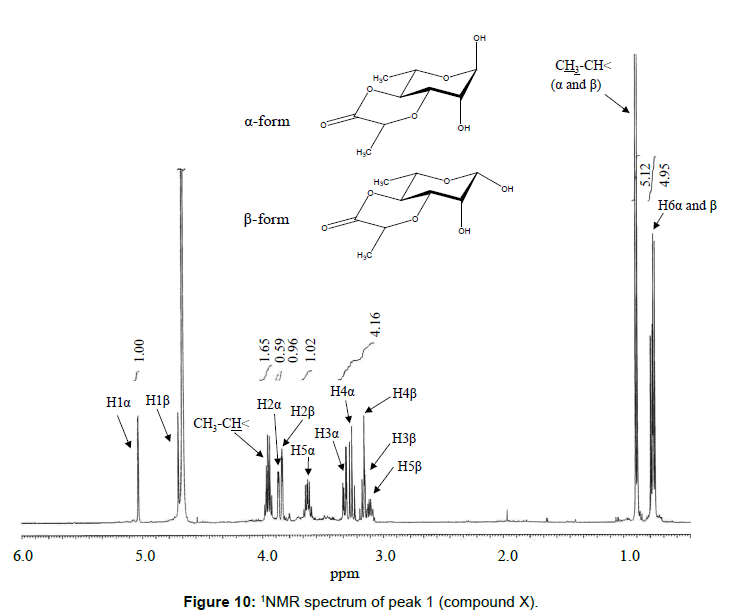
Data for the purified peak 1 in Figure 1, designated as compound X (3-O-[(?)-1-carboxyethyl]-L-rhamnopyranose (intramolecular ester form)): 1H-NMR (D2O, sodium 3-(trimethylsilyl)-1-propanesulfonate): δ 1.26 (α-form, 3H, d, J5,6=6.42, H6), 1.28 (β-form, 3H, d, J5,6=5.96, H6),1.39 (α-form, 3H, d, J CH3-CH<, CH3-CH<=6.87, CH3-CH<), 1.39 (β-form, 3H, d, JCH3-CH<, CH3-CH<=6.87, CH3-CH<), 3.38 (β-form, 1H, dd, J4,5=9.17, J5,6=5.96,H5), 3.42 (β-form, 1H, dd, J2,3=2.75, J3,4=8.76, H3), 3.45 (β-form, 1H, dd, J3,4=8.76, J4,5=9.17, H4), 3.52 (α-form, 1H, dd, J3,4=9.62, J4,5=9.17, H4), 3.58 (α-form, 1H, dd, J2,3=3.21, J3,4=9.62, H3), 3.86 (α-form, 1H, dq, J4,5=9.17, J5,6=6.42, H5), 4.04 (α-form, 1H, dd, J1,2=1.83, J2,3=3.21, H2), 4.07 (β-form, H2, 1H, dd, (broad), J1,2=0.92, J2,3=2.75, H2), 4.13 (α-form, 1H, q, J CH3-CH<, CH3-CH<=6.87, CH3-CH<), 4.15 (β-form, 1H, q, J CH3-CH<, CH3-CH<=6.87, CH3-CH<), 4.82 (β-form, H1, 1H, d, J1,2=0.92, H1), 5.12 (α-form, 1H, d, J1,2=1.83, H1); 13C-NMR (D2O, sodium 3-(trimethylsilyl)-1-propane-sulfonate): δ 19.57 (C6, α-form or β-form), 19.60 (C6, α-form or β-form), 21.49 (CH3-CH< , α-form or β-form), 70.72 (C2, α-form), 71.02 (C2, β-form), 71.14 (C5, α-form), 73.36 (C4, β-form), 73.67 (C4, α-form), 74.70 (C5, β-form), 77.84 (CH3-CH<, α-form or β-form), 78.05 (CH3-CH<, α-form or β-form), 80.81 (C3, α-form), 83.29 (C3, β-form), 96.22 (C1, β-form), 96.64 (C1, α-form), 183.94 (>C=O, α-form or β-form).
CI-GCMS analysis: CI-GCMS analyses were carried out with the JEOL SUM300 using an HP-1 capillary column (30 m × 320 μm; thickness, 0.25 μm) and helium as the carrier gas according to the manufacturer’s instructions. Iso-butane was used as the reagent gas. The column was initially heated at 60ºC for 1 min and the temperature was increased by 10ºC min-1 to 160ºC followed by further increase by 2ºC min-1 to 180ºC and finally by 10ºC min-1 to 230ºC where it was maintained for 4 min. The injection temperature was 200ºC.
Physiological study
Acute toxicity test: Acute toxicity test was performed according to the method as previously described with some modifications [28]. BL58 polymer and xanthan gum (250 mg kg-1, 500 mg kg-1, 1000 mg kg-1 as 1% of aqueous solution) were orally administrated to 4 weeks old SPE male mice. The weight of the mice was 15-17 g. Before the administration was started, mice were normally bred for 4 days. From the evening of the day before administration, mice were completely deprived of food, except for water, for 15 h. Mice were divided into 5 per subgroup. The average weight of the food-deprived mice was adjusted to be 20 g in each subgroup. Mice were fed at 23 ± 3ºC of room temperature, at 55 ± 5% of relative humidity, at 13 cycle h-1 of air-ventilation and at 12 h d-1 of artificial lightning. The feedstuff used was commercially available material sterilized by γ ray irradiation, the Solid Feedstuff MF (Oriental yeast Co. Ltd.). The drinking-water was filtrated by RO membrane and fed freely to the mice. Who were observed for 2 weeks following the administration of test polymers. The weight of mice was measured on days 0, 2, 3, 6, 9 and 13. Mice were sacrificed by cervical dislocation, and megascopic pathological check for internal organs was conducted in detail.
Eye irritancy test: Eye irritation test was performed according to the Draise rabbit eye test [29]. One ml of 1% aqueous solution of BL58 polymer was instilled into the right eyes of three 10 weeks old Japanese white male rabbits. The left eyes were used as controls. Anterior region of the right eye (cornea, iris and conjunctiva) was megascopically and ophtalmoscopically checked at 1, 24, 48 and 72 h after the instillation and compared with those of the left eye used as controls. At 24 h after the instillation, abnormalities of cornea were examined by using the FLUORES Ocular Examination Test Paper and the eye irritancy was evaluated according to the Draize standard.
Primary rabbit skin irritation test: Three Japanese white male rabbit (10 weeks old) were used for rabbit skin irritation test [30]. Fur on neck of the rabbit was removed and cotton pad with 1% of BL58 polymer was applied to the naked skin of the rabbit. The irritancy for rabbit skin was evaluated according to the Draize standard at 24, 48 and 72 h after the application.
Primary human skin irritation test: BL58 polymer was applied to medial side of the arm of 45 of healthy adults (men, 16; women, 29) for 24 h by the Finn chamber test. At 0.5 (24 h assessment) and 24 h (48 h assessment) after the test was finished, skin irritancy was megascopically checked [30]. The symptom was assessed in 6 levels: (-), no reaction; (±), light erythematous; (+), erythematous; (2+), and papula; (3+), erythematous, infiltratous, papula, and emphlysis; (4+), bullosum. In control experiments, methyl paraben was used instead of BL58 polymer.
Skin sensitization test: Male guinea pig (5 pigs/subgroups) was used for the skin sensitization test. Induction of sensitization was carried out by the application of a filter paper immersed emulsion of 2% of DNCB and 0.1% or 0.5% of BL58 polysaccharide to the skin of the guinea pig three times a week. At 2 weeks after final induction, induction of sensitization was further evoked by the application of the emulsion of 2% of DNCB and 0.1% or 0.5% of BL58 polymer to the skin. In the control experiment, saline was used instead of BL58 polymer.
Micronucleus test (Clastogenicity test): ICR male mice (6 weeks old, SPF) were used in for micronucleus test [31]. Mice were divided into subgroups and each contains 5 mice and BL58 polymer (0.25 g, 0.5 g and 1.0 g kg-1 as 1% aqueous solution) were orally administrated once a day. At 24 h after the final administration, mice were killed and myeloid cells were collected from the femur. Smears of myeloid were immobilized with methanol for 3 min and finally stained with Giemsa-stain solution for 35 min. The number of micronucleated polychromatophilic erythrocytes on the smears was examined under microscope. Orthochromatic normoblasts were also examined.
Rec assay: BL58 polymer was autoclaved at 121ºC for 5 min and suspended in DMSO to obtain 250 mg ml-1 of final concentration solution. This suspension was successively diluted to 1.22-5000 mg disk-1 and used for the Bacillus subtilis H17 Rec+ and M45 Rec- assay [32]. Eight mm in diameter of filter-paper disks impregnated 20 ml of BL58 polysaccharide were applied in the middle of the streak on the plate and the incubation was carried out at 37ºC for 24 h. The result was judged as positive when the inhibition zone against H17 Rec+ was 0-1 mm and the difference in zones of H17 Rec+ and M45 Rec– was more than 3 mm.
Antigenicity test: First, male guinea pig (5 pigs per subgroups) was used for the skin sensitization test [33]. Induction of sensitization was carried out by the application of a filter paper immersed in emulsion of 2% of DNCB and 0.1% or 0.5% of BL58 polymer to the skin of the guinea pig three times a week. At 2 weeks after final induction, induction of sensitization was further evoked by the application of the emulsion of 2% of DNCB and 0.1% or 0.5% of BL58 polysaccharide to the skin. In the control experiment, saline was used instead of BL58 polymer. For the antigenicity of BL58, active systemic anaphyla (ASA) response for guinea pig was investigated. Egg albumin was use as a positive control. Both subgroups lowly sensitized by 1 mg/kg and highly sensitized by 5 mg/kg of BL58 polysaccharide were further injected BL58 polymer (1 mg kg-1) for the induction.
Anti-bacterial and anti-virus test: Escherichia coli K12 was incubated in NB culture overnight, and seeded to the freshly prepared culture containing 0-2000 μg ml-1 of BL58 polymer. OD 600 was measured to quantify turbidity after the overnight incubation. Human influenza virus (H1N1, H3N2) was infected to MDCK cells, and lactate dehydrogenase activity in the culture medium was checked [34].
Anti-proliferation test of carcinoma cells: 0.5% of BL58 polymer aqueous solution was autoclaved, and precipitate was removed by centrifugation. The supernatant was used for the repression test of human acute promyelocytic leukemia cells (HL60: JCRB0085 purchased from JCRB Cell Bank), human monocytoid leukemia cells (U-937: JCRB9021), human liver carcinoma cells (HepG2: JCRB1054) and human normal pulmonic cells (MRC5: JCRB9008). Cells (0.5- 1.0 × 104 cells well-1) were added to 100 μl of culture medium. Ten % of BL58 polymer was added to the culture media (RPMI-1640 or DMEM+FBS 10%). Fifty, 5 and 0.5 mg% of the polymer was contained in the medium and cultivated at 37ºC for 72 h and the number of cells were counted by MTT assay measuring tetrazorium dye reduction at Abs 550 nm [35].
Results and Discussion
Isolation and identification of strain BL58 secreting exopolysaccharide
A bacterial strain that produced a novel polysaccharide was isolated employing an enrichment technique under alkaline conditions using 5% ethanol as a sole carbon source. The bacteria that caused the culture broth to gel was isolated and designated as strain BL58. A preliminary analysis of the accumulated metabolites by colorimetric tests for saccharides, such as Molish [36], Phenol-sulfate and Anthrone-sulfate tests [37], all indicated that the accumulated extracellular metabolite was carbohydrate polymer (BL58 polymer).
From the taxonomical studies, strain BL58 was Gram-negative, rod-shaped (0.5 to 0.8 by 1.6 to 1.8 μm) and had polar flagella. It grew well in aerobic conditions. Nitrate reduction and indole production were negative, and catalase, oxidase and cytochrome oxidase were positive. The strain was able to utilize glucose, maltose, gluconate, caprate, malate, citrate and starch as carbon sources. The base sequence of 16S rDNA of strain BL58 exhibited 99.5% homology to that of Pseudomonas stutzeri. From the results, the isolated strain was identified to be P. stutzeri BL58.
Cultural conditions for BL58 polymer production
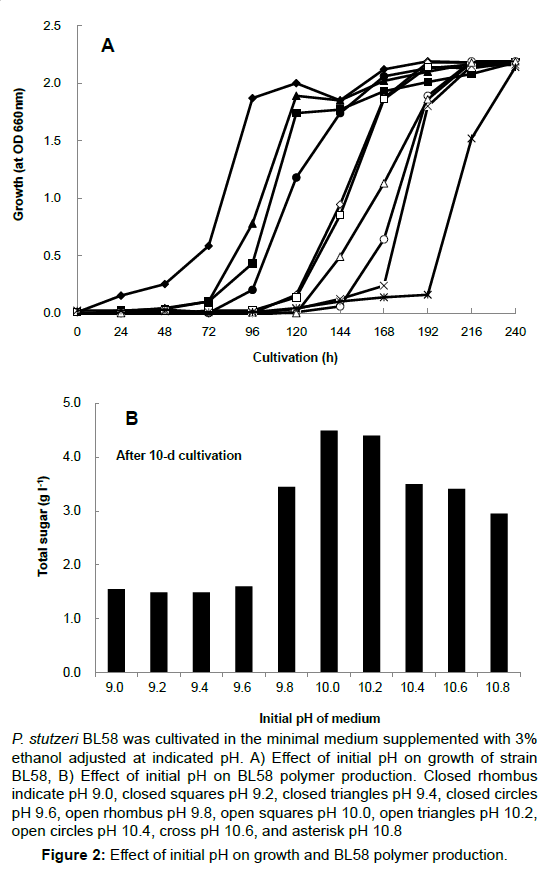
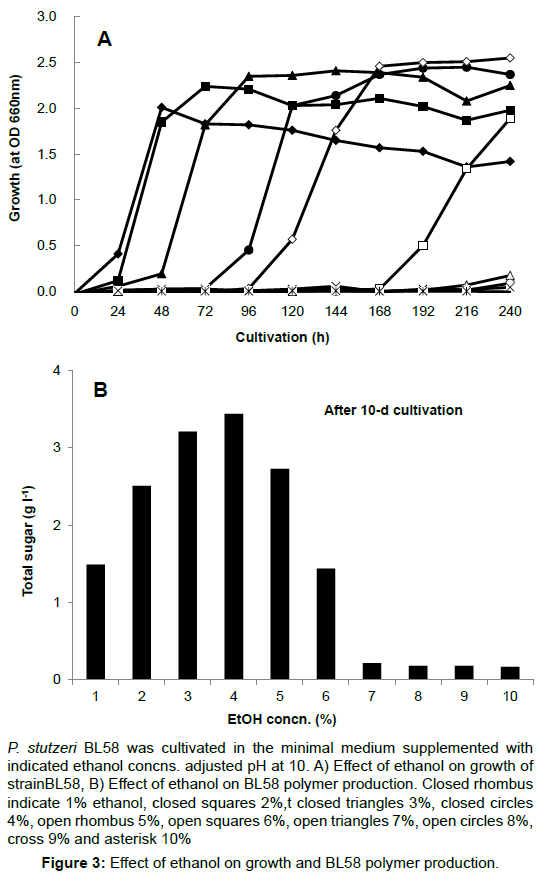
The opious production of exopolysaccharides by P. stutzeri under strong alkaline conditions and using ethanol as the carbon source had not yet been reported. Therefore, the culture conditions for accumulation of exopolysaccharides by strain BL58 was studied with regard to the effect of initial pH and ethanol concentration in the medium. Using minimal medium containing 3% ethanol as sole carbon source, initial pH of the medium was varied from 7 to 11. The strain exhibited growth in the range of pH 8 to 10, and the gelation of culture broth with accumulation of BL58 polymer was observed at pH 10 (data not shown). A more detailed examination of the growth and polymer production was made from 9 to 10.8 (Figure 2). Although growth was delayed with pH rise, BL58 polymer was accumulated at pH above 9.8, optimally at pH10, as measured by total sugar of cultured broth. After 120 h cultivation, the accumulation of BL58 polymer reached 4.5 g l-1 of total sugar at pH10. The effect of ethanol as sole carbon source was examined from 1 to 10% in the medium (Figure 3). Up to 6% ethanol, the turbidity derived from growth reached similar levels (OD 610 nm>2.0) independent of the period of lag phase. During the cultivation at pH 10, gelation of cultured broth was observed for the addition of 3% to 5% ethanol, indicating that BL58 polymer was excreted outside of P. stutzeri BL58 cells at relatively high concentration of ethanol. The highest concentration of polymer production was obtained with 3-4% ethanol in the medium after 120 h cultivation.
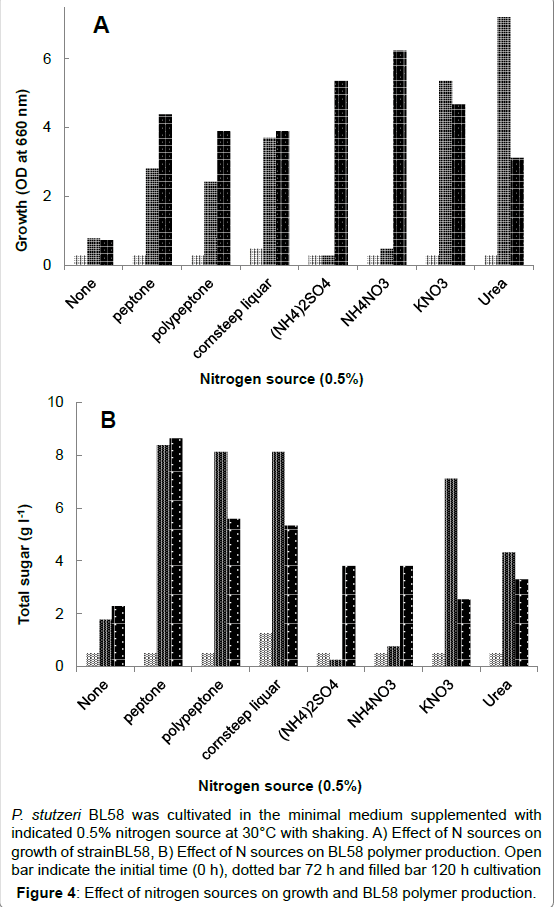
In order to optimize culture conditions for production of BL58 polymer in a shorter cultivation time, we focused on nitrogen source, because ammonium salts were released as ammonia gas from alkaline medium during sterilization by autoclaving. As shown in Figure 4 0.5% organic- and inorganic nitrogen sources was added to the medium containing 3% ethanol as a carbon source. Both growth and BL58 polymer production of the strain were observed in a medium supplemented with organic nitrogen sources, such as peptone, polypeptone and cornsteep liquor. Of the tested nitrogen sources, the strain produced 12.5 g l-1 of BL58 polymer in the medium supplemented with 0.5% peptone, and both growth and polymer accumulation depended on peptone concentration (data not shown).
P. stutzeri BL58 was cultivated in the minimal medium supplemented with 3% ethanol adjusted at indicated pH. A) Effect of initial pH on growth of strain BL58, B) Effect of initial pH on BL58 polymer production. Closed rhombus indicate pH 9.0, closed squares pH 9.2, closed triangles pH 9.4, closed circles pH 9.6, open rhombus pH 9.8, open squares pH 10.0, open triangles pH 10.2, open circles pH 10.4, cross pH 10.6, and asterisk pH 10.8
Figure 2: Effect of initial pH on growth and BL58 polymer production.
Batch fermentation for BL58 polymer
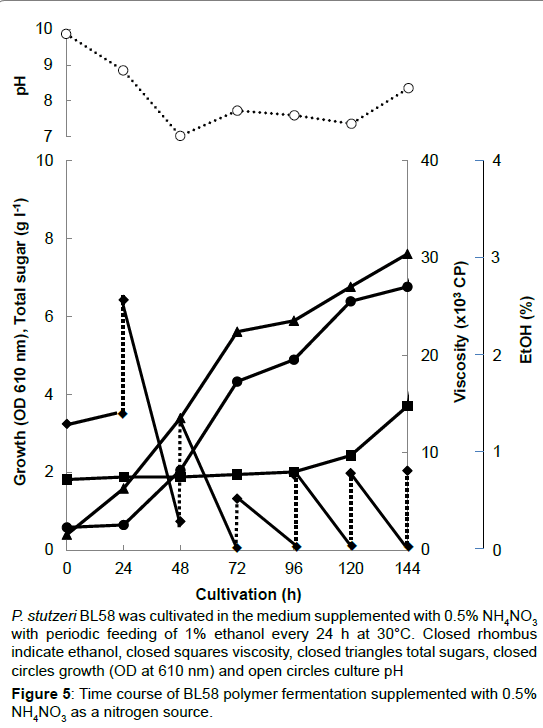
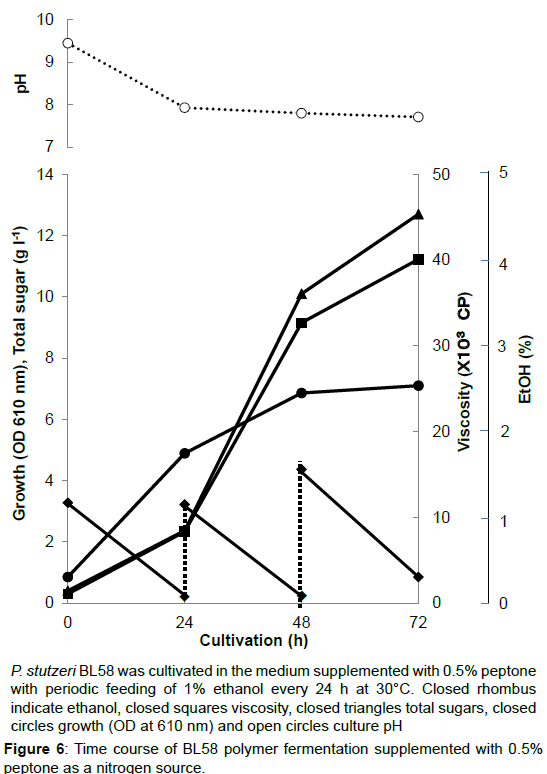
During the shaking cultivation in the test tube, the accumulated BL58 polymer increased the viscosity of culture broth and seemed to limit the aeration for growth of the strain. Therefore, a large scale fermentation of BL58 polymer was evaluated with a jar fermenter capable of aerated mixing. In addition, considering the release of volatile ethanol from culture broth, ethanol as the sole carbon source was added every 24 h to give a final concentration of 1%. As the control, 0.5% NH4NO3 was used as a nitrogen source (Figure 5). After 48 h cultivation, the added ethanol began to be assimilated for growth of the strain and for production of BL58 polymer. Although the amount of added ethanol reached 60 g l-1 at 144 h cultivation, total sugar of the culture broth was still 7 g l-1 representing about 10% recovery from ethanol. Since the growth of the strain was enough to produce BL58 polymer, the remainder of utilized ethanol seemed to be catabolized to other metabolites. In the case when 0.5% peptone was used as a nitrogen source, P. stutzeri BL58 grew rapidly and produced 12.5 gl-1 polymer within 72 h of cultivation (Figure 6). Although the strain seemed to be able to produce more BL58 polymer, further fermentation was difficult because of gelation of the broth by accumulated polymers. We established the most efficient fermentation condition for BL58 polymer as one in which ethanol was added sequentially to the medium supplemented with 0.5% peptone as a nitrogen source.
P. stutzeri BL58 was cultivated in the minimal medium supplemented with indicated ethanol concns. adjusted pH at 10. A) Effect of ethanol on growth of strainBL58, B) Effect of ethanol on BL58 polymer production. Closed rhombus indicate 1% ethanol, closed squares 2%,t closed triangles 3%, closed circles 4%, open rhombus 5%, open squares 6%, open triangles 7%, open circles 8%, cross 9% and asterisk 10%.
Figure 3: Effect of ethanol on growth and BL58 polymer production.
Physical properties of BL58 polymer
Typically, microbial polysaccharides are precipitated with an organic solvent, but BL58 polymer was swollen by the addition of organic solvent of ethanol or acetone. An effective purification method for the exopolysaccharide was developed by combining adsorption with active charcoal, filtration with diatomaceous earth and dialysis with UF membrane described Materials and Methods. Using this method, BL58 polymer was purified from 1 l of the 72 h culture broth of the jar-fermenter, and isolated as lyophilized powder. The purified polymer was white, odorless-powder and the purity (total sugar content) was estimated to be 96.6% by the phenol-sulfate method. The purified polymer had a mean molecular weight of approximately 1,800 kDa and its viscosity and pH of the 1% solution were 160,000-250,000 cp and 6.3, respectively. The number of viable bacteria in the 1% solution was <10 cells ml-1. The decrease degree of the weight of the polymer after desiccation at 105ºC for 4 h was <10%. Concentration of Pb and AS2O3 were <10 μg g-1 and <4 μg g-1, respectively.
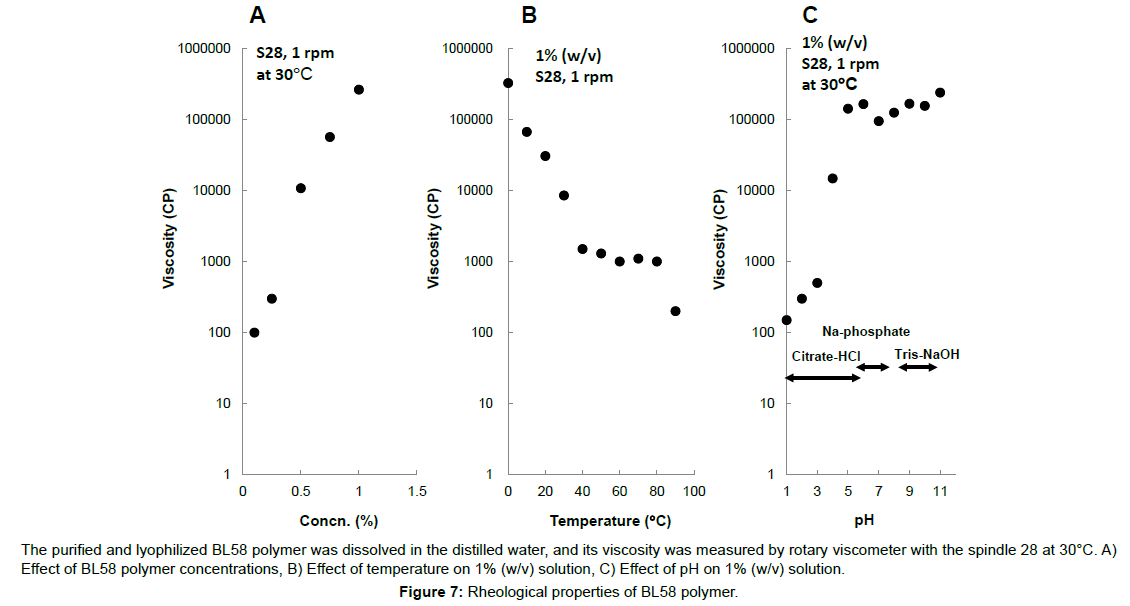
Effect of concentration, temperature and pH on viscosity of the polymer was shown in Figure 7. The viscosity of the polymer solution logarithmically increased above 0.6% (w/v) and the solution formed viscoelastic gel. The viscosity of 1% (w/v) of the polymer solution was estimated to be 280,000 cp and it was 10 times higher value than 1% (w/v) xanthan solution. The viscosity of the polymer decreased in the temperature and reached a constant value (ca. 1000 cp) at 40 to 80ºC. Further decrease was observed over 80ºC. Cooling of the polymer solution resulted in the logarithmical increase of viscosity (especially, under 40ºC) and formation of the viscoelastic gel was observed, suggesting that conformation of the polymer might change by repeated heating-cooling cycle. Formation of viscoelastic gel was observed from pH 5 to 12.
P. stutzeri BL58 was cultivated in the minimal medium supplemented with indicated 0.5% nitrogen source at 30°C with shaking. A) Effect of N sources on growth of strainBL58, B) Effect of N sources on BL58 polymer production. Open bar indicate the initial time (0 h), dotted bar 72 h and filled bar 120 h cultivation
Figure 4:Effect of nitrogen sources on growth and BL58 polymer production.
Structure analysis of BL58 polymer
NMR analysis of the polysaccharide produced by P. stutzeri BL58: To obtain initial information about the polysaccharide, 1H-NMR analysis was carried out and the spectrum was compared to those of the poly- and oligosaccharides produced by P. stutzeri ATCC 17588 and Pseudomonas sp. OX1 (previously known as P. stutzeri OX1) [38- 41], which were previously reported by Osman et al. and Leone et al., respectively [38,39]. Three broad peaks were observed at 5.32, 5.16 and 4.98 ppm, suggesting that these are anomeric protons. The peaks around 3.2 and 4.4 ppm are thought to be protons of the pyranose or furanose rings (data not shown). Osman et al. reported that signals for three anomeric protons were observed at 4.95, 4.75 and 4.45 ppm in the 1H-NMR analysis of the native polysaccharide from P. stutzeri ATCC19588 [38]. These results indicated that the BL58 polymer has a different structure from the already known polymers.
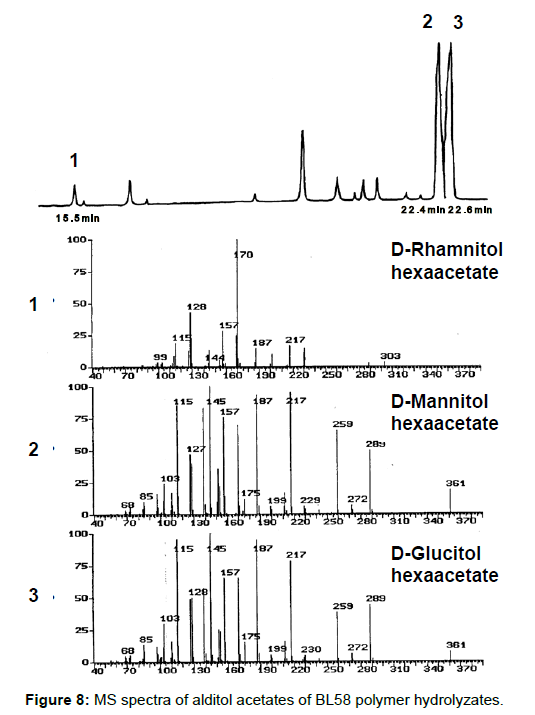
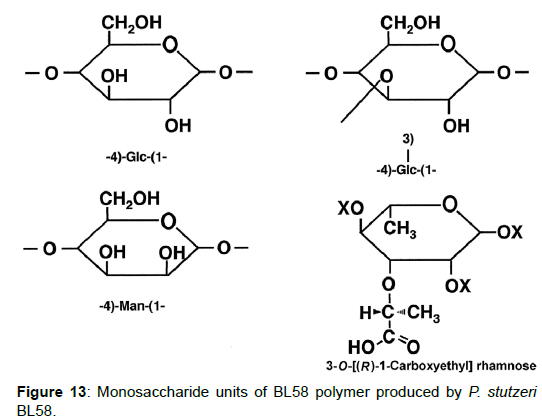
Analysis of the composition and bonding types of the sugars in the BL58 polymer: To investigate the constituent sugars of the BL58 polysaccharide, acid hydrolysis and alditol-acetate analysis were attempted. TLC analysis of the completely hydrolyzed products showed that glucose, mannose and rhamnose were the main components of the novel polysaccharide. Alditol-acetate analysis supported the results of the TLC analysis, and the ratio of these sugars was estimated to be D-rhamnitol hexaacetate (peak 1): D-mannitol hexaacetate (peak 2): D-glucitol hexaacetate (peak 3)=0.07: 0.6: 1.0 (Figure 8). In addition to the peaks of the three monocarbohydrates, an unknown peak was observed in the alditol-acetate analysis.
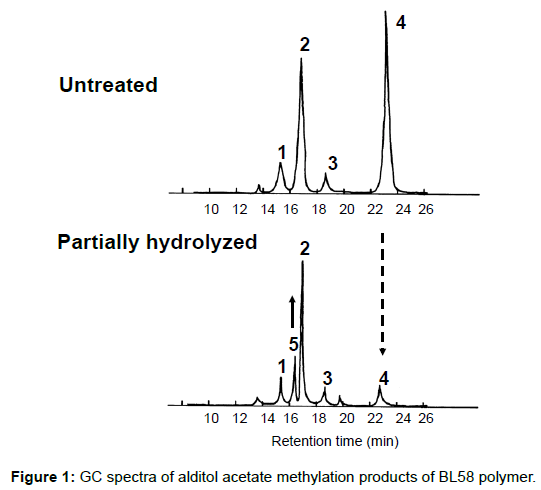
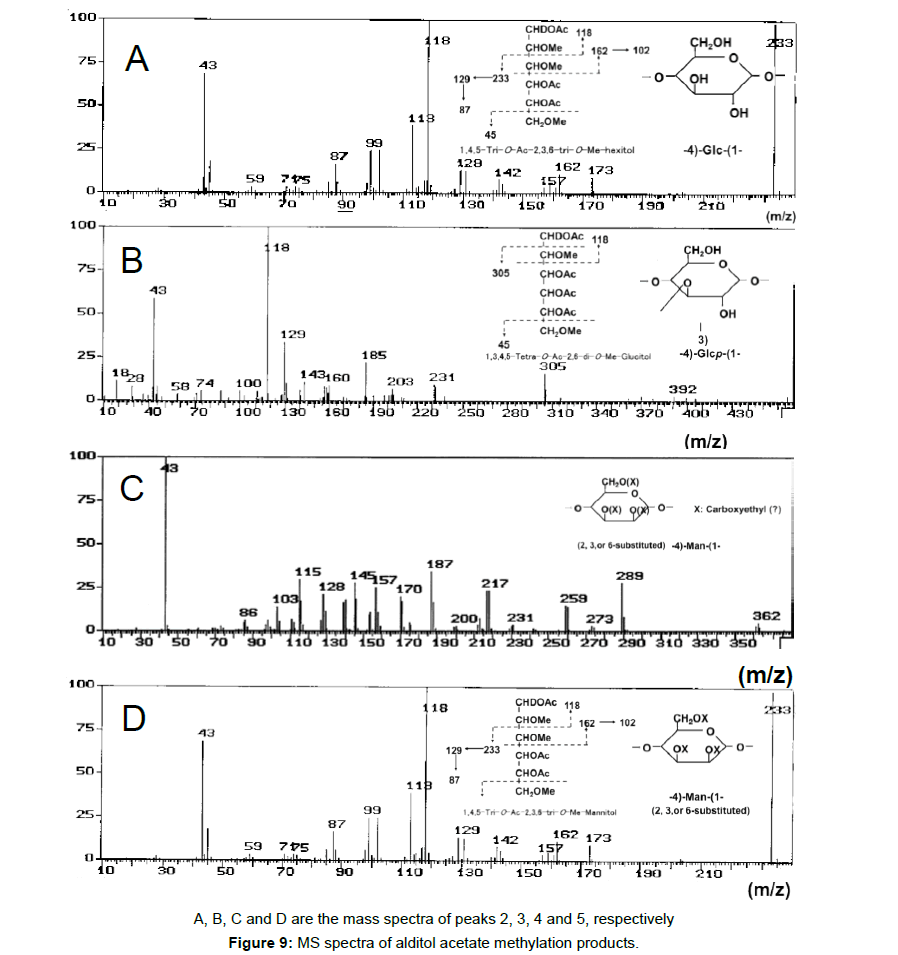
To obtain information about the bonding between these constituent sugars, alditol-acetate analysis of methylated BL58 polymer and its hydrolyzates was attempted by using gas chromatography-mass spectrometry (GC-MS) [42]. The GC-MS spectra of alditol-acetate analysis of methylated non-hydrolyzed (MW: ca.1,800,000) and partially-hydrolyzed (MW: ca. 180,000) BL58 polymers are shown in Figure 1. In both spectra, four peaks (peaks 1, 2, 3 and 4 in Figure 1) were observed at the same retention times. In the spectrum of the partially hydrolyzed products, one new peak (peak 5 in Figure 1) increased remarkably with the decrease of peak 4. Based on the analysis of the mass spectra and comparison of retention times, peaks 2 and 3 were assigned to 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl-glucitol and 1,3,4,5-tetra-O-acetyl-2,6-di-O-methyl-glucitol, respectively (Figures 9A and 9B). Peak 2 was the strongest signal in the spectrum of the partially hydrolyzed BL58 polymer. These results indicate that the polysaccharide has 1,4-linked glucose units as the main chain and has a branched chain or substituted group at the 3 position of glucose.
P. stutzeri BL58 was cultivated in the medium supplemented with 0.5% NH4NO3 with periodic feeding of 1% ethanol every 24 h at 30°C. Closed rhombus indicate ethanol, closed squares viscosity, closed triangles total sugars, closed circles growth (OD at 610 nm) and open circles culture pH.
Figure 5:Time course of BL58 polymer fermentation supplemented with 0.5% NH4NO3 as a nitrogen source.
P. stutzeri BL58 was cultivated in the medium supplemented with 0.5% peptone with periodic feeding of 1% ethanol every 24 h at 30°C. Closed rhombus indicate ethanol, closed squares viscosity, closed triangles total sugars, closed circles growth (OD at 610 nm) and open circles culture pH.
Figure 6:Time course of BL58 polymer fermentation supplemented with 0.5% peptone as a nitrogen source.
The chemical structure of the compound in peak 4 was not assigned from the mass spectrum analysis (Figure 9C). However, the compounds in peaks 4 and 5 are expected to be structurally related, because peak 5 increased with the decrease of peak 4 after acid hydrolysis of the BL58 polymer. The compound in peak 5 was assigned to 1,4,5-tri-Oacetyl- 2,3,6-tri-O-methyl-hexitol (Figure 9D) based on the analysis of the mass spectrum and comparison of retention times. These results suggest that the mannose unit is also one of the constituents of the main chain of the BL58 polymer and branched chains or substituted groups are present at the 2,3,6-positions of the mannose units.
The purified and lyophilized BL58 polymer was dissolved in the distilled water, and its viscosity was measured by rotary viscometer with the spindle 28 at 30°C. A) Effect of BL58 polymer concentrations, B) Effect of temperature on 1% (w/v) solution, C) Effect of pH on 1% (w/v) solution.
Figure 7:Rheological properties of BL58 polymer.
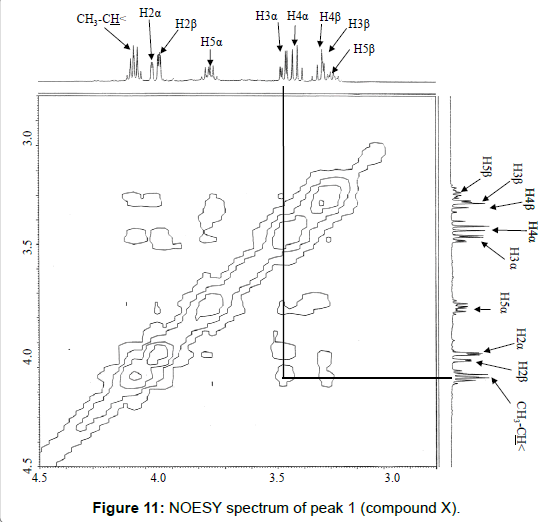
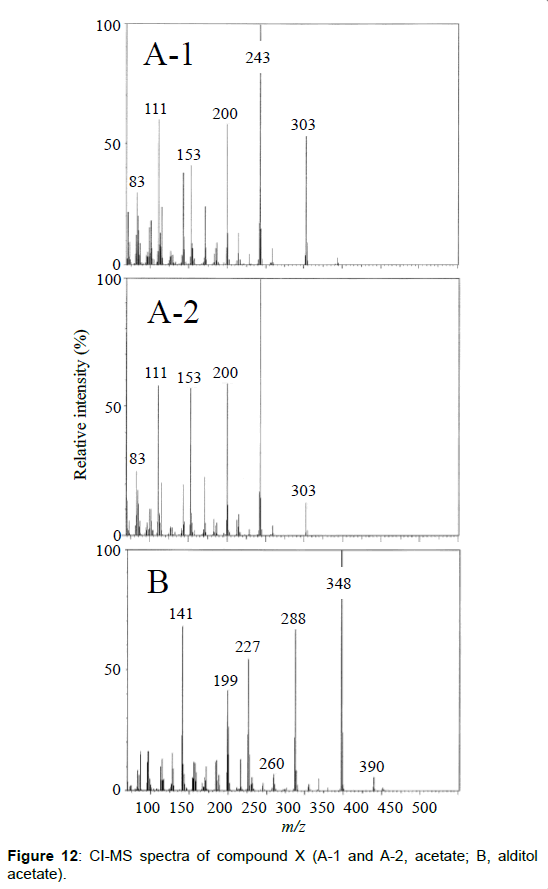
The chemical structure of peak 1 was not also assigned on the basis of the mass spectrum analysis. Therefore, peak 1 (designated as the compound X) was chromatographically isolated from the partial hydrolysate of the BL58 polymer, and compound X was analyzed by 1H- and 13C, 2D-NMR and EI- and CI-MS spectroscopy. To estimate coupling constants of the signals in the 1H-NMR spectrum, a decoupling technique was applied in the analysis. In the 1H-NMR spectrum of compound X (Figure 10), two doublet signals (5.12 and 4.82 ppm) were observed. Coupling constants (1.83 Hz for 5.12 ppm, 0.92 Hz for 4.82 ppm) indicate that signals at 5.12 and 4.82 ppm are assigned to α and β anomeric protons, respectively, at the 1 position of the sugar ring. Based on the information from 2D-NMR (HH-COSY, HMQC and HMBC) spectra (data not shown), the signals at 4.04 (dd), 3.86 (dq), 3.58 (dd), 3.52 (dd) and 1.26 ppm (d) in the 1H-NMR spectrum were assigned to the H2, H5, H3, H4 and H6 protons of the α-form. The doublet signal at 1.39 ppm and quartet at 4.13 ppm were assigned to the three protons of CH3-CH< and one proton of CH3-CH<. Similarly, the signals of the β-form appeared at 1.28 (H6, d), 3.38 (H5, dd), 3.42 (H3, dd), 3.45 (H4, dd), 4.07 (H2, broad dd), 1.39 (CH3-CH<, d) and 4.15 ppm (CH3- CH<, q). The 13C-NMR spectrum also supports the assignment of the 1H-NMR spectrum (data is summarized in the Experimental section). Another important finding from the completely decoupled 13C-NMR and DEPT spectra is the presence of >C=O structure (183.9 ppm). In the NOESY spectrum, correlation between the proton of CH3CH< and H3 was observed (Figure 11), suggesting that the intermolecular ester of compound X is the R form [43]. From the NMR analyses, compound X is strongly suggested to be an intramolecular ester form of the rhamnose derivative, 3-O-[(?)-1-carboxyethyl]-L-rhamnopyranose. To confirm this structure, CI-GCMS analyses of the acetylated form of the compound were carried out by using iso-butane as a reaction regent. In the CI mass spectrum of acetate, two main peaks were observed and these can be tentatively be assigned to be α and β anomers. The [M+1]+ peak at 303 was observed in the MS spectra of both anomer peaks (Figures 12A-1 and 12A-2). Furthermore, CI-GCMS analysis was performed for the acetylated alditol (compound X reduced by NaBD4) (Figure 12B). Significant peaks at m/z 348 ([M+1]+) and at m/z 390 ([M+43]+) were observed. These GCMS data supported the molecular weight (302) of the proposed chemical structure, the intramolecular ester form of 3-O-[(?)-1-carboxyethyl]-L-rhamnopyranose.
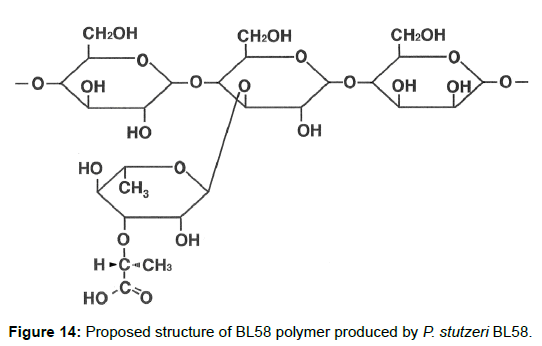
Proposed structure of the BL58 polymer
Taken together, the results indicate that the BL58 polymer is comprised of four types of monosaccharide units: a “-4)-glucose-(1- “unit, a “-4)-mannose-(1-“ unit, a “-4)glucose(1-“ unit with a branch at the 3 position and a 3-O-carboxyethyl rhamnose unit (Figure 13). Most likely, compound X is an intramolecular ester form of 3-O-[(?)-1- carboxyethyl]-L-rhamnopyranose, which is supposed to be a secondary metabolite produced from 3-O-carboxyethyl rhamnose during the hydrolysis of the BL58 polysaccharide. The final proposed structure is shown in Figure 14. Previously, Osman et al. and Maalej et al. reported that a major structural motif of the polysaccharides produced by P. stutezeri ATCC17588 and AS22 was assigned as follows: it is composed of a main chain consisting -4)-β-Glc-(1- and -3,4)-Man-(1-residues, in which a considerable part of the -3,4)-Man-(1-resides is branched at C-4 position with 3-O-carboxyethyl rhamnose and with an acetyl group on O-2 [38,44]. The proposed structure of BL58 polymer differed from the previous P. stutzeri polysaccharides in that 3-O-carboxyethyl rhamnose was branched at 3 position of glucose unit. Because the spectrum data was not assigned completely, further work is needed to determine the carbohydrate structure of highly polymerized BL58 polymer.
Physiological properties of BL58 polymer
Maalej et al. reported that the polysaccharides from P. stutzeri AS22 strains exhibited the physiological functions, such as antioxidant activity and dermal wound healing [45]. In addition, the rheological properties such as a pseudoplastic behavior, high elasticity, good mechanical strength and stability with high-absorption ability, were expected to apply to food and cosmetic additives [46]. Consequently, we evaluated further physiological properties of BL58 polymer, which may have similar rheological properties to the AS22 strain polysaccharide, starting with toxicity testing using several safety tests.
Acute toxicity test: Mice were orally administrated 250 mg kg-1, 500 mg kg-1 and 1000 mg kg-1 of BL58 polysaccharide or xanthan gum. Death of individual mice was not observed in any cases at 2 weeks after the administration of these polysaccharides. The weight of individual mouse steadily increased during experiments. Remarkable pathological change of internal organs was not observed except for slight decolorization of liver. The slight decolorization of liver is assumed to be due to the bleeding when mouse was killed by cervical dislocation; because same result was observed in subgroup administrated distilled water. These results suggest that BL58 polymer has no acute toxicity.
Eye irritancy test: At 1, 24, 48 and 72 h after 1% solution of BL58 polymer was instilled into the anterior eye region (cornea, iris, and conjunctiva) of the rabbit’s right eye was megascopically and ophtalmoscopically checked and compared with those of the left eye used as a control. At 24 h after the instillation, abnormalities of cornea were examined by using the FLUORES Ocular Examination Test Paper, and the eye irritancy was evaluated according to the Draize standard. No changes or irritancy were observed in any cases during observation period. Since the criterion of Draize was zero, the class of eye irritation according to Draize Kay and Calanda was judged “Non-irritation”.
Primary rabbit skin irritation test: The irritancy for rabbit skin was evaluated according to the Draize standard at 24, 48 and 72 h after the BL58 polymer was administrated to the naked skin. There are no changes of rabbit skin or irritancy observed in any cases during observation period.
Primary human skin irritation test: One % solution of BL58 polymer was applied to medial side of the arm of 45 healthy adults (16 men, 29 women) for 24 h by the Finn chamber technique. Four persons became erythematous within 30 min after the patch test was finished, and three of them were very light symptoms. After 48 h, all persons showed no symptoms. Skin irritation index of BL58 polysaccharide was 5.6, indicating that the polysaccharide has no irritancy for human. In control experiments, skin irritation index of 0.1% of methyl paraben, which is generally recognized by the FDA as safe for use as preservative in food and cosmetics, was 6.7.
Skin sensitization test: Five male guinea pig per subgroup were used for the skin sensitization test. Induction of sensitization was carried out by the application of a filter paper immersed emulsion of 2% of DNCB and 0.1% or 0.5% of BL58 polymer to the skin of guinea pigs three times a week. At 2 weeks after final induction, sensitization was further evoked by the application of the emulsion of 2% of DNCB and 0.1% or 0.5% of BL58 polymer to the skin. In the control experiment, saline was used instead of BL58 polysaccharide. At 24 and 48 h, BL58 polysaccharide has no repression effect on skin sensitization.
Micronucleus test (Clastogenicity test): After the oral administration of BL58 polymer (0.25, 0.5, or 1.0 g kg-1) to the ICR male mouse, no increase of micronucleated polychromatophilic erythrocytes was observed (1 to 2 polychromatophilic erythrocytes per 1000 orthochromatic erythrocytes). In the case of administration of mitomycin (2 mg kg-1) as positive control, the increase in number of micronucleated polychromatophilic erythrocytes was statistically significant (tens of polychromatophilic erythrocytes per 1000 orthochromatic erythrocytes). These results indicated that BL58 polymer has no clastogenic or genotoxicity potential.
Rec assay: The growth inhibition zone for both Bacillus subtillis H17 Rec+ and M45 Rec- by the BL58 polymer treatment (1.22 to 5000 μg disk-1) was not observed. Therefore, BL58 polymer had no damaging effect on DNA.
Antigenicity test: Since BL58 polymer has no repression effect on skin sensitization, in order to investigate antigenicity of BL58, active systemic anaphylaxis (ASA) response for guinea pig was investigated. Both test groups, sensitized with 1 and 5 mg kg-1 BL58, developed ASA response, such as scratching nose, decrease of tension level, dyspnea, emiction, defecation and collapse was delayed to 3-4 h after the injection of 1 mg kg-1. Two dead animals were observed in lowly sensitized subgroup. In the positive control experiments (2 mg kg-1 of egg albumin), ASA response was immediate after the induction injection. Decrease of tension level, twitch and collapse were still continued after 4-5 h. In the positive control, death of one animal was observed. BL58 polymer showed ASA response and delay compared with the positive control with egg albumin, suggesting that BL58 polymer has weak antigenicity.
From the results obtained here, a certain level of safety of the BL58 polymer was confirmed and then, the physiological activities of BL58 polymer were evaluated as follows.
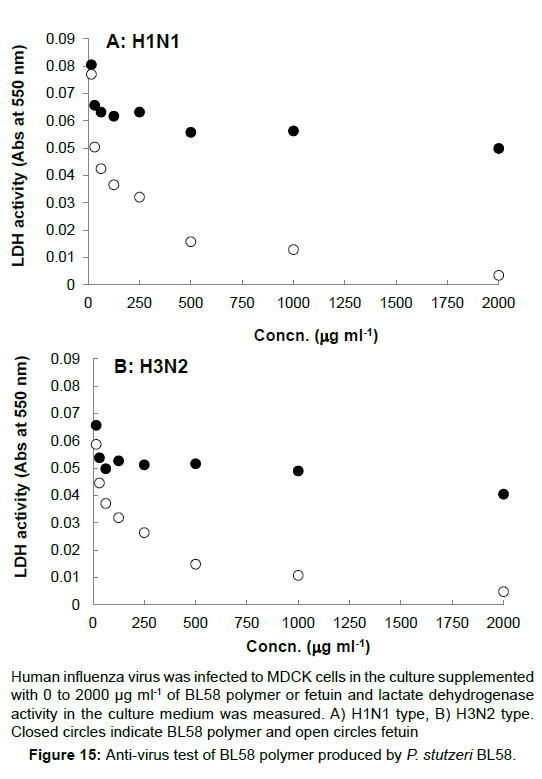
Anti-bacterial and anti-virus test: E. coli K12 was incubated in NB culture overnight, and inoculated into the freshly prepared culture containing 0 to 2,000 μg ml-1 of BL58 polymer. When the growth at OD 600 nm was measured after the overnight incubation, more than 500 μg ml-1 of BL58 polymer exhibited anti-bacterial effect against E. coli cells. For the anti-virus test, Human influenza virus (H1N1, H3N2) was infected to MDCK cells and lactate dehydrogenase activity in the culture medium was measured, because infection lysed host cells and resulted in leakage of lactate dehydrogenase into the culture medium (Figure 15). BL58 polysaccharide exhibited concentration-dependent inhibition of viral infection, but the activity of the polysaccharide was lower than that of positive control, fetuin.
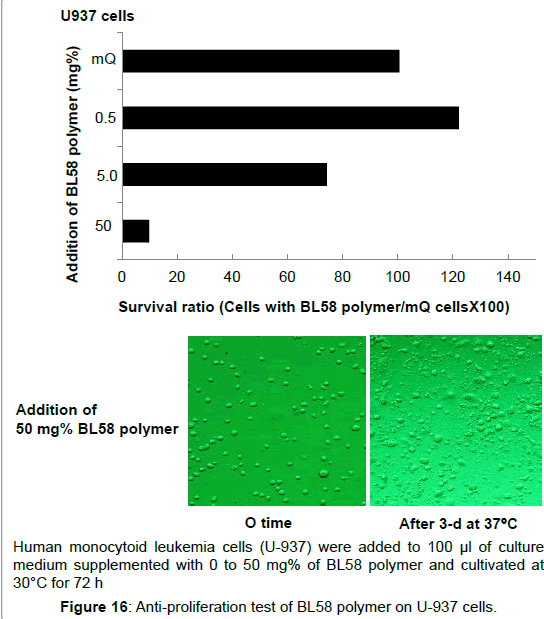
Antiproliferative activity on human acute promyelocytic leukemias cells (HL60) and human monocytoid leukemia cells (U- 937): Effect of P. stutzeri BL58 polymer on growth number of human acute promyelocytic leukemia cells (HL60) was examined. Suppression of the cell growth was not observed up to the addition of 50 mg% polysaccharide, and the cells almost survived after 72 h (data not shown). On the other hand, ratio of survival human monocytoid leukemia cells (U-937) decreased to 70% by the addition of more than 5 mg% of polysaccharide (Figure 16). Addition of 50 mg% of polymer resulted in further decrease to 10%, and destruction of the cells was observed. Both HL60 and U-937 are classified as same leukemia cell groups, however, susceptibility for the BL58 polysaccharide was clearly different. The reason is still unclear, however, addition of the polysaccharide might affect differentiation or signaling related to proliferation of these cells.
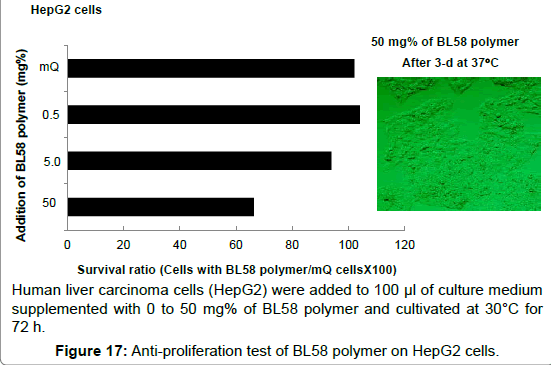
Antiproliferative activity on human liver carcinoma cells (HepG2) and human normal pulmonic cells (MRC5): Effect of BL58 polysaccharide on the number of human liver carcinoma cells (HepG2) was shown in Figure 17. The ratio of survival cells was not changed up to the addition of 5 mg% polymer; however, addition of 50 mg% of polymer resulted in decrease to 65%. This value is same as the number of new-onset cells and the destruction of HepG2 was not observed after the addition of the polysaccharide, suggesting repression of cell proliferation. These results for HepG2 cells were very similar to those for human normal pulmonic cells (MRC5). Addition of 50 mg% of polymer resulted in decrease to 58% (data not shown), suggesting repression of cell proliferation for this non-transformed cell line as well.
Human influenza virus was infected to MDCK cells in the culture supplemented with 0 to 2000 µg ml-1 of BL58 polymer or fetuin and lactate dehydrogenase activity in the culture medium was measured. A) H1N1 type, B) H3N2 type. Closed circles indicate BL58 polymer and open circles fetuin.
Figure 15:Anti-virus test of BL58 polymer produced by P. stutzeri BL58.
From the results obtained here, the BL58 polymer exhibited interesting physiological properties, suggesting potential applications in the pharmaceutical field. We are currently studying the mechanism of proliferation repression for the human cell lines tested here.
Conclusion
The microbial polysaccharides have been studied because of their beneficial bioactive functions as well as their unique rheological properties. Using a severe growth condition, 5% ethanol under alkaline conditions of pH 10, we isolated a P. stutzeri strain BL58 capable of producing large amounts of a novel exopolysaccharide and developed an effective purification method for the exopolysaccharide, not using organic solvent. Typically, microbial polysaccharides are precipitated with an organic solvent, but the highly branched BL58 polymer having a MW of 1,800 kDa was swollen by the addition of ethanol or acetone, so solvent precipitation could not be used. The adsorption of organic solvent into BL58 polymer may be a useful property for industrial chemistry applications in the future.
The rheology of BL58 aqueous solution exhibited a transition from high viscosity sol to viscoelastic gel dependent on the temperature and the pH, suggesting that this behavior is due to its unique carbohydrate structure. Although the constituent sugars of BL58 polymer were the same as those of already reported for P. stutzeri polysaccharides by Maalej et al. [44,46], the estimated structure of BL58 polymer differed from the previously reported P. stutzeri polysaccharides in that 3-O-carboxyethyl rhamnose was branched at 3 position of the glucose rather than mannose residues. These results suggested that Pseudomonas strains synthesize the exopolysaccharides via a common pathway, but the expression of enzymes involved in glycogenesis might be regulated at different levels in each strain and under different culture conditions. The different conditions could control molecular size, ratio of constituent sugars and carbohydrate structure of P. stutzeri exopolysaccharides.
Anticipating usage as a base material for cosmetics and pharmaceuticals, we evaluated BL58 exopolysaccharide in preliminary safety and physiological tests. The acute toxicity, eye irritation, skin irritation, and mutagenicity were negative and the polymer exhibited both antibacterial and antiviral activities. The anti-bacterial activity may be due to the divalent cation chelating activity of exopolysaccharides observed by viscosity increase and gelation in the presence of Mg+ and Ca2+, cations important for cell growth (data not shown). Anti-viral activity has been reported in the past for exopolysaccharides [47]; however the mechanisms are not clearly understood. Perhaps, the most intriguing observation was the anti-proliferative effect on leukemia cells, HL60 and U-927 that were affected at different levels of the polymer. Since both HL60 and U-937 are classified as having monocytic cell lineage, the difference in susceptibility to polymer inhibition may reflect physiological differences in the two leukemia cell lines. We are presently attempting to clarify the mechanism for anti-proliferation activity using several tumor cell lines.
Acknowledgement
The authors thank Dr. Henry G Wada for his helpful and valuable discussions.
References
- Ge Z, Yang F, Goh JC, Ramakrishna S, Lee EH (2006) Biomaterials and scaffolds for ligament tissue engineering. J Biomed Mater Res A 77: 639-652.
- Chen GQ1, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26: 6565-6578.
- Heilmann SM, Rasmussen JK, Krepski LR (2001) Chemistry and technology of 2-alkenyl azlactones. J PolymSciA 39: 3655-3677.
- Chiellini E, Cinelli P, Chiellini F, Imam SH (2004) Environmentally degradable bio-based polymeric blends and composites. MacromolBiosci 4: 218-231.
- Uyama H (2007) Artificial polymeric flavonoids: Synthesis and applications. MacromolBiosci 7: 410-422.
- De Boer B, Facchetti A (2008) Semiconducting polymeric materials. Polym Rev 48: 423-431.
- Das-Gupta DK (1991) Pyroelectricity in polymers. Ferroelectrics 118: 165-189.
- North EJ, Halden RU (2013) Plastics and environmental health: The road ahead. Rev Environ Health 28: 1-8.
- Iwata T (2005) Strong fibers and films of microbial polyesters. MacromolBiosci 5: 689-701.
- Kim DY, Rhee YH (2003) Biodegradation of microbial and synthetic polyesters by fungi. ApplMicrobiolBiotechnol 61: 300-308.
- Puskas JE, Chen Y (2004) Biomedical application of commercial polymers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement. Biomacromolecules 5: 1141-1154.
- Muetgeert J (1961) The fractionation of starch. AdvCarbohydrChem 16: 299-333.
- Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: Fascinating biopolymer and sustainable raw material. AngewChemInt Ed Engl 44: 3358-3393.
- Turvey JR, Williams EL (1976) The agar-type polysaccharide from the red alga Ceramiumrubrum. Carbohydr Res 49: 419-425.
- Mattescu M, Ispas-Szabo A, Mulhbacher P (2006) Polysaccharides for drug delivery and pharmaceutical applications. J ACS SympSer 934: 121-137.
- Heinze T, Liebert T (2001) Unconventional methods in cellulose functionalization. ProgPolymSci 26: 1689-1762.
- Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, et al. (2001) Advances in cellulose ester performance and application. ProgPolymSci 26: 1605-1688.
- Bhardwaj TR, Kanwar M, Lal R, Gupta A (2000) Natural gums and modified natural gums as sustained-release carriers. Drug DevInd Pharm 26: 1025-1038.
- Paul F, Morin A, Monsan P (1986) Microbial polysaccharides with actual potential industrial applications. BiotechnolAdv 4: 245-259.
- Singh RS, Saini GK, Kennedy JF (2008) Pullulan: Microbial sources, production and applications. CarbohydrPolym 73: 515-531.
- Gacesa P (1998) Bacterial alginate biosynthesis--recent progress and future prospects. Microbiology 144: 1133-1143.
- Sutherland IW (1998) Novel and established applications of microbial polysaccharides. Trends Biotechnol 16: 41-46.
- Andrew TR (1977) Extracellular microbial polysaccharides. ACS SympSer 45: 231-241.
- Sanderson GR (1982) The interactions of xanthan gum in food systems. Prog Food NutrSci 6: 77-78.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350-356.
- Hakomori S (1964) A rapid permethylation of glycolipid and polysaccharidecatalyzed by methysulfinylcarbanion in dimethyl sulfoxide. The Journal of Bio-chemistry 55: 205-208.
- Walum E (1998) Acute oral toxicity. Environ Health Perspect 106: 497-503.
- York M, Steiling W (1998) A critical review of the assessment of eye irritation potential using the Draize rabbit eye test. J ApplToxicol 18: 233-240.
- Sekizawa J, Yasuhara K, Suyama Y, Yamanaka S, Tobe M, et al. (1994) A simple method for screening assessment of skin and eye irritation. J ToxicolSci 19: 25-35.
- Liu C, Li X, Li Y, Feng Y, Zhou S, et al. (2008) Structural characterisation and anti-mutagenic activity of a novel polysaccharide isolated from Sepiellamaindroni ink. Food Chem 110: 807-813.
- Mazza G (1982) Bacillus subtilis "rec assay" test with isogenic strains. Appl Environ Microbiol 43: 177-184.
- Kouchi Y, Imamura K, Izumi K, Yoshida R (1989) Antigenicity tests on propiverine hydrochloride in guinea pigs and mice. J ToxicolSci 14: 143-151.
- Allen MJ, Rushton N (1994) Use of the CytoTox 96â?¢ Assay in routine biocompatibility testing in vitro. Promega Notes 45: 7-10.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Morris DL (1948) Quantitative determination of carbohydrates withDreywood'sAnthrone Reagent. Science 107: 254-255.
- Osman SF, Fett WF, Dudley RL (1994) Structure of the exopolysaccharide of Pseudomonasstutzeri strain ATCC 17588. Carbohydr Res 265: 319-322.
- Leone S, Izzo V, Sturiale LR, Garozzo D, Lanzetta R, et al. (2004) Structure of minor oligosaccharides from the lipopolysaccharide fraction of Pseudomonas stutzeri OX1.Carohydr Res 339: 2657-2665.
- Leone S, Izzo V, Lanzetta R, Molinaro A, Parrilli M, et al. (2005) The structure of the O-polysaccharide from Pseudomonas stutzeri OX1 containing two different 4-acylamido-4,6-dideoxy-residues, tomosamine and perosamine. Carbohydr Res 340: 651-656.
- Leone S, Lanzetta R, Scognamiglio R, Alfieri F, Izzo V, et al. (2008) The structure of the O-specific polysaccharide from the lipopolysaccharide of Pseudomonas sp. OX1 cultivated in the presence of the azo dye Orange II. Carbohydr Res 343: 674-684.
- Björndal H, Hellerqvist CG, Lindberg B, Sevensson S (1970) Gas-liquid chromatography and mass spectrometry in methylation analysis of polysaccharides. AngewChemInternat Edit 9: 610-619.
- Severn WB, Richards JC (1993) A novel approach for stereochemical analysis of 1-carboxyethyl sugar ethers by NMR spectroscopy. J Am ChemSoc 115: 1114-1120.
- Maaleja H, Boissetb C, Hmideta N, Buonb L, Heyraudb A, et al. (2014) Purification and structural data of a highly substituted exopolysaccharide from Pseudomonas stutzeri AS22. Carbohydrate Polymers 112: 404-411.
- Maaleja H, Moalla D, Boissetb C, Bardaa S, Ayed HB, et al. (2014) Colloids and surfaces B: Rhelogical, dermal wound healing and in vitro antioxidant propertiesof exopolysaccharide hydrogel from Pseudomonas stutzeriAS22. Biointerfaces 123: 814-824.
- Maaleja H, Hmideta N, Boissetb C, Bayma E, Heyraud A, et al. (2016) Rheological and emulsifying properties of a gel-like exopolysaccharide produced by Pseudomonas stutzeriAS22. Food Hydrocolloids 52: 634-647.
- Wu Y, Li S, Li H, Zhao C, Ma H, et al. (2016) Effect of a polysaccharide from Poriacocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines. Int J BiolMacromol 91: 248-257.
Citation: Yamada H, Yoshida S, Nozaki K, Doi T, Nakamura H, et al. (2016) Structural Analysis and Physiological Properties of Exopolysaccharides Secreted by a Newly Isolated Pseudomonas stutzeri Bl58 under Alkaline Conditions. J Biotechnol Biomater 6:250. DOI: 10.4172/2155-952X.1000250
Copyright: © 2016 Yamada H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12607
- [From(publication date): 12-2016 - Aug 23, 2025]
- Breakdown by view type
- HTML page views: 11616
- PDF downloads: 991