Telomere Length in Children with Acquired Severe Aplastic Anemia
Received: 10-Dec-2018 / Accepted Date: 02-Jan-2019 / Published Date: 15-Jan-2019 DOI: 10.4172/2161-1165.1000364
Abstract
Objective: This study aimed to assess telomere length in children with severe aplastic anemia and to correlate this parameter with the response to immunosuppressive therapy
Method: The study group consisted of 18 children aged with severe aplastic anemia (SAA) treated with rabbit antithymocyte globulin and cyclosporine from one of hematology center. Telomere length was analyzed in all patients and in 20 parents. Control group was composed of 12 healthy children and 12 healthy adults. Telomere length in peripheral blood was assessed using polymerase chain reaction (PCR).
Result: There was statistical significance between telomere length in patients with SAA and healthy children (p=0.03852) as well as in parents of SAA children and adults from control group (p=0.01086). There was no difference concerning response to IST in relation to telomere length (p=0.7859).
Conclusion: Our studies confirmed that pediatric patients with SAA and their parents have shorter telomeres compared to healthy population. The assessment of telomere length in diseases of short telomeres, including aplastic anemia, should be a diagnostic standard. Future studies are necessary to confirm the role of telomere length as an independent prognostic risk factor and a good predictor of prognosis in SAA.
Keywords: Aplastic anemia; Children; Telomere length; Immunosuppressive treatment
Introduction
Augured severe aplastic anemia (SAA) is rare disorder of bone marrow failure which if not appropriately treated is highly fatal. SAA is characterized by morphologic marrow features, namely hypocellularity, and resultant peripheral cytopenias [1-7]. Now most of the patients survive because they can be successfully treated with either hematopoietic stem cell transplantation (HSCT) or immunosuppressive therapy (IST) [8-12]. The choice of the treatment is depended on a patients’ age and availability of HLA-matched family donor. Hematopoietic stem cell transplantation is considered as the treatment of choice in children with HLA-matched family donor and in young adults before 40 years of age. In other cases immunosuppressive therapy is carried out [4,8-17]. The long-term results of those two methods are comparable. Long-lasting remission in 60-75% of patients above 5 years of age, treated with HSCT as well as IST, was proven by numerous studies [8-13,17-25]. However, serious adverse effects of above-mentioned therapies still exist. Graft-versus- Host Disease (GvHD) is one of the major issues after HSCT, on the other hand after IST non-response occurs. Additionally IST is connected with higher relapse rate and higher probability of transformation in to myelodysplastic syndrome (MDS) or malignant disease, including acute myeloblastic leukemia (AML) [17-21]. Despite multiple attempts, a factor enabling to qualify children to proper therapeutic arm in terms of treatment outcome and the occurrence of adverse effects has not been established yet [22-25]. The prognostic value of initial neutrophil, reticulocyte or platelet count, presence of paroxysmal nocturnal hemoglobinuria clone is questionable [14,16,17,22,23,25]. Only the patient’s age at the onset of the disease seem to influence the clinical outcome. Younger patients respond better to IST in comparison with older patients. The search for a reliable prognostic factor is still being conducted [14,16,17,22-25]. Telomere shortening is observed in about 1/3 of patients with SAA, and mutations in genes of telomerase complex (TERC, TERT) were identified in about 5-10% of SAA patients [26-34]. Initially telomere shortening was considered to be a consequence of oxidative stress in hematopoietic system [35,36]. Moreover, the mutations in TERC and TERT were identified as etiological background of telomere shortening. Patients with those mutations are found to possess significantly shorter telomeres than age-matched healthy individuals [34-36]. As SAA belongs to the short telomeres diseases, an attention has been paid to telomere length as potential predictor of long-term treatment outcome [37-41].
This study was aimed to assess telomere length in children with severe aplastic anemia (SAA) and to determine whether this parameter can be used to predict response to immunosuppressive therapy.
Material And Methods
Eighteen patients with severe aplastic anemia on immunosuppressive treatment with no HLA-matched family donor were enrolled in one polish pediatric hematology and oncology center in years 2009-2013. The mean age of enrolled subjects was 14.35 years (range: 7-19), study group included 9 boys and 9 girls. All patients fulfilled criteria of severe aplastic anemia: bone marrow cellularity <25% of reference value for the age or 25-50% with <30% of hematopoietic reservoir and additionally neutrophil count (ANC) 38]. All patients had congenital aplastic anemia. In all cases paroxysmal nocturnal hemoglobinuria was excluded. Diagnostic tests for PNH were performed with the use of flow cytometry with CD55 and CD59 antigen expression analysis on neutrophils and red blood cells. The chromosomal fragility test was performed as Fanconi anemia screening. In all patients had bone marrow cytogenetic analysis was performed to diagnose chromosomal defects.
Protocol therapy
Patients received rabbit antithymocyte globulin (r-ATG, Lymphoglobulin, Genzyme) at 3.75 mg/kg/mc intravenously at 1-5 days, cyclosporine (CSA, Sandimmun Neoral, Novartis Pharma) at 5 mg/kg orally at days 1-180. CSA dose was modified to maintain serum drug level of 100-200 ng/ml. Granulocyte colony stimulating factor (GCSF, Filgrastim– Neupogen, Hoffmann-La Roche) was administered subcutaneously or intravenously only to patients with severe infection non-responding to antibiotics and antifungal medications. The criteria of remission were evaluated at days 112, 180 and 360 after treatment initialization. Complete remission (CR) was defined as ANC >1.5 × 109/L, PLT >100 × 109/L and hemoglobin level >11.0g/L. Criteria for partial remission (PR) were defined as follows: ANC >0.5 × 109/L, PLT >20 × 109/L and hemoglobin level >8.0 g/L. Children were divided into three groups: non responders (NR) – 6 children with refractory disease, early remissions (E-CR) – 6 children with early response to treatment defined as complete or partial remission at day 112, and late remissions (L-R) – 6 children who achieved CR or PR at days 180-360. Telomere length was analyzed in all patients and in 20 parents of 11 children with SAA (11 mothers and 9 fathers aged 39-55 years). Control group was composed of 12 healthy children (3 girls and 9 boys, aged 3-17) and 12 healthy adults aged 31-57 years. Each participant/parents signed informed consent and agreed for blood sampling.
Local ethical committee approved the protocol of the study
Telomere length was measured in peripheral blood using quantitive PCR (qPCR) method described by Pavesi et al. [40]. Genomic DNA was extracted using AxyPrep Blood Genomic DNA Miniprep Kit (Axygene). Two 96-well plates were prepared for measurements, first plate was used for amplification of telomere product and second one was used for single copy gene (SEP15) product amplification. Each reaction contained: Power SYBR1 Green PCR Master Mix (Applied Biosystems), DNA and primers (270nM Tel1 and 900nM Tel2 or 500nM each SEP15 primers). Reactions were performed in Real Time PCR CFX96 system (Biorad) with thermal cycling conditions: 95°C - 10 minutes; 95°C - 15 seconds, 54°C - 2 minutes (35 cycles).
Statistical analysis
Telomere/single copy gene (T/S) ratio for samples was calculated from human reference DNA (Applied Biosystems), standard curve and T/S ratios values were expressed in relation to telomere length in controls. Control quartiles leukocyte telomere length (LTL) were categorized into quartiles based on the weighted sample distribution of LTL. The R Project for Statistical Computing was used for statistical analysis, p value <0.05 was considered statistically significant [41]. Mann–Whitney U test or Kruskal-Wallis test was employed as appropriate. Linear regression analysis was carried out with the use of Excel (Microsoft) and Statistica software.
Results
Telomere length assessment
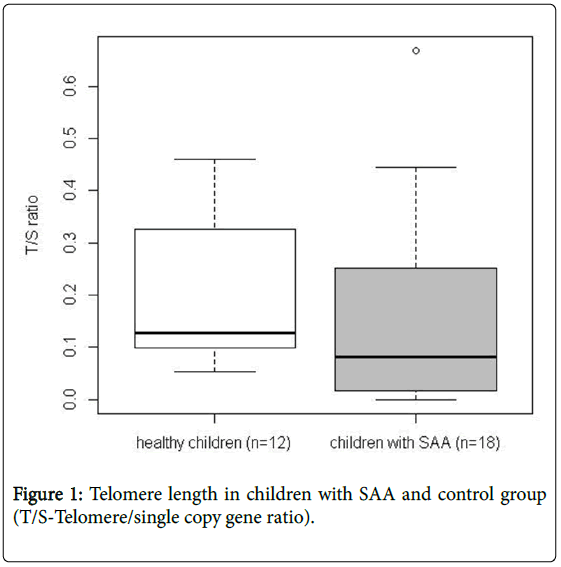
Tables 1 and 2 presents mean values of telomere length in SAA and healthy children as well as in parents of SAA children and adults established as their control group. Telomere length in leukocytes of SAA children were significantly shorter (p=0.001136) than in healthy individuals (Figure 1).
| Healthy children, control group (n=12) |
SAA children, study group (n=18) | Group difference (p-value) |
|
|---|---|---|---|
| T/S median | 0.164191 | 0.086838 | 0.001136 |
| (mean) | (0.22242) | (0.11742) | |
| T/S min. value | 0.055424 | 0.027866 | |
| T/S max. value | 0.508752 | 0.253397 | |
| Control quartiles LTL | range | n(%) | |
| I | <0.121459 | 9(50%) | |
| II | 0.121459 ≤ n <0.164191 | 0 | |
| III | 0.164191 ≤ n <0.350899 | 7(39%) | |
| IV | 0.350899 ≥ n | 2(11%) |
T/S-Telomere/single copy gene ratio
Control quartiles leukocyte telomere length (LTL) were categorized into quartiles based on the weighted sample distribution of LTL
Table 1: Telomere length values in severe aplastic anemia (SAA) and healthy children.
| Adults, control group (n=12) | SAA parents (n=20) |
Group difference (p-value) |
|
|---|---|---|---|
| T/S median | 0.192916 | 0.058040 | 0.01086 |
| (mean) | (0.1961) | (0.13268) | |
| T/S min. value | 0.001233 | 0.002637 | |
| T/S max. value | 0.522416 | 0.774778 | |
| Control quartiles LTL | range | n(%) | |
| I | <0.113909 | 13(65%) | |
| II | 0.113909 ≤ n<0.192916 | 3(15%) | |
| III | 0.192916 ≤ n<0.240131 | 1(5%) | |
| IV | 0.240131 ≥ n | 3(15%) |
T/S-Telomere/single copy gene ratio
Control quartiles leukocyte telomere length (LTL) was categorized into quartiles based on the weighted sample distribution of LTL.
Table 2: Telomere length values in parents of children with severe aplastic anemia (SAA) and healthy adult individuals.
When analyzing telomere length between healthy adults and parents of children with SAA, it was found that the parents of our patients have shorter telomeres and these differences are statistically significant (0.01086).
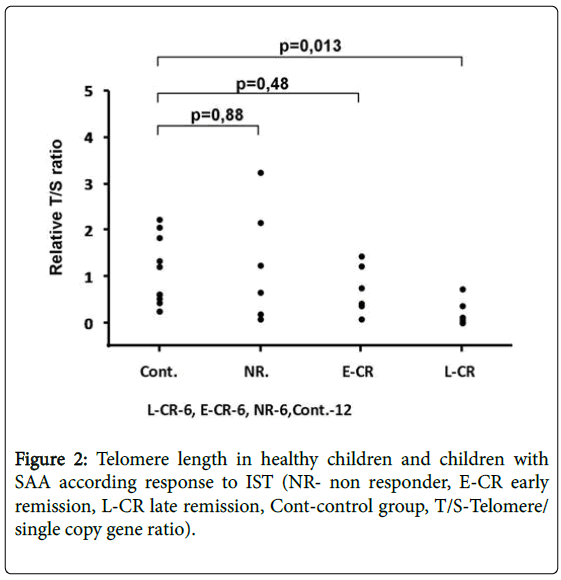
In the whole group of 18 SAA patients treated with IST, 12 (66.6%) of them responded to the therapy. Seven patients out of 9 achieved remission in the group of children with short telomeres, 4 out of 7 in the group of children with medium telomeres and 1 out of 2 in children with long telomeres. No significance between telomere length and response to treatment was found (p=0.7859). There was a significant difference (p=0.013) between telomere length in healthy children and children with SAA who responded to treatment after day 180 (Figure 2). The difference was not detected between nonresponders and late responders (L-CR) (p=0.065).
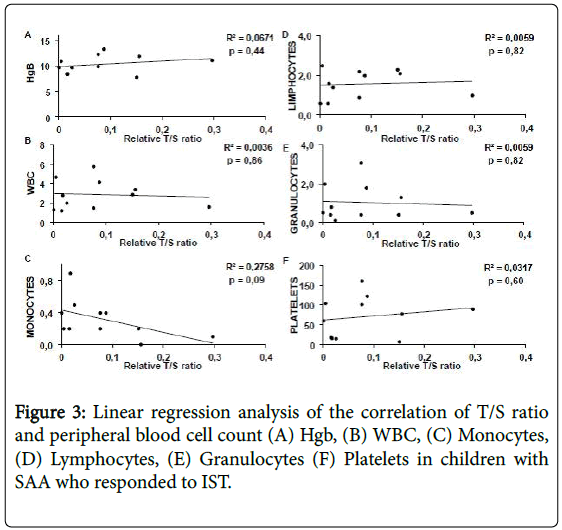
Complete blood count and telomere length analysis in children with SAA and control group. There was no correlation between telomere length and complete blood count parameters In 12 children with SAA who responded to treatment (both early and late responders) (Figure 3).
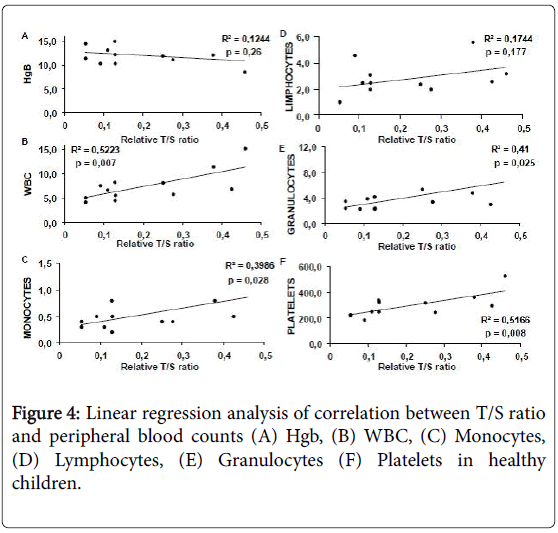
In healthy children, telomere length correlated with platelet (p=0.008, R2=0.5166), white blood cell (p=0.007, R2=0.5223) neutrophil (p=0.025, R2=0.41) and monocyte (p=0.02, R2=0.3986) count and all the correlations were statistically significant (Figure 4).
In the course of 5 year follow-up, one relapse 3 years after remission was noted. No clonal disease or transformation into malignant disease was observed. No chromosomal abnormalities or PNH clones were found. All patients live in remission: 17 after IST and one after IST and BMT from unrelated donor.
Discussion
Congenital aplastic anemia’s such as Dyskeratosis congenital (DS), Fanconi anemia (FA), Diamond-Blackfan anemia (DBA) belong to a group of short telomeres diseases [26-31]. Short telomeres are detected in around one third of patients with acquired severe aplastic anemia [25-30,35,37]. Our study confirms significantly shorter telomeres in children with SAA in comparison to healthy controls (p=0.001136) and indicates that parents of SAA children have shorter telomeres than adults from control group. Other studies also highlighted this phenomenon in adults with SAA [25-30]. Tulelman et al. confirmed telomere shortening in neutrophils, T- and B-lymphocytes as well as NK cells in the group of 28 patients with SAA [37]. Pavesi et al. assessed telomere length in patients with bone marrow failure syndromes (BMFS) such as congenital bone marrow failure (DS, DBA, FA) acquired aplastic anemia and concluded that telomere length was a handy clue in a diagnostic process of children with no phenotypic features [40]. Young observed correlation between length of telomeres in SAA patients and blood cell count in pancytopenic patients. Clonal transformation to myelodysplasia and acute leukemia and long-term survival were also dependent to the telomeres length [32]. In the observed group there was only one relapse and no clonal transformations, thus such observation could not be confirmed. Surprisingly in the control group (but not in patients) a significant correlation between leukocyte, platelet, neutrophil, monocyte count and telomere length was observed. In a search for prognostic factors for IST response, we have shown that there was no relationship between telomere length and treatment response (p=0.7859). Sakaguchi et al. study demonstrated that the measurement of telomere length of lymphocytes is useful for predicting the response to IST in patients with AA [35]. Multivariate analysis showed that telomere length shorter than -1.0 SD (hazard ratio (HR): 22.0; 95%CI: 4.19-115; P<0.001), platelet count at diagnosis less than 25 × 109/L (HR: 13.9; 95%CI: 2.00- 96.1; P=0.008), and interval from diagnosis to immunosuppressive therapy longer than 25 days (HR: 4.81; 95%CI: 1.15-20.1; P=0.031) were the significant variables for poor response to immunosuppressive therapy. But in our study we not found correlation between this parameters and response to IST. To begin with, there are several differences between the current study and Sakaguchi et al. study, including the methods of telomere length measurement and patients’ characteristics [35]. In our study, the telomere length of pretreatment total leukocytes was assessed by quantitative polymerase chain reaction (PCR) but in Chinese study measured the telomere length of lymphocytes using flow-FISH. Another difference between the two studies was the distribution of patients’ diagnosis. Patients in our study was restricted to children with severe AA, and very severe AA, patients with moderate AA were not included. In contrast, 20 of 64 AA patients in Sakaguchi et al. study had moderate disease [35].
A large retrospective analysis of 183 SAA patients on ATG-based IST assessing association of treatment results with initial telomere length in peripheral blood leukocytes was conducted by National Institutes of Health (NIH) [36]. A special attention was drawn to relation between telomere length and hematological response 6 months after treatment initialization, SAA relapse, chromosomal disorders and subsequent after-effects (clonal and malignant diseases). There was no association between initial telomere length and hematological response 6 month after treatment initiation, however relapse rate was significantly higher in patients with shorter telomeres. Higher rate of clonal cytogenetic abnormalities was observed in patients with shorter telomeres in comparison to patients with longer telomeres. Moreover, the risk of chromosomal abnormalities such as monosomy 7 was also noted in group of patients with shorter telomeres. Overall survival differed six years after IST initiation and was connected with initial telomere length. TERC/TERT mutation was found only in one person among 183 SAA patients [35].
Tutelman et al. showed that short telomeres and PNH clone in children with SAA is a predictive factor of good IST response [37]. In addition, an increased frequency of chromosomal breaks and chromosomal aberrations, particularly monosomy 7, is commonly found in AA patients. In our group no chromosomal abnormalities or PNH clones were found.
During the 5-years follow-up we observed only one patient with relapse. None of the children developed MDS or AML. All patients in our study group live in remission.
Conclusion
Our studies confirmed that patients with SAA and their parents have shorter telomeres compared to healthy population. The assessment of telomere length in diseases of short telomeres, including aplastic anemia, should be a diagnostic standard. Future studies are necessary to confirm the role of telomere length as an independent prognostic risk factor and a good predictor of prognosis in SAA.
References
- Benson-Quarm N, Gandhi S, Kulasekararaj A, Marsh J (2014) Aplastic anaemia. Hematology 19: 60-61.
- Young NS (2013) Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program 2013: 76-81.
- Scheinberg P, Young NS (2012) How I treat acquired aplastic anemia. Blood 120: 1185–1196.
- Scheinberg P (2012) Aplastic anemia: therapeutic updates in immunosuppression and transplantation. Hematology Am Soc Hematol Educ Program 2012: 292–300.
- Marsh JC (2000) Hematopoietic growth factors in the pathogenesis and for the treatment of aplastic anaemia. Semin Hematol 37: 81-90.
- Pawelec K, Rokicka-Milewska R (2002) Nabyta niedokrwistość plastyczna. Standardy Medyczne 5: 303-307.
- Pawelec K, Jackowska T, Matysiak M (2005) Nabyta niedokrwistość aplastyczna u dzieci. Klinika Pediatryczna 3: 322-326.
- Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, et al. (2000) Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO). Blood 95: 1931–1934.
- Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, et al. (2011) Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 365: 430-438.
- Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Wu CO, et al. (2012) Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood 119: 345–354.
- Tichelli A, Passweg J, Nissen C, Bargetzi M, Hoffmann T, et al. (1998) Repeated treatment with horse antilymphocyte globulin for severe aplastic anaemia. Br J Haematol 100: 393–400.
- Scheinberg P, Nunez O, Young NS (2006) Retreatment with rabbit antithymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol 133: 622–627.
- Gluckman E, Devergie A, Poros A, Degoulet P (1982) Results of immunosuppression in 170 cases of severe aplastic anaemia Report of the European Group of Bone Marrow Transplant (EGBMT). Br J Haematol 51: 541–550.
- Chang MH, Kim KH, Kim HS, Jun HJ, Kim DH, et al. (2010) Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur J Haematol 84: 154–159.
- Afable MG, Shaik M, Sugimoto Y, Elson P, Clemente M, et al. (2011) Efficacy of rabbit anti-thymocyte globulin in severe aplastic anemia. Haematologica 96: 1269–1275.
- Yoshida N, Yagasaki H, Hama A, Takahashi Y, Kosaka Y, et al. (2011) Predicting response to immunosuppressive therapy in childhood aplastic anemia. Haematologica 96: 771–774.
- Führer M, Rampf U, Baumann I, Faldum A, Niemeyer C, et al. (2005) Immunosuppressive therapy for aplastic anaemia in children: a more severe disease predicts better survival. Blood 106: 2102-2104.
- Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, et al. (2000) Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood 96: 2049-2054.
- Pawelec K, Matysiak M, Niewiadomska E, Rokicka-Milewska R, Kowalczyk J, et al. (2006) Results of immunosuppressive therapy in children with severe aplastic anaemia. Report by the Polish Paediatric Leukaemia and Lymphoma Study Group. Med Wieku Rozwoj 10: 832-839.
- Pawelec K, Matysiak M, Niewiadomska E, Rokicka-Milewska R, Kowalczyk J, et al. (2008) Results of immunosupressive therapy in children with severe aplastic anaemia. Report of the Polish Paediatric Haematology Group. Med Wieku Rozwoj 12: 1092-1097.
- Locasciulli A, Oneto R, Bacigalupo A, Socié G, Korthof E, et al. (2007) Outcome of patients with acquired aplastic anemia given firts bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 92: 11-18.
- Scheinberg P, Wu CO, Nunez O, Young NS (2009) Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol 144: 206–126.
- Yoshida N, Yagasaki H, Takahashi Y, Yamamoto T, Liang J, et al. (2008) Clinical impact of HLA-DR15, a minor population of paroxysmal nocturnal haemoglobinuria-type cells, and an aplastic anaemia-associated autoantibody in children with acquired aplastic anaemia. Br J Haematol 142: 427–435.
- Pawelec K, Salamonowicz M, Panasiuk A, Demkow U, Kowalczyk J, et al. (2015) First-line immunosuppressive treatment in children with aplastic anemia: rabbit antithymocyte globulin. Adv Exp Med Biol. 836: 55-62.
- Scheinberg P (2013) Prognostic value of telomere attrition in patients with aplastic anemia. Int J Hematol 97: 553–557.
- Calado RT (2009) Telomeres and marrow failure. Hematology Am Soc Hematol Educ Program 2009: 338-343.
- Calado RT, Young NS (2008) Telomere maintenance and human bone marrow failure. Blood 111: 4446–4455.
- Calado RT, Young NS (2009) Telomere diseases. N Engl J Med 361: 2353–2365.
- Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, et al. (1998) Progressive telomere shortening in aplastic anemia. Blood 91: 3582–3592.
- Brümmendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM (2001) Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood 97: 895–900.
- Calado RT, Cooper JN, Padilla-Nash HM, Sloand EM, Wu CO, et al. (2012) Short telomeres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia 26: 700-707.
- Young NS (2012) Bone marrow failure and the new telomere diseases: practice and research. Hematology Suppl 1: 18-21.
- Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, et al. (2003) Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet 362: 1628–1630.
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, et al. (2005) Mutations in TERT, the gene for telomerase reverse transcriptase in aplastic anemia. N Engl J Med 352: 1413–1424.
- Sakaguchi H, Nishio N, Hama A, Kawashima N, Wang X, et al. (2014) (Japan Childhood Aplastic Anemia Study Group). Peripheral blood lymphocyte telomere length as a predictor of response to immunosuppressive therapy in childhood aplastic anemia. Haematologica 99: 1312-1316.
- Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, et al. (2010) Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA 304: 1358–1364.
- Tutelman PR, Aubert G, Milner RA, Dalal BI, Schultz KR, et al. (2014) Paroxysmal nocturnal haemoglobinuria phenotype cells and leucocyte subset telomere length in childhood acquired aplastic anaemia. Br J Haematol 164: 717-721.
- Hemann MT, Strong MA, Hao LY, Greider CW (2001) The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107: 67–77.
- Camitta BM (2000) What is the definition of cure for aplastic anemia? Acta Haematologica 103: 16-18.
- Pavesi E, Avondo F, Aspesi A, Quarello P, Rocci A, et al. (2009) Analysis of Telomeres in Peripheral Blood Cells From Patients With Bone Marrow failure. Pediatric Blood & Cancer 53: 411–416.
- R Core Team (2013) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Citation: Pawelec K, Janiak M, Wlodarski P, Matysiak M (2019) Telomere Length in Children with Acquired Severe Aplastic Anemia. Epidemiology (Sunnyvale) 9: 364. DOI: 10.4172/2161-1165.1000364
Copyright: © 2019 Pawelec K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4207
- [From(publication date): 0-2019 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 3223
- PDF downloads: 984