Tendencies towards a C4 Leaf: Quantitative Studies on Leaf Anatomy of Selected C3 and C4 Grasses
Received: 19-Dec-2017 / Accepted Date: 12-Jan-2018 / Published Date: 15-Jan-2018 DOI: 10.4172/2375-4338.1000188
Abstract
Grass family (Poaceae) is one of the largest families that represent monocots; containing species like Oryza sativa, Triticum aestivum, Zea mays, Eleusine coracana, Panicum miliaceum, Panicum sumatrense and Sorghum bicolor which are important to humans since they, directly or indirectly provide more than 3/4 of our food. C4 photosynthesis is a complex physiological adaptation, that is believed to have resulted from a series of anatomical and biochemical modifications to the ancestral C3 pathway. It is believed that C4 photosynthesis to have evolved in a stepwise manner nearly 22-24 times creating intermediates with different combinations of C4 -like components in grasses. C4 photosynthesis confers greater productivity than the C3 photosynthetic type in environments under high irradiance and high temperature. Accompanying higher photosynthetic rate C4 plants possess a vascular system in leaves with higher export rates which permit efficient translocation of photosynthates.
In the current study, selected 11 grass species were subjected to analysis of leaf vascular system in relation to their C3 and C4 photosynthesis. The leaf vascular network in both C3 and C4 grasses consist of LLV, SLV and TV that are connected to longitudinal veins. Analysis carried out using the mature leaves of selected C3 and C4 grasses have shown that there are significant differences in anatomical features such as venation, stomata and bundle sheaths. The comparison of DLLV, DSLV and DTV of C3 and C4 grasses demonstrate that C4 grasses have a denser vascular pattern compared to C3 species (respective distances of C4 grasses were 71.74 μm, 11.85 μm and 81.4 μm). It has been found that these reduced distances between veins have led to significant low stomatal density among C4 grasses. Further, characteristic large bundle sheath structure of C4 species has been clearly seen from significantly high values obtained for BSD, ISD and OSD (respectively 10.4 μm, 4.32 μm and 7.15 μm).
Furthermore, this screening has found Oryza nivara (a C3 species) having C4 like anatomical characters in leaf venation and bundle sheath structure. This species showed significantly low mean values for the distance between transverse veins (41.75 μm) resulting in significantly high total vein length per unit leaf area (81.77μm-1). The C4 tendency of its leaf anatomy was further supported by significantly large bundle sheaths, inner sheath and outer sheath distances (13.1 μm, 5.22 μm and 8.22 μm respectively).
At the end of the analysis we were able to demonstrate significant differences of anatomical characters like venation, stomata and bundle sheaths in C3 and C4 plants. In addition, observations suggest characteristic leaf anatomy that is compatible with C4 anatomy may have evolved in Oryza nivara resulting it to be considered as C3- C4 intermediate.
Keywords: C3-C4 intermediate; Grasses; Leaf vascular system; Longitudinal veins; Poaceae
Abbreviations
LLV: Large longitudinal veins; SLV: Small longitudinal veins; TV: Transverse veins; DLLV: Distance between large longitudinal veins; DSLV: Distance between small longitudinal veins; DTV: Distance between transverse veins; BSD: Bundle sheath distance; ISD: Inner sheath distance; OSD: Outer sheath distance; GPWG: Grass phylogeny working group; FAO: Food and Agricultural organization; PGRC: Plant Genetic Resource Center; RRDI: Rice research and Development Institute.
Introduction
Photosynthesis is a highly regulated, multistep process. It encompasses the harvest of solar energy, transfer of excitation energy, energy conversion, electron transfer from water to NADP+, ATP generation and synthesis of carbohydrates. Majority of plant species perform C3 photosynthesis [1] where CO2 is initially fixed by the enzyme Rubisco (ribulose-1, 5- bisphosphate carboxylase⁄oxygenase). However, Rubisco has the same affinity to react with CO2 as well as O2, which leads to photorespiration. It is known that photorespiration which consumes energy and releases previously fixed CO2 (causing a loss of 40% of photosynthates), is thought to be the driving force behind the evolution of C4 [2,3].
C4 leaves are characterized by Kranz-type anatomy, in which the vascular bundle is surrounded by bundle sheath (BS) cells and this tissue layer is further surrounded by radially arranged mesophyll cells. In contrast, C3 leaves have mesophyll cells that are well developed relative to the BS cells. However, these mesophyll cells of C3 plants contain only a few organelles [4]. In C4 photosynthesis, atmospheric CO2 is initially fixed in the mesophyll cells, then decarboxylation and refixation of CO2 occur in the BS cells [5]. Along with the changes in BS anatomy, some studies have reported that C4 leaves have a denser vascular system than C3 leaves [6]. This is clearly seen in leaves of grasses, which possess parallel venation. Leaves of C3 and C4 species usually express a distinct difference in the interveinal distances leading to the denser vascular system of C4 species. Denser vascular system may also be needed for the expression of photosynthetic enzymes in the mesophyll and bundle sheath cells [7]. C4 plants exhibit higher photosynthetic rates than C3 plants even under high irradiance and at high temperatures [8].
Evolution of C4 plants from C3 has been accompanied by several anatomical and biochemical modifications of leaves. A change in the vein density has evidently occurred during the evolution from C3 to C4. Veins of grass leaves show a hierarchical order and they are differentiated into large, small and transverse veins. Further, they show a clear division of labour. The large longitudinal veins (LLV) serve primarily in longitudinal transport of photosynthate. The small longitudinal veins (SLV) serve primarily in collecting photosynthate from nearby photosynthetic cells whereas transverse veins (TV) connect the longitudinal veins and play an important role in the lateral transport of photosynthate from the small to the large longitudinal veins [9]. In studying the vascular architecture of the leaf, localization of the veins and their distinct functions are important. Most of the previous studies on C3 and C4 grass leaves have mainly focused only on large longitudinal veins [6,10-12], while studies of Ueno et al. [4] and Oguro et al. [13] had given equal emphasis to studying transverse vein densities as well.
C4 origins are not randomly distributed across the plant tree of life. Most large (species rich) clades of plants completely lack C4 taxa where as others contain an astonishingly high number of C3 to C4 transitions. This is observed predominantly in grasses, in which C4 photosynthesis has evolved independently 22-24 times [13]. Repeated origins of C3 and C4 photosynthesis in grasses occurred only in PACMAD clade (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae and Danthonioideae) [14,15].
Previous studies have shown that stomatal characters vary predictably between C3 and C4 species, clearly attributed to the functional convergence based on photosynthetic pathway [16,17]. Since C4 species have high photosynthetic rates even at low intercellular CO2 concentrations, they maintain lower stomatal conductance rates than C3 species to reduce their transpiration rate and further increase their water-use efficiency (the ratio of photosynthesis to transpiration) [18]. Generally, C4 plants have achieved lower stomatal conductance rates by producing smaller stomata for a given stomatal density. It has been found that some C4 lines have reduced stomatal density, while others have a reduced stomatal aperture [2].
To the best of our knowledge, there have been very few studies conducted focusing on leaf anatomy in relation to the photosynthetic mechanism in grass species in Sri Lanka. Therefore, this study was carried out with the main objective of studying differences in the venation, bundle sheath anatomy and stomatal characters of selected C3 and C4 grass species of Sri Lanka.
Materials and Methods
Plant materials
Eleven representative species of both C3 and C4 photosynthetic groups were used for the study (Table 1). C4 species were further divided into two subcategories as NADP-ME and NAD-ME, according to their decarboxylation mechanisms.
| Photosynthetic mechanism | Plant species |
|---|---|
| C3 | Oryza sativa (BG 357, BG 403, BG 94-1), Oryza nivara, Isachne globosa |
| C4 | |
| NADP-ME | Setaria italica, Sorghum bicolor, Zea mays |
| NAD-ME | Panicum sumatrense, Panicum miliaceum, Eleusine coracana |
Table 1: Plant species used in the study.
Experimental design
Seeds of selected plant species were obtained from PGRC (Plant Genetic Resource Centre, Sri Lanka) and RRDI (Rice Research and Development Institute, Bathalagoda, Sri Lanka). Seeds were sown in pots filled with a mixture of sand, compost and soil (1:1:2 volume ratios). They were allowed to germinate in a glass house. Temperature was recorded as 30.25°C ± 1.7°C and relative humidity as 72% ± 2.16% throughout the experimental period. Plants were watered daily with adequate amounts.
Sampling of leaves and fixation
Fully expanded leaves were used for the study [14]. Leaves were tagged and sampled at twenty days. Leaf blades were cut and immediately fixed in a mixture of formaldehyde, acetic acid and ethanol in water (1:1:18 volume ratios).
Venation studies
Leaf clearing: Cleared leaf blades were prepared according to the method described by Ueno [19]. The middle portions of fixed leaves were boiled in 70% ethanol for about 10-20 minutes. After washing in distilled water several times, they were transferred to boiling 85% lactic acid for 20 min, and then stored in chloral hydrate-saturated in ethanol before analysis. Slides were prepared and observed (without staining) under a light microscope.
As a modified methodology some leaves were cleared by soaking in a solution of formaldehyde: propionic acid: ethanol (1:2:18) over night. Slides prepared by this method were stained using safranin and observed under light microscope.
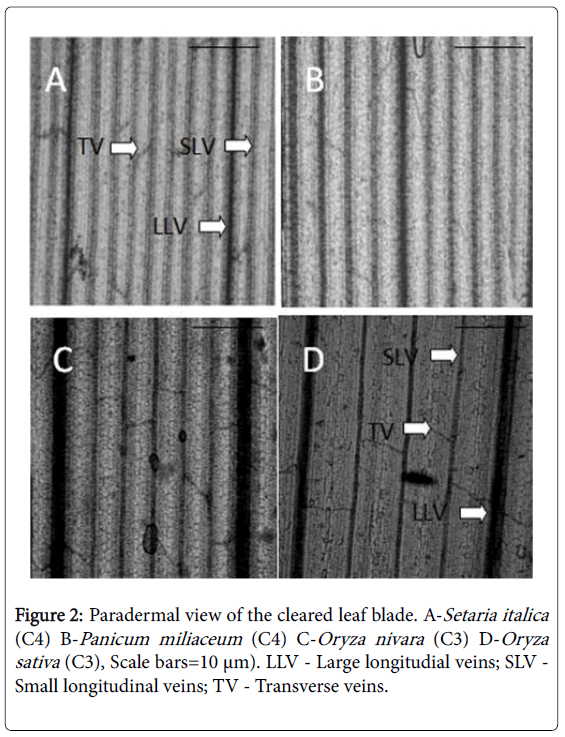
Quantitative data of leaf vascular systems: Leaf veins were identified as three types: large longitudinal veins (LLV), small longitudinal veins (SLV) and transverse veins (TV) [19,20]. The two types of longitudinal veins were distinguished by diameter under the light microscope. The distances between SLV, LLV and TV were represented by means of 30 measurements of middle portions of five leaf blades taken from five plants. The distance between longitudinal veins was measured between the centers of adjacent veins. The transverse veins are usually curved, unlike the parallel longitudinal veins. For the distance between TV, therefore, the mean of the minimum and maximum distances between adjacent transverse veins running between a pair of longitudinal veins was calculated. The distance between SLV was multiplied by that between TV for each species to obtain areolar area, which represents the minimum area of photosynthetic tissue surrounded by veins. Total vein length was measured using the lengths of large, small and transverse veins in one field.
Stomatal studies
Stomatal imprints were obtained using the method described by [21]. Stomatal index (Stomatal density/(Stomatal density+Epidermal cell density) and stomatal density were calculated using nail polish imprints obtained from abaxial and adaxial surfaces of leaves. These measurements were represented by means of thirty measurements of five leaf blades taken from five different plants.
Bundle sheath observations
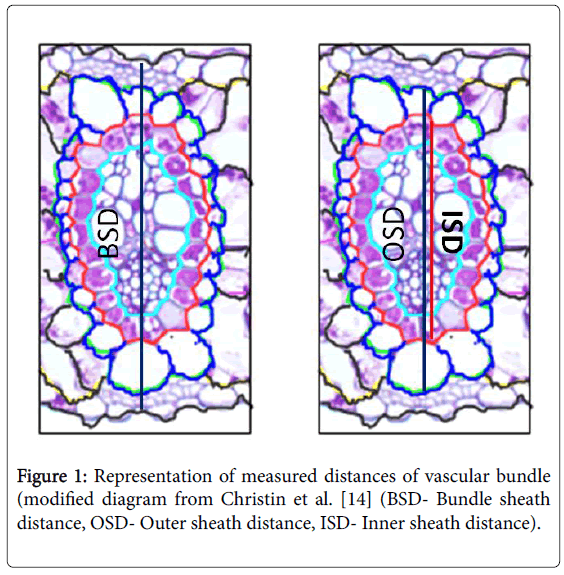
Hand sectioning was done to make observations on vascular bundles. Bundle sheath distance, outer sheath distance and inner sheath distances were obtained from the leaf cross sectioning. Distance measurements were calculated by obtaining average of three diameters. Bundle sheath, outer and inner sheath areas were calculated using the distance measurements (Figure 1). Demonstration of these areas was carried out following Christin et al. [14]. Means of these measurements were represented by fifteen measurements of five leaf blades taken from five different plants.
Figure 1: Representation of measured distances of vascular bundle (modified diagram from Christin et al. [14] (BSD- Bundle sheath distance, OSD- Outer sheath distance, ISD- Inner sheath distance).
Statistical analysis
Differences in the mean values generated for each species were tested at 0.05 significance for each species between the C4 and C3 groups using one- way analysis of variance (ANOVA) with Tukey’s honesty significance difference test (statistical software SPSS version 16.0).
Principal component analysis
Principle Component analysis was carried out with PAST (Paleontological statistics software package for education and data analysis) version 2.17C.
Results
The leaf vascular network in both C3 and C4 grasses consist of LLV, SLV and TV that are connected to longitudinal veins (Figure 2). The comparison of distances measured between large longitudinal veins, small longitudinal veins and transverse veins of C3 and C4 grasses demonstrate that C4 grasses have a denser vascular pattern compared to C3 species. Comparison of mean values obtained for distances measured between large longitudinal veins, small longitudinal veins, transverse veins for the C3 and C4 species reveal that C3 species have a 1.3, 2.44 and 1.4 times significantly higher value than that of C4 grasses. In addition, compared to C3 species, C4 species recorded a 1.03 and 1.47 times significantly higher mean value for areolar area (minimum photosynthetic area surrounded by veins) and total vein length per unit leaf area respectively (Table 2).
| DLLV(µm) | DSLV(µm) | DTV(µm) | Areolar area (µm2) | Total vein length per unit leaf area (µm-1) | |
|---|---|---|---|---|---|
| C3 | 94.26 ± 3.04A | 28.97 ± A | 112.5 ± 6.14A | 1.89 ± 0.089A | 73.85 ± 0.8A |
| Oryza sativa | |||||
| BG 403 | 77.29a | 15.09c | 156.8a | 1.674a | 77.01a |
| BG 357 | 71.29a | 14.86a | 164.1a | 1.56a | 73.08a |
| BG 94-1 | 79.67a | 15.29a | 157.85a | 1.462a | 70.53a |
| Oryza nivara | 77.38a | 14.82a | 41.75b | 1.42a | 81.77b |
| Isachne globosa | 165.66c | 84.79d | 42b | 3.54c | 66.87c |
| C4 | 71.74 ± 2.68B | 11.85 ± 0.16B | 81.4 ± 1.9B | 1.95 ± 0.099B | 108.78 ± 1.66B |
| NADP-ME | 76.09 ± 1.99A' | 11.71 ± 0.2A' | 79.43 ± 2.91A' | 2.6 ± 0.15A' | 110.29 ± 1.83A' |
| Setaria italica | 64.3d | 9.62a | 91.8d | 1.2b | 130.21d |
| Sorghum bicolor | 77.64b | 10.65b | 115.3c | 1.56d | 117.34e |
| Zea mays | 86.34b | 14.86e | 31.2b | 4.99e | 83.33b |
| NAD-ME | 54.07 ± 1.92B' | 11.99 ± 0.26B' | 83.27 ± 0.87B' | 1.31 ± 0.02B' | 107.21 ± 2.88B' |
| Panicum miliaceum | 44.33e | 11.23b | 83.36e | 1.25b | 118.56f |
| Panicum sumatrense | 44.58f | 11.33b | 86.8f | 1.3b | 126.97g |
| Eleusine coracana | 73.29b | 13.42f | 79.7g | 1.37b | 76.27h |
Within columns, values followed by the same case letter are not significantly different according to Tukey’s honesty significant difference (HSD) test (P<0.05).
Bold-face values indicate significant difference among the mean values of species representing genus Oryza.
Table 2: Comparison of vein parameters among C3 and C4 species.
There have been several studies focused on the leaf vasculature of C3 and C4 grasses [4,12,14]. In a extensive study on leaf vasculature system in C3 and C4 grasses [4] reported a trend similar to the trend recorded in the present study where a 1.1, 2.2 and 1.9 times higher value for distance between large, small and transverse veins of C4 species were measured compared to C3 plants. Furthermore, Ueno et al. [4] had reported a 4.3 and 2.1 times higher value for areolar area and total vein length per unit leaf area in C4 plants.
All the other vein parameters except distance between TV and total vein length per unit leaf area were similar among O. nivara and the other varieties of O. sativa . However, values obtained for these two parameters of O. nivara and Zea mays (C4 grass) were not significantly different. Thus, the present study has been figured out for some vein parameters there is similar tendency between O. nivara and C4 species where it was not so for the species among same genus Oryza . The results obtained have further shown that the significantly low mean value of distance between transverse veins (41.75 μm) of O. nivara has led to a significantly high total vein length per unit leaf area of 81.77 μm-1.
The C4 photosynthetic carbon cycle is an elaborated addition to the C3 photosynthetic pathway. In C4 plants the CO2 concentration mechanism is achieved by division of labor between two distinct, specialized leaf cell types, the mesophyll and the bundle sheath cells [22]. Thus, bundle sheaths play a major role in evolution of C3 pathway to C4. Christin et al. [14], in a study of quantitative bundle sheath anatomy has shown that C4 evolvability strongly increases when the proportion of vascular bundle sheath tissue is higher than 15%, which results from a combination of short distance between bundle sheaths and large bundle sheath cells. Indicating large bundle sheath structure of C4 species, the present study has found 15.9 percent increment of bundle sheath thickness in C4 plants (Table 3), a similar trend that has been recorded in previous studies as well [12,14,17].
| Bundle sheath distance (µm) | Outer sheath distance (µm) | Inner sheath distance (µm) | Outer sheath area (µm2) | Inner sheath area (µm2) | |
|---|---|---|---|---|---|
| C3 | 8.9 ± 0.03A | 6.21 ± 1.02A | 3.7 ± 0.11A | 29.6 ± 2.92A | 10.87 ± 1.11A |
| Oryza sativa | |||||
| BG 403 | 6.13a | 5.2a | 2.2a | 19.63a | 3.14a |
| BG 357 | 6.23a | 5.1a | 2.5a | 19.63a | 4.9a |
| BG 94-1 | 8.2a | 5.3a | 3.4c | 19.63a | 7.07c |
| Oryza nivara | 13.1b | 8.22b | 5.22b | 50.24b | 19.63b |
| Isachne globosa | 11.2b | 7.23b | 5.12b | 38.47b | 19.63b |
| C4 | 10.4 ± 0.01B | 7.15 ± 0.03B | 4.32 ± 0.72B | 44.7 ± 1.12B | 12.95 ± 2.12B |
| NADP-ME | 12.1 ± 1.21A' | 8.76 ± 0.72A' | 4.4 ± 0.23A' | 65.8 ± 2.01A' | 15.2 ± 3.02A' |
| Setaria italica | 9.84c | 5.78a | 3.09a | 26.41a | 7.64a |
| Sorghum bicolor | 12.0d | 8.1b | 4.3c | 50.24b | 12.56c |
| Zea mays | 14.3e | 12.41d | 5.7b | 120.7e | 25.5b |
| NAD-ME | 8.81 ± 0.89B' | 5.54 ± 0.71B' | 3.9 ± 0.51B' | 23.6 ± 1.51B' | 10.7 ± 2.81B' |
| Panicum miliaceum | 8.1c | 5.22a | 4.2c | 19.625a | 12.56c |
| Panicum sumatrense | 8.21a | 4.3c | 3.1a | 12.56c | 7.07a |
| Eleusine coracana | 10.11b | 7.1b | 4.41c | 38.47d | 12.56c |
Within columns, values followed by the same case letter are not significantly different according to Tukey’s honesty significant difference (HSD) test (P<0.05). Bold-face values indicate significant difference among the mean values of species representing genus Oryza
Table 3: Comparison of bundle sheath distances and area among C3 and C4 species.
Among the few representatives of genus Oryza , O. nivara showed a significantly high value for the Oryza nivara and other Oryza varieties, a few C4 species had shown no significant difference for the respective parameters (Table 3). Furthermore, a similar trend has been observed in mean values of outer sheath and inner sheath areas. Bundle sheath distance (13.1 μm), outer sheath distance (8.22 μm) and inner sheath distance (5.22 μm). While mean values of bundle sheath, outer sheath and inner sheath distances were significantly different.
Stomatal characters vary predictably between C3 and C4 species attributing to their photosynthetic pathway [17]. Among the C3 and C4 representatives, C4 plants have achieved low stomatal conductance by low density of smaller stomata [2]. The present research has clearly demonstrated a significant difference in stomatal density and index between C3 and C4 species (Table 4).
| Species name | Stomatal index | Stomatal density (µm-2) |
|---|---|---|
| Oryza sativa | ||
| BG 403 | 0.0203a | 1100a |
| BG 357 | 0.0302a | 1000a |
| BG 94-1 | 0.023a | 1210a |
| Oryza nivara | 0.0404e | 1150a |
| Panicum miliaceum | 0.1521b | 310d |
| Panicum sumatrense | 0.1727c | 260c |
| Sorghum bicolor | 0.1353b | 270c |
| Eleusine coracana | 0.1432b | 100e |
| Zea mays | 0.1493b | 150b |
| Isachne globosa | 0.1016d | 140b |
| Setaria italica | 0.161c | 160b |
Within columns, values followed by the same case letters are not significantly different according to Tukey’s honesty significant difference (HSD) test (P<0.05).
Bold-face values indicate significant difference among the mean values of species representing genus Oryza.
Table 4: Comparison of stomatal index and stomatal density of C3 and C4 grasses.
Among the C3 and C4 species, genus Oryza had the highest density of stomata (1000-1210 μm-2). While there have been several studies that explain the theory behind this phenomenon, Kawamitsu et al. [23] has reported rice plants growing in wet places may have high evaporative demand (due to high density of stomata) in order to lower the surface temperature of the leaf on a hot dry day. On the other hand this may lead to intense transpiration from the leaf blade resulting in water stress conditions in the plant. It has been reported that in some paddy fields rice plants are subjected to water stress at mid-day even with adequate supply of water due to high density of stomata [4].
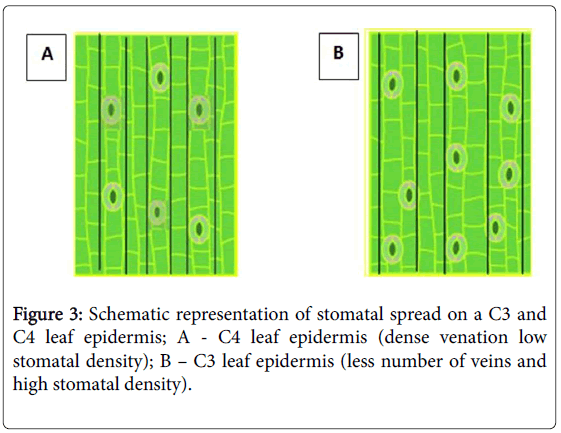
Furthermore, it has been identified that high density of veins and the shortest distance between them have resulted in low stomatal density in C4 plants (Figure 3).
Unlike the bundle sheath anatomy and venation, there were no records for similar stomatal density values among O. nivara and C4 species. Thus, it has been found that these significant changes of Oryza nivara leaf anatomy have only been due to its bundle sheath anatomy and venation.
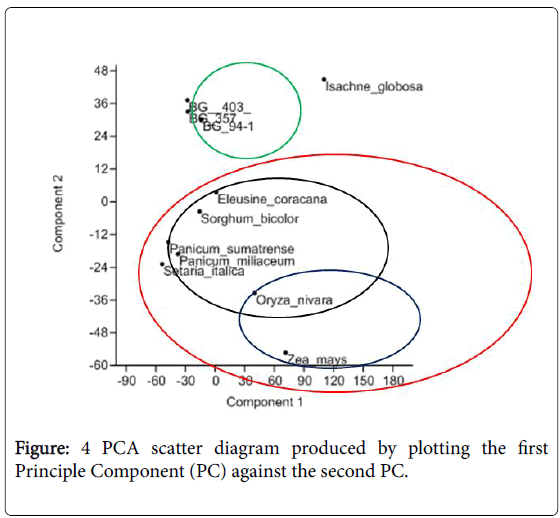
From the principal component analysis (PCA) a clear association of C3 and C4 species (Figure 4) is seen. Most weighted characters that had contributed to the main principle components were distance between large longitudinal veins, distance between small longitudinal veins and outer sheath distance. Reflecting the differences of traits associated to the leaf anatomy, all the varieties of Oryza sativa (BG 403, BG 357 and BG 94-1) clustered together while Oryza nivara has clustered with the other C4 species.
In the classification of grasses, genus Oryza is placed within the BEP clade, one of the major clades that contain only C3 origins [24]. During the last year, percentage increment of annual rice yield has been recorded as 0.9%. Further, it is expected that the world rice inventories might drop by 3% in 2017, which if confirmed, would be the second consecutive season of declines [25]. Thus, there is a strong evidence that yield potential in rice (Oryza sativa L.) is becoming limited by ‘source’ capacity (photosynthetic capacity or efficiency), and hence the ability to further increase the yield in modern varieties [26]. Thus, among different approaches in developing enhanced photosynthetic capacity of rice, introducing C4 pathway has become one of the focal points and challenges [3]. Thus, screening selected C3 and C4 grasses, in the context of understanding developed characteristic features of C4 species is important to lay a good foundation in manipulating the anatomy and the physiology of the rice leaf.
The current study has revealed that anatomical features like venation and bundle sheaths of Oryza nivara have contributed to its improved leaf anatomy; so called “C4 tendency”. This, as mentioned before can be considered as one of the important facts that might open up new insights in to engineering the rice leaf. Oryza nivara is one of the common and widely distributed wild Oryza species found in Sri Lanka. It is generally found in shallow water and swampy areas. These plants have shown resistance to different biotypes of brown plant hoppers, green leaf hopper, grassy stunt virus and blast and partial resistance to stem rot [27]. The study of Rizal et al. [28] has reported several accessions of Oryza nivara with low CO2 compensation points, a developed physiological tendency compared with the rest of C3 species. Further, several studies have focused on Oryza nivara where the plant been used in breeding strategies to enhance the yield of Oryza sativa [29,30]. But, no records have been found related to the anatomical traits of this species. Thus, this paper mainly emphasizes the developed leaf anatomy of this species showing a potential “C4- ness”.
Conclusion
Based on the results of this study, it can be concluded that C4 grasses have denser vein system, large bundle sheaths and low stomatal density compared to C3 grasses that were used in this study. In addition, observations suggest that some characteristic features of C4 leaf anatomy may have evolved in Oryza nivara resulting it to be considered as C3-C4 intermediate. Furthermore, it is also suggest that possession of C4 leaf anatomical features in Oryza nivara could be used as a source of germplasm to bring about a change in the leaf anatomy of Oryza sativa that would contribute towards“C4-ness” and subsequently enhancing the photosynthetic efficiency of rice.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
Conceived and designed the experiments: AJ, IC, HNW. Performed the experiments: HNW. Analyzed the data: HNW. Wrote or proofread the paper: AJ, IC, HNW. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Dr. A. Bentota, Director General, RRDI, Bathalagoda, Sri Lanka, Dr. K. Hettiarachchi, Director, Seed Certification & Plant Protection Centre, Gannoruwa, Sri Lanka and Prof. B. Marambe for their support given.
References
- Tanaka A, Makino A (2004) Photosynthetic research in plant science. Plant Cell Physiol 50: 681-683.
- Sage RF (2004) The evolution of Photosynthesis. New Phytol 161: 341-370.
- Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155: 56-63.
- Ueno O, Kawano Y, Wakayama M, Takeda T (2006) Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. Ann Bot 97: 611-621.
- Hatch M (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta- Rev Bioenerg 895: 81-106.
- Takeda T, Fukuyama M (1971) Studies on the photosynthesis of the Gramineae. 1. Differences in photosynthesis among subfamilies and their relations with the systematics of the Gramineae. Proc Crop Science Soc Jpn 40: 12-20.
- Wakayama M (2003) Photosynthetic Enzyme Accumulation during Leaf Development of Arundinella hirta, a C4 Grass Having Kranz Cells not Associated with Veins. Plant Cell Physiol 44: 1330-1340.
- Ehleringer J, Monson R (1993) Evolutionary and Ecological Aspects of Photosynthetic Pathway Variation. Annu Rev Ecol Syst 24: 411-439.
- Altus D, Canny M (1982) Loading of Assimilates in Wheat Leaves. I. The Specialization of Vein Types for Separate Activities. Aust J Plant Physiol 9: 571-581.
- Crookston R, Moss D (1974) Interveinal Distance for Carbohydrate Transport in Leaves of C3 and C4 Grasses1. Crop Sci 14: 123-125.
- Kawamitsu Y, Hakoyama S, Agata W, Takeda T (1985) Leaf interveinal distances corresponding to anatomical types in grasses. Plant Cell Physiol 26: 589–593.
- Dengler NG, Dengler RE, Donnelly PM, Hattersley PW (1994) Quantitative Leaf Anatomy of C3 and C4 Grasses (Poaceae): Bundle Sheath and Mesophyll Surface Area Relationships. Ann Bot 73: 241-255.
- Oguro H, Hinata K, Tsunoda S (1985) Comparative Anatomy and Morphology of Leaves between C3 and C4 Species in Panicum. Ann Bot 55: 859-867.
- Christin P, Osborne C, Chatelet D, Columbus J, Besnard, G, et al. (2013) Anatomical enablers and the evolution of C4photosynthesis in grasses. Proc Nat Acad Sci 110: 1381-1386.
- Grass Phylogeny Working Group II (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193: 304-312.
- Liu H, Osborne C (2014) Water relations traits of C4 grasses depend on phylogenetic lineage, photosynthetic pathway, and habitat water availability. J Exp Bot 66: 761-773.
- Way DA (2012) What lies between: the evolution of stomatal traits on the road to C4 photosynthesis. New Phytol 193: 291-293.
- Taylor S, Hulme S, Rees M, Ripley B, Ian Woodward F et al. (2010) Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytol 185: 780-791.
- Ueno O (1995) Occurrence of distinctive cells in leaves of C4 species in Arthraxon and Microstegium (Andropogoneae-Poaceae) and the structural and immunocytochemical characterization of these cells. Int J Plant Sci 156: 270–289.
- Chonan N, Kawahara H, Matsuda T (1974) Morphology on Vascular Bundles of Leaves in Gramineous Crops : I. Observations on vascular bundles of leaf blades, sheaths and internodes in rice plants. Jpn J Crop Sci 43: 425-432.
- Gitz D, Baker J (2009) Methods for Creating Stomatal Impressions Directly onto Archivable Slides. Agronomy Journal 101: 232-236.
- Edwards E, Osborne C, Stromberg C, Smith S, Bond W, et al. (2010) The Origins of C4 Grasslands: Integrating Evolutionary and Ecosystem Science. Science 328: 587-591.
- Kawamitsu Y, Agata W, Hiyane S, Murayama S, Nose A, et al. (1996) Relation between Leaf Gas Exchange Rate and Stomate. I. Stomatal frequency and guard cell length in C3 and C4 grass species. Jpn J Crop Sci 65: 626-633.
- Kellogg E (2001) Evolutionary History of the Grasses. Plant Physiol 125: 1198-1205.
- Food and Agricultural Organization of the United Nations. (2016). Rice Market Monitor 11: 1-26.
- Furbank R, Von Caemmerer S, Sheehy J, Edwards G (2009) C4rice: a challenge for plant phenomics. Functional Plant Biol 36: 845-856.
- Madurangi S, Samarasinghe W, Senanayake S, Hemachandra P, Ratnasekera D (2011) Resistance of Oryza nivara and Oryza eichingeri derived lines to brown planthopper, Nilaparvata lugens (Stal). J Nat Sci Found S Lanka 39: 175-181.
- Rizal G, Karki S, Thakur V, Chatterjee J, A Coe R, et al. (2012) Towards a C4 Rice. Asian J Cell Biol 7: 13-31.
- Gaikwad K, Singh N, Bhatia D, Kaur R, Bains N, et al. (2014) Yield-Enhancing Heterotic QTL Transferred from Wild Species to Cultivated Rice Oryza sativa L. PLOS ONE 9: e96939.
- Swamy B, Kaladhar K, Shobha Rani N, Prasad G, Viraktamath B, et al. (2012) QTL Analysis for Grain Quality Traits in 2 BC2F2 Populations Derived from Crosses between Oryza sativa cv Swarna and 2 Accessions of O. nivara. J Heredity 103: 442-452.
Citation: Weerasooriya HA, Jayasekera A, Caldera I (2018) Tendencies towards a C4 Leaf: Quantitative Studies on Leaf Anatomy of Selected C3 and C4 Grasses. J Rice Res 6: 188. DOI: 10.4172/2375-4338.1000188
Copyright: © 2018 Weerasooriya HN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11386
- [From(publication date): 0-2018 - Aug 25, 2025]
- Breakdown by view type
- HTML page views: 10306
- PDF downloads: 1080