The Implantable Miniature Telescope and Quality of Life Improvements in Patients with End-Stage Age-Related Macular Degeneration
Received: 09-Oct-2017 / Accepted Date: 12-Oct-2017 / Published Date: 16-Oct-2017 DOI: 10.4172/2161-0711.1000562
Abstract
Age-related macular degeneration (AMD) is a progressive, degenerative eye disease that leads to central vision loss in people older than 60. End-stage AMD, for which there is no cure, is the leading cause of blindness in highincome countries. Often underappreciated by physicians, AMD is an important public health problem as patients can experience significant emotional distress, reduced cognitive function and a decrease in quality of life from depression, isolation, reduced mobility, and independence. These patients are more likely to suffer from falls and injuries, which may result in serious, life-threatening complications and significant medical costs. There are no medical interventions that can halt the visual outcome of end-stage AMD; patients can only make the best of the vision they have left through low-vision rehabilitation and external devices and tools. In 2010, the U.S. Food and Drug Administration approved the implantable miniature telescope (IMT; VisionCare Ophthalmic Technologies, Saratoga, CA) prosthesis for severely visually impaired patients with bilateral end-stage AMD. Patients with no other treatment options may have improved quality of life after implantation due to visual gains, increased mobility and independence, and improved overall function.
Keywords: Age-related macular degeneration; Implantable miniature telescope; Low vision; Vision rehabilitation; Legal blindness; Depression; Falls
Introduction
Age-related macular degeneration (AMD) is a chronic, progressive, degenerative eye disease that leads to central vision loss and primarily affects people older than age 60 [1,2]. End-stage AMD is the leading cause of blindness in high-income countries. Approximately 1.75 million Americans are living with the advanced stages of the disease, and that number is projected to increase to nearly 3 million by 2020 [3,4]. With an overall global prevalence of 8.69%, about 228 million people will be diagnosed with AMD in 2040 [5].
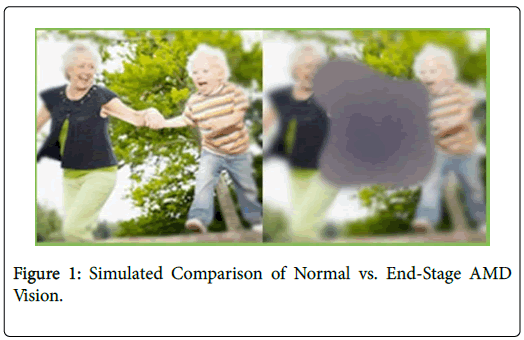
Patients with early- and intermediate-stage AMD can have mild to moderate visual acuity loss; however, patients with advanced-stage AMD typically develop severe vision loss (Figure 1) and can have a number of symptoms depending on the type of AMD they develop: neovascular or non-exudative (wet and dry, respectively). Wet AMD symptoms include spots in a patient’s central vision or complaints that straight lines appear wavy and distorted, greatly inhibiting the patient’s ability to read. Dry AMD symptoms include washed-out colors and difficulty seeing fine details. Objects in the patient’s central vision may appear distorted and faded. Approximately 80% of the people diagnosed with AMD will have the dry or atrophic subtypes, which accounts for about 20% of legal blindness (defined as 20/200 or worse in the better-seeing eye, best corrected with standard glasses or contacts) [3,6-9].
There is no known cure for AMD, but pharmacologic and laser treatments may help slow disease progression and temporarily restore some of the vision lost [10-15]. Visual gains from these interventions are difficult to maintain, however, and, anatomically, there remain patients who will progress to severe vision loss [16]. Pharmacologic treatments can be costly, entail ongoing vitreous cavity injections, may not be effective in all patients, and may result in a slow regression over the long-term [16]. Learning to live with low vision through rehabilitation and use of external devices and tools is often the only choice that remains for patients with advanced AMD.
The disease impacts quality of life (QoL), as patients often incur falls and injuries, depression, suicidal thoughts, stress, and feelings of social isolation [17]. There is a significant need for a treatment for advanced AMD that enhances patient QoL through improved everyday function. In 2010, the U.S. Food and Drug Administration approved the first prosthesis for severely visually impaired patients with bilateral endstage AMD, the implantable miniature telescope (IMT; VisionCare Ophthalmic Technologies, Saratoga, CA) [18,19]. The device was designed to allow patients to regain everyday functional abilities (e.g., reading, writing, and watching TV) and greater independence [20]. The IMT is currently the only treatment approved for patients with end-stage AMD in the United States. This paper discusses the QoL implications for patients with advanced AMD, as well as the current treatment and management options.
Quality of life
Advanced AMD is severely debilitating to patients, leading to reduced independence and significant emotional distress [21]. Patients with moderate vision loss from advanced AMD have a 32% decrease in QoL, which is similar to what patients experience after breaking a hip [22]. As the disease progresses, patients report a 60% decrease in QoL, which equates to the experience of someone with end-stage cancer who requires around-the-clock care. Despite this evidence, physicians often understate the impact end-stage AMD has on patients. Brown et al. for example, found that AMD patients have a reduced QoL ranging from 96% to 750% greater than what their eye care provider estimates [22].
Depression
It is well known that depression diminishes QoL [23-26]. It is the fourth major cause of disability worldwide [27] and a common side effect in people with vision loss [28-33]. Multiple studies have found close to a 30% prevalence rate of depression among elderly patients with advanced AMD, [27,34] and these patients are at an increased risk of suicide [35].
Patients with AMD struggle to read, eat, watch TV, recognize faces, and participate in hobbies and activities that they used to enjoy, often resulting in a feeling of hopelessness and clinical depression [17]. Many patients with AMD are legally blind and therefore cannot drive, which adds to social isolation and dependence on caregivers. Vision loss robs people of the joy of living, prevents them from pursuing pleasurable activities and engaging in their surroundings, and diminishes their self-esteem, autonomy, and perceived value to society [36,37].
Elderly patients with depression and end-stage AMD report extreme dissatisfaction in a variety of social and functional activities as compared with patients with either disorder independently, suggesting that the combination has a particularly negative impact on QoL [38]. Depression has been reported to exacerbate AMD symptoms, [39] causing many AMD patients to believe that their vision is worse than it is [33]. Patients with vision loss and depression often report challenges such as difficulty traveling to the clinic for appointments, trouble communicating with staff once there, and difficulty retaining the information provided [38]. Combined, these challenges and negative outlook lead to noncompliance, loss to follow-up, and worse outcomes overall [33].
In elderly patients, visual impairment is associated with increased cognitive decline [40,41]. A South Korean study of 170 AMD patients and 190 non-AMD community-based controls showed that visuospatial function, verbal memory, visual memory, and frontal function were impaired in AMD patients vs. controls. Mild cognitive impairment was also higher in AMD patients than in controls (52.4% vs. 26.8%; P<0.001), with the greatest cognitive impairment in those patients with poor visual acuity (≤ 20/100) [40].
Because depression has such a profound impact on QoL and AMD treatment and outcomes, it is now suggested that healthcare providers screen for depression among their elderly and vision-impaired patients. Relatively easy screening tests can be performed in-office in about 5 minute [32]. Once depression has been identified, supportive care is needed. Problem-solving therapy may help restore self-esteem by concentrating on the positive, or what the patient can do rather than what they cannot [42]. A self-management program consisting of health education and problem-solving skills may improve mood and self-efficiency and reduce emotional distress [26]. Furthermore, integrated mental health and low-vision intervention has been shown alleviate depressive disorders in patients with AMD by as much as half compared with low-vision rehabilitation alone [43].
The treatment options for patients with advanced AMD are limited and often unsuccessful long term, [16] therefore addressing the patient’s depression may have the biggest impact on overall QoL. Healthcare providers that recognize and evaluate depression symptoms in their patients can provide supportive care, and encourage patients to seek professional psychological help in concurrence with low-vision rehabilitation when appropriate.
Falls
Falls are the leading cause of injury-related death in the elderly [44] and can cause debilitating, often life-threating complications such as fractures, joint dislocations, and head and internal organ injuries that may incur significant medical costs in both lengthy hospital stays and nursing home admission [45].
Vision impairment is a well-known risk factor for falls and hip fractures in the older population, [46-49] and the risk is higher in patients with AMD than without [50]. AMD causes visual function loss, reduced contrast sensitivity, and difficultly viewing straight lines, all of which increases the risk of slips, stumbles, loss of balance, and falls caused by the patient’s inability to identify environmental hazards [51-53]. The Beaver Dam Study showed a correlation between poor visual acuity (20/25 or worse) and an increased risk of falls. The odds ratios for two or more falls in the past year for patients with poor vision were 2.02 (95% CI, 1.13–3.63) for current binocular acuity and 1.85 (95% CI, 1.10–3.12) for visual sensitivity [52]. Wood et al. found that 74% of patients with AMD reported sustaining a fall or non-fallrelated injury (e.g., lacerations or collisions with an object), while 30% of patients reported more than one fall [54].
Fear of falling is also higher in patients with AMD than without, which leads to anxiety, decreased mobility (e.g., walking, getting up and sitting down), and increased frailty [52,53,55]. Patients with AMD who fear falling are less likely to leave the house, which compounds social isolation and depressive symptoms. West et al. found that for every line of visual acuity lost, the odds for mobility limitations increased by 10%. Similarly, every 10% change in visual field equated to a 20% increase in the chance for mobility limitations [56].
Because falls and fear of falling have such a negative impact on QoL in patients with AMD, it becomes imperative to recognize those patients at high risk. Reduced contrast sensitivity has been shown to be the strongest predictor of falls and other injuries in patients with AMD [54] Melillo et al. developed a classification tree to help ophthalmologists and optometrists identify patients at high-risk of falling within 1 year [57]. Once high-risk patients have been identified, healthcare professionals should refer patients to rehabilitation specialists for guidance on interventions and modifiable risk factors such as exercise [58,59], video game technology [59,60], appropriate footwear, floor coverings, and hip protectors [61] to reduce the incidence of falls and the risk of injury.
End-stage amd
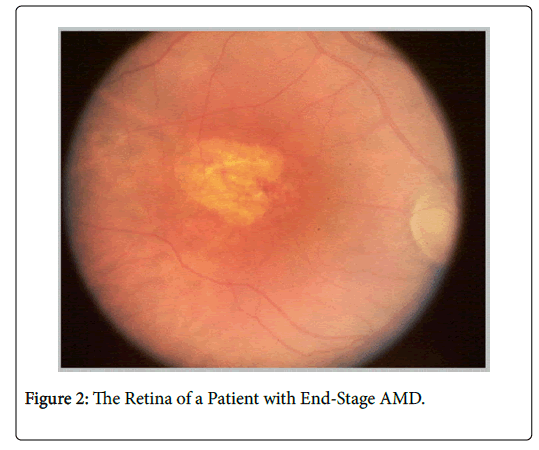
End-stage AMD is defined as moderate (≤ 20/80) to profound (20/600 or worse) vision impairment [62]. It is the most advanced form of AMD and is the leading cause of blindness in people over age 60 in high-income countries [1,2]. Patients with end-stage AMD experience central vision loss of and blurry peripheral vision. Figure 2 depicts the retina of a patient with end-stage AMD. These patients are most affected by diminished QoL and have difficulty with even the most simplistic of daily activities that rely upon vision.
Patients with end-stage AMD can maximize the impact of their remaining vision and learn how to use it to the best of their ability through low-vision training and vision rehabilitation. Low-vision specialists have shown some success in helping patients learn how to shift visual fields from central to peripheral vision. This specialized training has also led to a resurgence in the need for this subspecialty in the eye care field [63].
Current amd treatment and management landscape
The goal of AMD treatment is to preserve the patients’ remaining vision and prevent further visual decline. Pharmacologic treatment with intravitreal anti-vascular endothelial growth factor (VEGF) injections have become the mainstay of treatment for early- to intermediate-stage AMD; studies have found this treatment modality can reduce the odds of legal blindness from wet AMD by up to 70% over 2 years [36]. Unfortunately, the initial visual improvement is unlikely to be sustained over the long-term as the disease continues to progress, with a majority of patients losing those initial gains within 7 years (even with ongoing treatment adherence) [13]. Further, more than half of patients do not show signs of visual improvement with anti-VEGF therapy, and approximately 10% of patients do not respond at all to treatment [37]. There are currently no pharmacolgic treatments approved in the United States for end-stage AMD.
Laser treatments are used in some wet AMD cases, but their invasive nature may inadvertently destroy surrounding healthy tissue that exacerbates vision loss [64]. Laser treatments are predominantly used to slow the progression of the disorder rather than restore vision.
Low-vision rehabilitation
Low-vision specialists, along with a multidisciplinary team including other vision rehabilitation professionals, occupational therapists, and orientation and mobility specialists, are the primary caregivers for people with advanced vision loss. Low-vision rehabilitation can improve the QoL for patients with advanced AMD by teaching them skills such as how to use magnifying and adaptive devices like the IMT, how to navigate safely around their home and in public, and how to perform daily activities including getting dressed, cooking, and reading.
Patient compliance with low-vision rehabilitation services varies widely, as some people do not have access to reliable transportation services and/or must rely upon family members for help [65]. Yet there is ample evidence that low-vision rehabilitation has substantial utility and value [66]. Both the LOVIT and LOVIT II trials examined the impact of low-vision rehabilitation on veterans, concluding that patients who underwent rehabilitation showed significant improvement in reading, mobility, vision information processing, and visual motor skills compared to those who did not undergo rehabilitation [67-70].
Furthermore, the VITAL trial found that integrated low-vision and mental health intervention halved the frequency of depressive disorders in high-risk patients with AMD (12.6% vs. 23.4%), [43] thereby illustrating that low-vision rehabilitation can serve as a preventative measure to reduce the overall incidence of depression in the visually impaired.
Low-vision devices
Low-vision devices can improve daily function for patients with advanced AMD and help reduce depression. Large print books, highpowered lenses, desk, stand/handheld magnifiers, and electronic assistive technology can assist with reading, while telescopes and virtual reality devices can make watching TV and other distance activities more enjoyable. Large-button phones and keyboards with solid, contrasting numbers can help reduce social isolation, and talking and/or large-numbered clocks can help visually impaired patients easily keep track of their day. Other innovative, electronic vision enhancement systems (head-mounted, desktop, or handheld) can also assist patients with everyday activities such as applying makeup, getting dressed, and cooking.
Small telescopic devices, such as the Keplerian and Galilean telescopes, can be mounted on eyeglasses, and others are connected to computers so the person can see the object on a large computer screen. Adaptive computer software can also help improve visual performance [63].
Although these devices can enhance vision and improve QoL, many patents find them cumbersome to use and cosmetically unappealing. In addition, low-vision devices can be quite costly as most third-party insurance plans don’t pay for them [71]. With some prices reaching several thousands of dollars, this places many higher tech devices out of reach for many patients. Even when able to secure, training on use and optimal success may be hindered by previously mentioned barriers such as decreased cognition and motivation, depression, physical disabilities, and lack of transportation to ancillary providers for proper training.
Surgical options
Surgical options for the treatment of AMD are limited, regardless of disease stage. Cataract surgery may be helpful for patients with cataracts and early- to intermediate-stage AMD and result in improved QoL [72,73]. Specialized intraocular lenses (IOLs) and implantable telescopes (IMT) may help patients with late-stage AMD make the best of the vision they have left. IOLs for AMD are different from the monofocal IOLs used post-cataract surgery. These IOLs either improve vision through magnification or redirect images to the patient’s preferred retinal locus (PRL), thereby creating a prismatic effect. Implantable telescopes typically use a Galilean- or Cassegrain-style approach to magnify images in the patient’s central vision, allowing use of healthier areas of the patient’s retina and minimizing the effects of the central scotoma.
The IMT
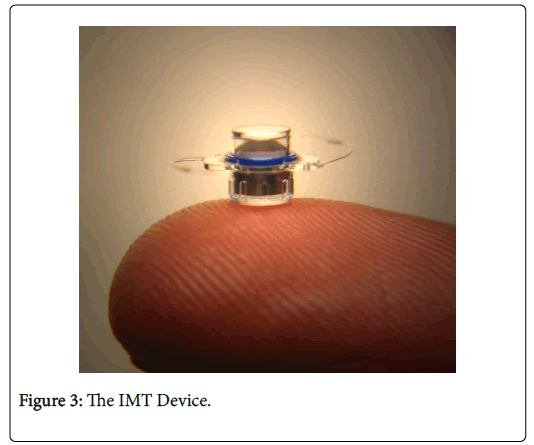
The IMT is about the size of a pea (Figure 3), and is a fixed-focus, monocular prosthetic telescope using ultraprecise, quartz glass, wideangle micro-optics. It is implanted in the anterior chamber of only one eye using standard procedures similar to cataract surgery, and it uses the eye’s natural movements to enhance vision by creating a wider field of view. The fellow eye is used for peripheral vision, while the IMTimplanted eye is used for central vision and details.
The device is available in two magnification powers: 2.2X and 2.7X, with full field view of 24° and 20°, respectively [74,75]. The 2.7X device is almost exclusively used in the United States, because 5-year data revealed more visual gains over time as disease progressed than with the 2.2X device [76]. The 2.2X model does have its advantages, however, providing more light and a larger field of view for patients with less advanced disease and less demanding visual goals
The IMT has been proven to safely provide end-stage AMD patients with clinically meaningful improvements in NEI VFQ-25 scores longterm (mean scores more than 7 points above baseline) [18-20,76]. Patients have experienced improved QoL and general vision (near and distance) as well as improved psychosocial vision-targeted subscales such as social functioning, mental health, and dependency. On average, IMT patients experienced a 12.5% QoL gain [77]. Long-term study data 60 months post-implantation revealed that 47.4% of patients maintained a 3-line gain in vision, while 61.8% maintained a 2-line gain [76]. Adverse events did occur, but were well within expectations, and included iris transillumination defects, iritis, posterior synechiae, and guttata [18].
The IMT has alleviated many of the issues facing patients with advanced AMD. Not all patients are qualified candidates for the device, and part of that lies with the patient’s dedication to a low-vision rehabilitation program as well as ensuring that the patient’s goals align with what the technology can offer. Patients must commit to a minimum of 6 low-vision rehabilitation visits in order to use the device effectively and adjust to the presence of diplopia, image size differences, and field-of-view restriction [20]. Patients will also require ongoing care under a vision rehabilitation specialist to monitor visual and functional status. Patients who are unwilling to commit to this level of care or who do not have reliable transportation and caregiver support are not optimal candidates for the implant.
Careful selection in choosing the eye for IMT implantation is also critical. The implanted eye should become the better-seeing eye, which will help patients achieve maximum visual gains. Potential candidates must be thoroughly vetted through a simulation test using an external telescope system administered by a qualified low-vision specialist, patient questionnaires, and dominance testing [20].
Other technologies
There are currently no other technologies approved for the treatment of end-stage AMD in the United States. A few investigational devices are in development outside of the United States (e.g., the iol-AMD, the IOL-VIP System, and the Lipshitz macular implant), but these devices do not provide the magnification of the IMT, [78-83] and QoL improvements are not uniform or verified.
Conclusion
Age-related macular degeneration is a chronic, debilitating eye disease affecting older and elderly patients and has a profound, often underappreciated impact on patient QoL. Patients may frequently experience reduced cognition, depression, social isolation, reduced independence and mobility, and a greater chance of falls and injuries with potentially catastrophic results. Because there is no cure, improving patient QoL should be the goal for healthcare professionals across disciplines. It becomes imperative for those physicians and providers caring for the elderly to understand the ancillary effects of vision loss, especially from AMD, and to have a general understanding of treatment and management options to improve QoL in their patients. The IMT can be an option to enhance QoL in patients with end-stage AMD for which no other medical or surgical option exists. Many patients can regain independence with improved visual function for a variety of activities.
Acknowledgements
VisionCare Ophthalmic Technologies supported the publication of this article.
References
- Rosenfeld PJ MA, Tennant MTS (2009) Age-related macular degeneration. In: Yanoff M DJ, Ophthalmology (3rd edn): Elsevier.
- Wang JJ, Mitchell P, Smith W, Cumming RG (1998) Bilateral involvement by age related maculopathy lesions in a population. Br J Ophthalmol 82: 743-747.
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. (2004) Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 122: 564-572.
- Bressler NM, Bressler SB, Congdon NG, Ferris FL, Friedman DS, et al. (2003) Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol 121: 1621-1624.
- Wong CW, Wong TY, Cheung CM (2015) Polypoidalchoroidalvasculopathy in Asians. J Clin Med 4: 782-821.
- Kahn HA, Leibowitz HM, Ganley JP, Kini MM, Colton T, et al. (1977) The framinghameye study. I. outline and major prevalence findings. Am J Epidemiol 106: 17-32.
- American Academy of Ophthalmology Retina Panel (2015) Preferred practice pattern: Age-related macular degeneration. San Francisco, CA: AAO.
- Hageman GS, Gehrs KM, Johnson LV, Anderson DF (2008) Age-related macular degeneration. In: Kolb H, Fernandez EJ, Nelson RW, eds. Webvision: The organization of the retina and visual system. Salt Lake City, UT: University of Utah Health Sciences Center.
- Valckenberg SS, Sahel JA, Danis R, Fleckenstein M, Jaffe GJ, et al. (2016) Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology 123: 361-368.
- Singer M (2014) Advances in the management of macular degeneration. F1000Prime Rep 6: 29.
- Mu Y, Zhao M, Su G (2014) Stem cell-based therapies for age-related macular degeneration: Current status and prospects. Int J ClinExp Med 7: 3843-3852.
- Geltzer A, Turalba A, Vedula SS (2013) Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age-related macular degeneration. Cochrane Database Syst Rev 1: CD005022.
- Jager RD, Mieler WF, Miller JW (2008) Age-related macular degeneration. N Engl J Med 358: 2606-2617.
- Fernandez-Robredo P, Sancho A, Johnen S, Recalde S, Gama N, et al. (2014) Current treatment limitations in age-related macular degeneration and future approaches based on cell therapy and tissue engineering. J Ophthalmol 2014: 510285.
- Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, et al. (2014) Guidelines for the management of neovascular age-related macular degeneration by the European society of retina specialists (EURETINA). Br J Ophthalmol 98: 1144-1167.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, et al. (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 120: 2292-2299.
- Schmier JK, Halpern MT, Covert D, Delgado J, Sharma S (2006) Impact of visual impairment on use of caregiving by individuals with age-related macular degeneration. Retina 26: 1056-1062.
- Hudson HL, Lane SS, Heier JS, Stulting RD, Singerman L, et al. (2006) Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology 113: 1987-2001.
- Hudson HL, Stulting RD, Heier JS, Lane SS, Chang DF, et al. (2008) Implantable telescope for end-stage age-related macular degeneration: Long-term visual acuity and safety outcomes. Am J Ophthalmol 146: 664-673.
- Primo SA (2010) Implantable miniature telescope: Lessons learned. Optometry 81: 86-93.
- Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI (1998) The psychosocial impact of macular degeneration. Arch Ophthalmol 116: 514-520.
- Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, et al. (2005) The burden of age-related macular degeneration: A value-based medicine analysis. Trans Am OphthalmolSoc 103: 173-84; discussion 84-86.
- Slakter JS, Stur M (2005) Quality of life in patients with age-related macular degeneration: Impact of the condition and benefits of treatment. SurvOphthalmol 50: 263-273.
- Murray CJ, Lopez AD, Jamison DT (1994) The global burden of disease in 1990: Summary results, sensitivity analysis and future directions. Bull World Health Organ 72: 495-509.
- Wells KB, Sherbourne CD (1999) Functioning and utility for current health of patients with depression or chronic medical conditions in managed, primary care practices. Arch Gen Psychiatry 56: 897-904.
- Brody BL, Roch-Levecq AC, Gamst AC, Maclean K, Kaplan RM, et al. (2002) Self-management of age-related macular degeneration and quality of life: A randomized controlled trial. Arch Ophthalmol 120: 1477-1483.
- Augustin A, Sahel JA, Bandello F, Dardennes R, Maurel F, et al. (2007) Anxiety and depression prevalence rates in age-related macular degeneration. Invest Ophthalmol Vis Sci 48: 1498-1503.
- Casten RJ, Rovner BW, Tasman W (2004) Age-related macular degeneration and depression: A review of recent research. CurrOpinOphthalmol 15: 181-183.
- Paz SH, Globe DR, Wu J, Azen SP, Varma R, et al. (2003) Relationship between self-reported depression and self-reported visual function in Latinos. Arch Ophthalmol 121: 1021-1027.
- Bazargan M, Hamm-Baugh VP (1995) The relationship between chronic illness and depression in a community of urban black elderly persons. J Gerontol B PsycholSciSocSci 50: 119-127.
- Boland MV, Chang DS, Frazier T, Plyler R, Friedman DS (2014) Electronic monitoring to assess adherence with once-daily glaucoma medications and risk factors for nonadherence: The automated dosing reminder study. JAMA Ophthalmol 132:838-844.
- Lee AG, Beaver HA, Jogerst G, Daly JM (2003) Screening elderly patients in an outpatient ophthalmology clinic for dementia, depression, and functional impairment. Ophthalmology 110: 651-657.
- Owsley C, McGwin G (2004) Depression and the 25-item National Eye Institute Visual Function Questionnaire in older adults. Ophthalmology 111: 2259-2264.
- Brody BL, Gamst AC, Williams RA, Smith AR, Lau PW, et al. (2001) Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology 108: 1893-900; discussion 900-901.
- Waern M, Rubenowitz E, Runeson B, Skoog I, Wilhelmson K, et al. (2002) Burden of illness and suicide in elderly people: Case-control study. Bmj 324: 1355.
- Heckhausen J, Schulz R (1995) A life-span theory of control. Psychol Rev 102: 284-304.
- Rovner BW, Casten RJ (2002) Activity loss and depression in age-related macular degeneration. Am J Geriatr Psychiatry 10: 305-310.
- Jones GC, Rovner BW, Crews JE, Danielson ML (2009) Effects of depressive symptoms on health behavior practices among older adults with vision loss. RehabilPsychol 54: 164-172.
- Rovner BW, Casten RJ, Tasman WS (2002) Effect of depression on vision function in age-related macular degeneration. Arch Ophthalmol 120: 1041-1044.
- Woo SJ, Park KH, Ahn J, Choe JY, Jeong H, et al (2010) Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology 119: 2094-20101.
- Chen SP, Bhattacharya J, Pershing S (2017) Association of vision loss with cognition in older adults. JAMA Ophthalmol 135: 963-970.
- Barrett JE, Williams JW, Jr., Oxman TE, Katon W, Frank E, et al. (1999) The treatment effectiveness project. A comparison of the effectiveness of paroxetine, problem-solving therapy, and placebo in the treatment of minor depression and dysthymia in primary care patients: Background and research plan. Gen Hosp Psychiatry 21: 260-273.
- Rovner BW, Casten RJ, Hegel MT, Massof RW, Leiby BE, et al. (2014) Low vision depression prevention trial in age-related macular degeneration: A randomized clinical trial. Ophthalmology 121: 2204-2211.
- Centers for Disease Control and Prevention (CDC) (2006) Fatalities and injuries from falls among older adults--United States, 1993-2003 and 2001-2005. MMWR Morb Mortal Wkly Rep 55: 1221-1224.
- Lilley JM, Arie T, Chilvers CE (1995) Accidents involving older people: a review of the literature. Age Ageing 24: 346-365.
- Anastasopoulos E, Yu F, Coleman AL (2006) Age-related macular degeneration is associated with an increased risk of hip fractures in the Medicare database. Am J Ophthalmol 142: 1081-1083.
- Abdelhafiz AH, Austin CA (2003) Visual factors should be assessed in older people presenting with falls or hip fracture. Age Ageing 32: 26-30.
- Chong EW, Wang Y, Robman LD, Aung KZ, Makeyeva GA, et al. (2015) Age related macular degeneration and total hip replacement due to osteoarthritis or fracture: Melbourne collaborative cohort study. PLoS One 10: 1371.
- Ivers RQ (2000) Visual impairment and hip fracture. American Journal of Epidemiology 152: 633-639.
- Klein BE, Klein R, Lee KE, Cruickshanks KJ (1998) Performance-based and self-assessed measures of visual function as related to history of falls, hip fractures, and measured gait time. The beaver dam eye study. Ophthalmology 105: 160-164.
- de Boer MR, Pluijm SM, Lips P, Moll AC, Völker-Dieben HJ, et al. (2004) Different aspects of visual impairment as risk factors for falls and fractures in older men and women. J Bone Miner Res 19: 1539-1547.
- Klein BE, Moss SE, Klein R, Lee KE, Cruickshanks KJ (2003) Associations of visual function with physical outcomes and limitations 5 years later in an older population: The Beaver Dam eye study. Ophthalmology 110: 644-650.
- Reed-Jones RJ, Solis GR, Lawson KA, Loya AM, Cude-Islas D, et al. (2013) Vision and falls: A multidisciplinary review of the contributions of visual impairment to falls among older adults. Maturitas 75: 22-28.
- Wood JM, Lacherez P, Black AA, Cole MH, Boon MY, et al. (2011) Risk of falls, injurious falls, and other injuries resulting from visual impairment among older adults with age-related macular degeneration. Invest Ophthalmol Vis Sci 52: 5088-5092.
- van Landingham SW, Massof RW, Chan E, Friedman DS, Ramulu PY (2014) Fear of falling in age-related macular degeneration. BMC Ophthalmol 14: 10.
- West CG, Gildengorin G, Haegerstrom-Portnoy G, Schneck ME, Lott L, et al. (2002) Is vision function related to physical functional ability in older adults? J Am GeriatrSoc 50: 136-145.
- Melillo P, Orrico A, Chirico F, Pecchia L, Rossi S, et al. (2017) Identifying fallers among ophthalmic patients using classification tree methodology. PLoS One 12: e0174083.
- Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: A meta-analytic study. PsycholSci 14: 125-130.
- Franco JR, Jacobs K, Inzerillo C, Kluzik J (2012) The effect of the NintendoWiifit and exercise in improving balance and quality of life in community dwelling elders. Technol Health Care 20: 95-115.
- Reed-Jones RJ, Dorgo S, Hitchings MK, Bader JO (2012) Vision and agility training in community dwelling older adults: Incorporating visual training into programs for fall prevention. Gait Posture 35: 585-589.
- Stern C (2014) Hip protectors for preventing hip fractures in older people. OrthopNurs 33: 297.
- Colenbrander A (2002) Visual standards: Aspects and ranges of vision loss with emphasis on population surveys. Sydney, Australia: International Council of Ophthalmology.
- Agarwal A, Rhoades WR, Hanout M, Soliman MK, Sarwar S, et al. (2015) Management of neovascular age-related macular degeneration: Current state-of-the-art care for optimizing visual outcomes and therapies in development. ClinOphthalmol 9: 1001-1015.
- Morris B, Imrie F, Armbrecht AM, Dhillon B (2007) Age-related macular degeneration and recent developments: New hope for old eyes? Postgrad Med J 83: 301-307.
- Chan TL, Goldstein JE, Massof RW (2013) Comparison of clinician-predicted to measured low vision outcomes. Optom Vis Sci 90: 776-787.
- Goldstein JE, Jackson ML, Fox SM, Deremeik JT, Massof RW, et al. (2015) Clinically meaningful rehabilitation outcomes of low vision patients served by outpatient clinical centers. JAMA Ophthalmol 133: 762-769.
- Stelmack JA, Tang XC, Reda DJ, Moran D, Rinne S, et al. (2007) The veterans affairs low vision intervention trial (LOVIT): design and methodology. Clin Trials 4: 650-660.
- Stelmack JA, Tang XC, Reda DJ, Rinne S, Mancil RM, et al. (2008) Outcomes of the veterans affairs low vision intervention trial (LOVIT). Arch Ophthalmol 126: 608-617.
- Stelmack JA, Tang XC, Reda DJ, Stroupe KT, Rinne S, et al. (2012) VA LOVIT II: A protocol to compare low vision rehabilitation and basic low vision. Ophthalmic Physiol Opt 32: 461-471.
- Stelmack JA, Tang XC, Wei Y, Wilcox DT, Morand T, et al. (2016) Outcomes of the veterans affairs low vision intervention trial II (LOVIT II): A randomized clinical trial. JAMA Ophthalmol.
- Morse AR, Massof RW, Cole RG, Mogk LG, O'Hearn AM, et al. (2010) Medicare coverage for vision assistive equipment. Arch Ophthalmol 128: 1350-1357.
- Shuttleworth GN, Luhishi EA, Harrad RA (1998) Do patients with age related maculopathy and cataract benefit from cataract surgery? Br J Ophthalmol 82: 611-616.
- Pham TQ, Cugati S, Rochtchina E, Mitchell P, Maloof A, et al. (2007) Age-related maculopathy and cataract surgery outcomes: Visual acuity and health-related quality of life. Eye (Lond) 21: 324-330.
- Peli E (2002) The optical functional advantages of an intraocular low-vision telescope. Optom Vis Sci 79: 225-233.
- Grzybowski A, Wasinska-Borowiec W, Alio JL, Amat-Peral P, Tabernero J (2017) Intraocular lenses in age-related macular degeneration. Graefes Arch ClinExpOphthalmol255: 1687–1696.
- Boyer D, Freund KB, Regillo C, Levy MH, Garg S (2015) Long-term (60-month) results for the implantable miniature telescope: efficacy and safety outcomes stratified by age in patients with end-stage age-related macular degeneration. ClinOphthalmol 9: 1099-1107.
- Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS, et al. (2011) Comparative effectiveness and cost-effectiveness of the implantable miniature telescope. Ophthalmology 118: 1834-1843.
- Hengerer FH, Artal P, Kohnen T, Conrad-Hengerer I (2015) Initial clinical results of a new telescopic iOLimplanted in patients with dry age-related macular degeneration. J Refract Surg 31: 158-162.
- Orzalesi N, Pierrottet CO, Zenoni S, Savaresi C (2007) The IOL-Vip System: A double intraocular lens implant for visual rehabilitation of patients with macular disease. Ophthalmology 114: 860-865.
- Qureshi MA, Robbie SJ, Tabernero J, Artal P (2015) Injectable intraocular telescope: Pilot study. J Cataract Refract Surg 41: 2125-2135.
- Singer MA, Amir N, Herro A, Porbandarwalla SS, Pollard J (2012) Improving quality of life in patients with end-stage age-related macular degeneration: Focus on miniature ocular implants. ClinOphthalmol 6: 33-39.
- Tabernero J, Artal P (2012) Optical modeling of a corneal inlay in real eyes to increase depth of focus: Optimum centration and residual defocus. J Cataract Refract Surg 38: 270-277.
- Agarwal A, Lipshitz I, Jacob S, Lamba M, Tiwari R, et al. (2008) Mirror telescopic intraocular lens for age-related macular degeneration: Design and preliminary clinical results of the Lipshitz macular implant. J Cataract Refract Surg 34: 87-94.
Citation: Primo SA (2017) The Implantable Miniature Telescope and Quality of Life Improvements in Patients with End-Stage Age-Related Macular Degeneration . J Community Med Health Educ 7: 562. DOI: 10.4172/2161-0711.1000562
Copyright: © 2017 Primo SA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7225
- [From(publication date): 0-2017 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 6173
- PDF downloads: 1052