The Potential of Statins for Buccal Delivery
Received: 24-Feb-2014 / Accepted Date: 14-Mar-2014 / Published Date: 24-Mar-2014 DOI: 10.4172/2329-9053.1000111
Abstract
Statins minimize the endogenous synthesis of cholesterol preventing the onset and development of artherosclerosis and are therefore used as an effective treatment against primary hypercholesterolemia. Statins do exhibit antiinflammatory, vasculoprotective, and antioxidant effects. The promising delivery of statins across the buccal mucosa poses a continuing challenge as well as a great opportunity. Buccal delivery has progressed far beyond the use of traditional dosage forms with novel approaches emerging continuously. Buccal drug delivery system in which drug enters directly in systemic circulation thereby by passing the first pass effect. The buccal route has been researched for a wide variety of drugs and has gained significant attention and momentum since it offers remarkable advantages. Buccal adhesive systems offer innumerable advantages in terms of accessibility, administration and withdrawal, retentivity, enhanced bioavailability, avoid first pass effect. At the current global scenario, scientists are finding ways to develop buccal adhesive systems through various approaches to improve the bioavailability of antihyperlipidemic drugs by manipulation of the formulation strategies like inclusion of pH modifiers, enzyme inhibitors, permeation enhances. This review highlights the buccal delivery approaches of statins.
Keywords: Statins; Buccal; Absorption; Mucoadhesive; Polymers
1356Introduction
Several drugs are currently available for the treatment of hyperlipidemia, but the most potent agents are known as 3-hydroxy- 3-methylglutaryl-coenzyme (HMG CoA reductase) inhibitors or statins. Statins like Atorvastatin, fluvastatin, lovastatin, Pravastatin, Simvastatin and Rosuvastatin are effective in modifying low-density lipoproteins (LDL), high-density lipoproteins (HDL), total cholesterol (TC) and triglycerides (TG) levels. All agents lower LDL in a dose dependent manner, approximately 20-38% with initial doses and 35- 61% with maximal doses [1]. Statins act by competitively inhibiting HMG-CoA reductase. On a molecular level, statins are similar to HMGCoA occupying the place of HMG-CoA in the enzyme and thereby reduce the rate of mevalonate production which in turn reduces the cholesterol production, as well as a number of other compounds via several mechanisms.
Simvastatin appears to have the ability to reduce low-density lipoprotein cholesterol (LDC-C) and increase HDL cholesterol (HDL-C) to a greater degree than the other approved statins providing a 63% LDL-C reduction at a dose of 40 mg [2]. Simvastatin is also considered as a stimulator for bone formation [3]. Statins are widely prescribed as cholesterol-lowering therapy, and are considered firstline therapeutic agents for the prevention of coronary heart disease and atherosclerotic disorders related to hypercholesterolemia [4]. Statins also have immunomodulatory, neuroprotective and antiinflammatory properties that are being explored for potential benefits in central nervous system disorders [5,6]. Statins may also be used to reduce mortality and neurological disability from stroke and reduce the incidence of dementia, although the latter is controversial [7,8]. All statins, both fungal metabolites and synthetic compounds, reduce the coronary heart disease similarly when adjusted for differences in lipid changes [9].
Statins are categorized based on their origin, hydrophilicity/hyrophobicity and specificity. Lovastatin, pravastatin and simvastatin are all obtained by fungal fermentation while atorvastatin, fluvastatin, rosuvastatin and cerivastatin (withdrawn from the market in 2001) are entirely synthetic [10].
Statins exhibit multiple non lipid-lowering actions or “pleiotropic” effects. In addition to cholesterol lowering, they also exhibit other beneficial effects like vasodilation, stabilization of plaque, thrombogenesis inhibition, oxidative stress attenuation, and inflammation reduction. The indications of different statins are listed in Table 1 [11].
| Indication | Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Rosuvastatin | Simvastatin |
|---|---|---|---|---|---|---|
| Primary Hypercholesterolemia | + | + | + | + | + | + |
| Mixed dyslipidemia | + | + | - | + | + | + |
| Hypertriglyceridemia | + | - | - | + | + | + |
| Primary Dysbetalipoproteinemia | + | - | - | + | - | + |
| Homozygous familial Hyperlipidemia | + | - | - | - | + | + |
| Primary prevention Coronary events | - | - | + | + | - | + |
| Secondary prevention Cardiovascular event(s) | - | + | + | + | - | + |
Table 1: Indications of statins.
Statins And Efflux Proteins
The bio-availability of statins is low due to the affinity towards efflux proteins and is substrates for multiple efflux transporters. Statins including pitavastatin, pravastatin, rosuvastatin and cerivastatin are substrates for OATP-C (OATP1B1), a transporting polypeptide expressed exclusively in the basolateral membrane of hepatocytes. Atorvastatin, cerivastatin and simvastatin have been reported to be pgp substrates. Pravastatin is MRP2 substrate while rosuvastatin is transported efficiently by BCRP in membrane vesicles and suggest that BCRP (ABCG2) may play a significant role in disposition of rosuvastatin [12]. Lovastatin and simvastatin interact with p-glycoprotein (ABCB1) while rosuvastatin, pravastatin and cerivastatin interact with ABCG2 (Table 2).
| Drug | Bioavailability (%) | First pass effect | Efflux Protein |
|---|---|---|---|
| Atorvastatin | 14 | CYP3A4 | ABCB1 |
| Fluvastatin | 24 | CYP2C9 | ABCG2 |
| Lovastatin | 5 | CYP3A4 | ABCB1 |
| Pravastatin | 17 | CYP3A5 | MRP2 |
| Simvastatin | 5 | CYP3A4 | ABCB1 |
| Rosuvastatin | 20 | CYP2C9 | ABCG2 |
Table 2: Pharmacokinetics of Statins.
Oral cavity is an attractive site for the delivery of drugs. It is possible through this route to realize mucosal (local effect) and transmucosal (systemic effect) drug administration to achieve a site-specific release of the drug on the mucosa and drug absorption through the mucosal barrier to reach the systemic circulation. The oral mucosa is highly vascularized and presents a reduced enzymatic activity with respect to nasal, intestinal and rectal mucosa and is less sensitive to damage and irritation than the nasal epithelium. The transmucosal route utilizes sublingual and buccal mucosa as absorption sites with two different therapeutic goals. In particular, the sublingual route is generally employed for the delivery of drugs characterized by high permeability across the mucosa and used in the treatment of acute disorders, whereas the buccal route is generally used in the treatment of chronic disorders when a prolonged release of the active substance is required. Although the sublingual route has been extensively investigated in the past, the interest on buccal drug delivery is comparatively more recent and coincides with the advances in biotechnology that have made peptides readily available for therapeutic use. The interest in novel routes of drug administration occurs from their ability to enhance the bioavailability of drugs impaired by the narrow absorption window in the gastrointestinal tract. Delivery of drugs via the buccal route using bioadhesive dosage forms offers such a novel route of drug administration. Many of the statins cause adverse effects (AEs) like neuropathy, cognitive loss, sexual dysfunction and pancreatic and hepatic dysfunction [13]. Statins are usually taken in one daily dose in the evening, to coincide with cholesterol synthesis, which is at its peak in the early morning hours. Patients should take this class of medication with or without food. It is recommended to take lovastatin with meals while fluvastatin, simvastatin, pravastatin and atorvastatin may be taken without regard to meals. Problems such as high first-pass metabolism, multi drug resistant (MDR) proteins and drug degradation in the gastrointestinal environment observed with many of the statins can be circumvented by administering via the buccal route. Moreover, buccal drug delivery offers a safe and easy method since the absorption of drug can be easily terminated in cases of toxicity by removing the dosage form from the buccal cavity immediately. Bilayered buccal mucoadhesive approach can overcome other drawbacks like loss of drug resulting from wash out with saliva to the GIT by applying the impermeable bilayer. Therefore, adhesive mucosal dosage forms for statins are suggested for buccal delivery, including adhesive gels, adhesive patches and adhesive tablets.
Mucoadhesive drug delivery systems
These drug delivery systems utilize the property of bioadhesion of certain water soluble polymers which become adhesive on hydration and hence can be used for targeting a drug to a particular region of the body for extended periods of time [14]. The mucosal layer lines a number of regions of the body including gastrointestinal tract, urogenital tract, airway, ear, nose and eye. These represent potential sites for attachment of any bioadhesive system and hence, the mucoadhesive drug delivery system such as Nasal delivery system, Oral delivery system, Vaginal delivery system, Ocular delivery system, Buccal delivery system and Rectal delivery system (Table 3).
| PARAMETER | Gastro-intestinal | Dermal | Nasal | Oral mucosa | Vaginal |
|---|---|---|---|---|---|
| Accessibility | + | +++ | ++ | ++ | + |
| Surface area | +++ | +++ | + | ++ | +++ |
| Surface environment | + | ++ | ++ | +++ | + |
| Permeability | +++ | + | +++ | ++ | +++ |
| Reactivity | ++ | ++ | + | +++ | ++ |
| Vascular drainage | +++ | + | +++ | ++ | +++ |
| First pass clearance | + | +++ | +++ | +++ | + |
| Patient acceptability | ++ | +++ | ++ | +++ | ++ |
Table 3: Comparison of different routes of drug delivery.
Buccal drug delivery was introduced by Orabase in 1947 when gum tragacanth is mixed with dental adhesive powder to supply penicillin to the oral mucosa [15]. Buccal delivery of drugs provides an alternative to the oral drug administration, mainly in overcoming deficiencies associated with the latter mode of dosing. Buccal mucosa consisting of stratified squamous epithelium supported by a connective tissue lamina propia [16] is investigated as a site for drug delivery several decades ago and the interest in this area for the trasmucosal drug administration is still growing with time.
Advantages of statins for buccal delivery
a. Direct access to the systemic circulation through the internal jugular vein prevents drugs from the hepatic first pass metabolism leading to high bioavailability.
b. Easy drug withdrawal, Low enzymatic activity, painless administration, suitability for drugs or excipients that mildly and reversibly damages or irritates the mucosa and facility to include permeation.
c. Among the various transmucosal routes, buccal mucosa has, relatively immobile mucosa, excellent accessibility and presence of smooth muscle, hence suitable for administration of retentive dosage forms [17].
For drugs with half-lives of several hours and wide therapeutic indices, maintaining therapeutic levels is not a major problem. In order to maintain therapeutic levels of such short half-life at very frequent intervals, obviously, retaining a delivery system at the oral mucosa for a period of several hours or more can be major problem, due to challenges such as mastication, beverages, salivary flow, speech and ingestion of food.
Buccal mucosa
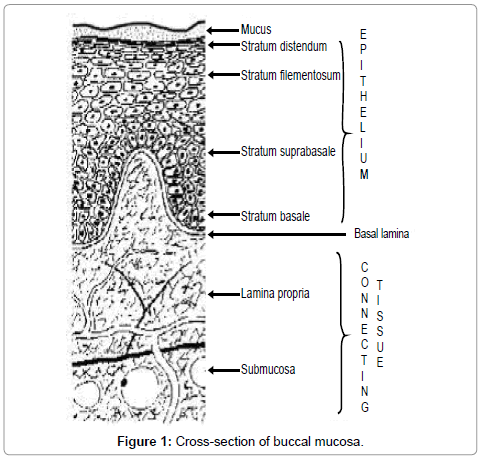
Buccal region is that part of the mouth bounded posteriorly and medially by the teeth and/or gums, anteriorly and laterally by the lips and the cheeks, and above and below by the reflections of the mucosa from the lips and cheeks to the gums [18]. Maxillary artery supplies blood to buccal mucosa and blood flow is faster and richer (2.4 ml/ min/cm2) than that in the gingival, palatal and sublingual regions, thus facilitating passive diffusion of drug molecules across the mucosa. The thickness of the buccal mucosa is measured to be 500–800 μm and is rough textured, hence suitable for retentive delivery systems. The turnover time for the buccal epithelium has been estimated at 5–6 days. Buccal mucosa composed of several layers of different cells as shown in Figure 1. The epithelium is similar to stratified squamous epithelia found in rest of the body and is about 40–50 cell layers thick.
Lining epithelium of buccal mucosa is the nonkeratinized stratified squamous epithelium that has thickness of approximately 500–600 μm and surface area of 50.2 cm2 (Table 4).
| Mucosa | Human | Porcine |
|---|---|---|
| Buccal | 500-600 | 772 |
| Sublingual | 100-200 | 192 |
| Gingival | 200 | 208 |
| palatal | 200 | N.A |
Table 4: Study on oral epithelium (thickness in µm).
Lamina propria, Basement membrane followed by the submucosa is present below the epithelial layer. Lamina propria is rich with blood vessels and capillaries that open in the internal jugular vein. Lipid analysis of buccal tissues shows the presence of ceramide NS at 0.72%, glucosphingolipid of 23.0% and phospholipid of 76.3%. The primary function of buccal epithelium is the protection of the underlying tissue. In nonkeratinized regions, lipid-based permeability barriers in the outer epithelial layers protect the underlying tissues against fluid loss and entry of potentially harmful environmental agents such as microbial toxins, carcinogens, antigens and enzymes from foods and beverages.
Absorption pathways
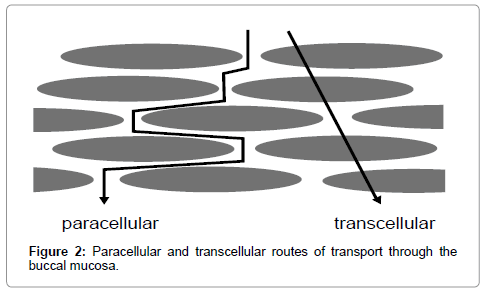
Studies with microscopically visible tracers such as small proteins and dextrans suggest that the major pathway across stratified epithelium of large molecules is via the intercellular spaces and that there is a barrier to penetration as a result of modifications to the intercellular substance in the superficial layers. However, rate of penetration varies depending on the physicochemical properties of the molecule and the type of tissue being traversed. This has led to the suggestion that materials uses one or more of the following routes simultaneously to cross the barrier region in the process of absorption, but one route is predominant over the other depending on the physicochemical properties of the diffusant [19].
a. Passive diffusion occurs in two ways
1. Transcellular or intracellular route (crossing the cell membrane and entering the cell)
2. Paracellular or intercellular route (passing between the cells)
b. Carrier mediated transport
c. Endocytosis
The existence of hydrophilic and lipophilic regions in the oral mucosa has lead the majority of researchers to postulate the existence of two routes of drug transport through the buccal mucosa i.e; Paracellular and Transcellular. This is analogous to the two routes of transport through intestinal epithelium, as is shown in Figure 2 [20].
In very few cases, absorption also takes place by the process of endocytosis where the drug molecules are engulfed by the cells. It is unlikely that active transport processes operate within the oral mucosa.
Permeability of buccal mucosa and drug permeation
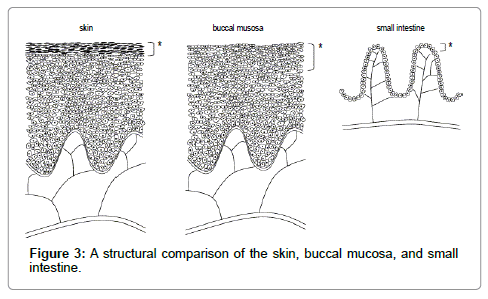
The permeability of the buccal mucosa is greater than that of the skin, but less than that of the intestine [18]. This does not only result from the greater surface area provided by the small intestine, but also from the structural differences between each of the tissues, as demonstrated in Figure 3. Based on epithelial structure alone, it is not surprising that the simple columnar epithelium covering the small intestine provides less resistance to drug transfer than the stratified squamous epithelium covering the skin and buccal mucosa [21].
Unlike the skin, and other keratinized regions of the oral cavity (such as the gingiva and palate), the epithelium lining the buccal mucosa lacks a cornified surface. The superficial cells of the non-keratinized buccal mucosa retain their nuclei and somecytoplasmic function, and are surrounded by a cross-linked protein envelope [16-21].
Pharmaceutical considerations for formulation design
The critical factors to be considered in the design of buccal formulations are penetration, drug release, organoleptic factors, effect of additives on drug release, local drug irritation and great care need to be exercised to develop safe and effective buccal adhesive drug delivery device.
Buccal adhesive polymers
The important physicochemical features of bioadhesive polymers include hydrophilicity, flexibility for interpenetration with mucus and epithelial tissue and visco-elastic properties [22]. The main components constituting the mucosa include water and mucin (an anionic polyelectrolyte), while the other components include proteins, lipids and mucopolysaccharides. Water and mucin constitute >99% of the total composition of the mucus and out of this >95% is water. The gel-like structure of the mucus can be attributed to the intermolecular entanglements of the mucin glycoproteins along with the non-covalent interactions (e.g. hydrogen, electrostatic and hydrophobic bonds) which results in the formation of a hydrated gel-like structure and explains the viscoelastic nature of the mucus. Hydrophobic bonds are formed due to the interaction of the non-polar groups when the polymers are dispersed in an aqueous solution. Freeze-thawing of polyvinyl alcohol solution in water exhibits this kind of interaction. Ionic bonds are formed due to the electrostatic interactions amongst the polymers (e.g. instantaneous formation of gelled structure when alginate and chitosan dispersions in water are mixed) while covalent bonds are formed due to the sharing of electrons amongst the atoms (e.g. crosslinking reaction amongst genipin and amino groups). Of the various cellulose derivates, sodium carboxymethyl cellulose has been found to have excellent ocular mucoadhesive property. Cationic cellulose derivatives (e.g. cationic hydroxyethyl celluloses) have been used in conjunction with various anionic polymers (Table 5) for the development of sustained delivery systems.
| Type of polymer | Examples |
|---|---|
| Cationic polymers | Aminodextran, chitosan, DEAE, trimethylated chitosan. |
| Anionic polymers | Chitosan EDTA, CMC, Sodium CMC, sodium alginate, Xanthum gum, pectin, carbopol. |
| Non-ionic polymers | Hydroxyethyl starch, HPMC, PVA, PVP, polyethylene oxide |
Table 5: Types of polymers.
Ionic polymers: The commonly used ionic polymers used in mucoadhesive delivery system include chitosan, xanthum gum and cationic guar gum. Chitosan provides an excellent example of cationic polyelectrolyte, which has been extensively used for developing mucoadhesive polymer due to its good biocompatibility and biodegradable properties. Chitosan undergoes electrostatic interactions with the negatively charged mucin chains thereby exhibiting mucoadhesive property. Xanthan gum (XG) is a polysaccharide having polyanionic properties due to carboxylic groups and has very good bioadhesive strength. Anionic polyelectrolytes, e.g. poly (acrylic acid) and carboxymethyl cellulose, have been extensively used for designing mucoadhesive delivery systems due to their ability to exhibit strong hydrogen bonding with the mucin present in the mucosal layer. Cationic guar gum (CGG) is a modified guar gum in which hydroxyl groups are replaced with trimethyl ammonium groups. The introduction of trimethyl ammonium groups imparts cationic character to the gum. Due to ammonium groups, it carries a net positive charge and can be easily cross-linked with other anions. Xanthan gum (XG) is a polysaccharide having polyanionic properties due to carboxylic groups and has excellent bioadhesive strength. However, despite their biodegradable character neither CGG nor XG can be used alone to formulate a buccoadhesive formulation as both possess highly acidic or alkaline pH due to the presence of anionic or cationic groups, respectively.
Non-ionic polymers: The non-ionic polymers include poloxamer, hydroxyl propyl methyl cellulose, methyl cellulose, poly vinyl alcohol, polyvinyl pyrrolidone and carbopol 934. The ionic polymers may be used to develop ionic complex with the counter-ionic drug molecules. The non ionic polymers along with permeation enchancers are also used to enchance the permeability of the drug through mucosal layer. Carrageenan gum, Pluronic F 127 and PVP K30 significantly influence the invitro mucoadhesive strength which reveals no buccal mucosal damage [23]. Higher mucoadhesive property of chitosan- TBA conjugate reveal good permeation properties and sustained action of fluvastatin which is observed on combination of chitosan TBA conjugate with xanthum gum and tamrind gum [24]. The buccal bilayered tablets of fluvastatin are developed using chitosan, tamarind gum, xanthum gum, gellan gum. The mucoadhesive properties of the optimized formulations are evaluated using time based and force based technique which shows maximum detachment force [25]. Chitosan is a polycationic copolymer and thiolated chitosans are being more advantageous due to their high mucoadhesiveness and extended drug release properties [26]. The bilayer tablets of simvastatin sustained release tablets are developed using wet granulation method using hydrophilic and hydrophobic polymers and evaluated [27]. The buccoadhesive bilayered tablet of simvastatin is developed and evaluated using carbopol 934, HPMC mucoadhesive polymers. The carbopol 934 mucoadhesive polymer shows good bioadhesive strength [28].
Physiological considerations
The physiological considerations such as texture, its turn over time thickness of the mucus layer and effect of saliva are to be considered while designing buccal delivery systems. Saliva secretions contain moderate levels of esterases, carbohydrates, phosphotases and peptidases like aminopeptidase A, B and N are found in buccal tissue which act as a barrier for drug penetration thus having a negative impact on dissolution, bioavailability and drug degradation. The development of unidirectional release systems with backing layer results high drug bioavailability.
Formulation considerations
The partition coefficient of the drugs has a vital role in drug absorption. Lipophilic and hydrophilic drugs are absorbed through the transcellular and paracellular routes respectively. Modification of drug chemically may increase drug penetration through buccal mucosa. Increasing nonionized fraction of ionizable drugs increases drug penetration through transcellular route. In weakly basic drugs, the decrease in pH increases the ionic fraction of drug but decreases its permeability through buccal mucosa. Residence time and local concentration of the drug in the mucosa, transportation of amount of drug across the mucosa into the blood are responsible factors for local or systemic drug delivery. Attempts are made to deliver drugs via buccal route like buprenorphine, testosterone, fentanyl, nifedipine and several peptides such as insulin, thyrotropin-releasing hormone and oxytocin.
The epithelium that lines the buccal mucosa is a very effective barrier and membrane permeation is the limiting factor for the absorption of drugs. Permeation enhancers are chemicals that facilitate the permeation. The low flux which is the major disadvantage of buccal drug delivery results in low drug bioavailability, and it can be increased by the incorporation of the penetration enhancers [29]. Irritation and toxicity are always concerned with penetration enhancers, though the oral mucosa is more resistant to damage than other mucosal membranes [30]. To date, the information available on buccal absorption enhancement is much less than that for transdermal enhancement. The relationship among structure, irritation, and enhancement effect of the enhancers are not clearly elucidated. Few penetration enhancers are available for buccal delivery systems and these agents are not used in marketed preparations and thus owing to the lack of a satisfactory profile with respect to irritation and effectiveness.
Developments in buccal drug delivery
Buccal mucoadhesive formulations may prove to be an alternative to the conventional oral medications as they can be readily attached to the buccal cavity which can be retained for a longer period and removed at any point of time. Buccal adhesive drug delivery systems using, films, layered systems, matrix tablets, discs, microspheres, ointments and hydrogel systems have been studied and reported by several research groups. There are numerous important considerations that include biocompatibility (both the drug/device and device/environment interfaces), permeability, reliability, durability; environmental stability, accuracy, delivery scalability and are to be considered while developing such formulations. Several buccal adhesive delivery devices were developed at the laboratory scale by many researchers either for local or systemic actions.
Buccal adhesive formulation approaches of statins
Tablets: Several bioadhesive tablet formulations were developed in recent years either for local or systemic drug delivery. Tablets that are placed directly onto the mucosal surface have been demonstrated to be excellent bioadhesive formulations. However, size is a limitation for tablets due to the requirement for the dosage form to have intimate contact with the mucosal surface. These tablets adhere to the buccal mucosa in presence of saliva. They are designed to release the drug unidirectionally targeting buccal mucosa. Novel buccal tablets are reported for the release of Lovastatin in unidirectional manner with improved bioavailability [31]. The mucoadhesive bilayer buccal tablets of pravastatin sodium are developed and results suggest that good permeation of pravastatin sodium is observed and buccal route can be one of the alternatives available for administration of pravastatin sodium [23]. Mucoadhesive buccal bilayered tablets of Rosuvastatin Calcium using natural gums are developed to impart mucoadhesion as well as permeability to the formulation. The results of suggested that buccal mucoadhesive tablet is potential way of delivering Rosuvastatin in order to prevent its extensive first pass metabolism and to improve its bioavailability [32].
Microparticles: Bioadhesive microparticles offer the same advantages as tablets but their physical properties like paricle size enable them to make intimate contact with a larger mucosal surface area, less local irritation at site of adhesion, uncomfortable sensation of a foreign object within the oral cavity is reduced .They can also be delivered to less accessible sites including the GI tract and upper nasal cavity. The solid lipid nanoparticles (SLNs) of simvastatin developed to enhance its oral bioavailability by minimizing its first-pass metabolism which was evaluated for biodistribution and pharmacokinetics by technetium-99m (Tc-99m) radiolabeling technique in mice [33].
Semi-solid dosage forms
Gels: Gel forming bioadhesive polymers include cross-linked polyacrylic acid that has been used to adhere to mucosal surfaces for extended periods of time and provide controlled release of drugs. Advantages of gel formulations include their ability to form intimate contact with the mucosal membrane and their rapid release of drug at the absorption site. A limitation of gel formulations lies on their in ability to deliver a measured dose of drug to the site. They are therefore of limited use for drugs with narrow therapeutic window [20].
Patches/films: Flexible films being advantageous over creams and ointments provide a measured dose of drug to the site of action and deliver drugs directly to a mucosal membrane. Canker sores, cold sores and lip sores are treated with Zilactin which is commercially available buccal adhesive film. Mucoadhesive sustained release bilayered buccal patch of pravastatin sodium using eudragit S100 was developed and the results suggested that mucoadhesive buccal patches circumvent the hepatic first pass metabolism, gastric instability and improved the bioavailability of pravastatin sodium [34].
Liquid dosage forms: The buccal mucosal surface usually coated with protectants or drug vehicles which are viscous liquids useful for drug delivery to the mucosal surface. Pharmaceutically biocompatible polymers improve the viscosity of the formulations to have retentive action in the oral cavity. To provide lubrication for the dry mouth it is usually treated with artificial saliva solution containing sodium CMC as bioadhesive polymer. Redispersible dry emulsion of lovastatin was developed and studied for the oral absorptive efficacy and intestinal stability which suggested that lovastatin dry emulsion reduced the metabolism in the small intestine and improved its oral absorption in rats [35]. Poly (amidoamine) dendrimers (PAMAM) based simvastatin (SMV) for controlled release formulations shows better pharmacokinetic performance than pure SMV suspension [36]. The solid microemulsion of simvastatin shows significant reduction in the cholesterol levels in hyperlipidemic rats with reference to control rats [37]. The dry adsorbed emulsion of simvastatin formulated using colloidal silicon dioxide suggests significant increase in HDL levels [38].
Commercial buccal adhesive drug delivery systems
Recent reports suggest that the market share of buccal adhesive drug delivery systems are increasing in the American and European market with the steady growth rate of above 10% with an increasing demand now in India. Some of the commercially available buccal adhesive formulations are listed in Table 6 [22].
| Brand name | Bioadhesive polymer | Company | Dosage form |
|---|---|---|---|
| Suscard | HPMC | Forest | Tablet |
| Gaviscon liquid | Sodium alginate | Rickitt Benckiser | Oral liquid |
| Orabase | Pectin, gelatin | ConvaTech | Oral paste |
| Corcodyl gel | HPMC | Glaxosmithkline | Oromucosal gel |
| Corlan pellets | Acacia | Celltech | Oromucosal pellets |
| Fentanyl Oralet™ | CP 934, Sodium CMC | Lexicomp | Lozenge |
| Miconaczole Lauriad | Modified starch, CP-934 | Bioalliance | Tablet |
Table 6: Commercially available buccal dosage forms.
Conclusion
Stains have wide variety of advantages in the treatment using buccal delivery systems. Many steps have been taken in this direction, but research must continue to provide ever better controls, improved efficacy and targeting better drug loading and lowering of the drug dose to diminish side effects, toxicity and enhance bioavailability. The future research on buccal delivery of statins with ionic or nonionic polymers having excellent mucoadhesive properties, biocompatibility and stability is very important to meet the patient needs.
References
- Hsu I, Spinler SA, Johnson NE (1995) Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother 29: 743-759.
- Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Müller WE, et al. (2007) Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem 101: 77-86.
- Jiang L, Sun H, Yuan A, Zhang K, Li D, Li C, et al. (2013) Enhancement of Osteoinduction by Continual Simvastatin Release from Poly (lactic-co-glycolic acid)-Hydroxyapatite Simvastatin Nano-Fibrous Scaffold. J Biomed Nanotechnol 9: 1921-1928.
- Vaughan CJ, Gotto AM Jr, Basson CT (2000) The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol 35: 1-10.
- Zacco A Togo J, Spence K, Ellis A, Lloyd D, et al. (2003) 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci 23: 11104-11111.
- Halcox JP, Deanfield JE (2004) Beyond the laboratory: clinical implications for statin pleiotropy. Circulation 109: II42-48.
- Li G, Larson EB, Sonnen JA, Shofer JB, Petrie EC, et al. (2007) Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 69: 878-885.
- Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD (2008) Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 71: 344–350.
- Goldenberg N, Glueck C (2009) Efficacy, effectiveness and real life goal attainment of statins in managing cardiovascular risk. Vasc Health Risk Manag 5: 369-376.
- Manzoni M, Rollini M (2002) Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl Microbiol Biotechnol 58: 555-564.
- Rikitake Y, Kawashima S, Takeshita S, Yamashita T, Azumi H, et al. (2001) Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 154: 87-96.
- Huang L, Wang Y, Grimm S (2006) ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos 34: 738-742.
- Golomb BA, Evans MA (2008) Statin adverse effects : a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs 8: 373-418.
- Desai KG, Kumar TM (2004) Preparation and evaluation of a novel buccal adhesive system. AAPS PharmSciTech 5: e35.
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK (2006) Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release 114: 15-40.
- Squier CA, Wertz PW (1996) Structure and function of the oral mucosa and implications for drug delivery. In: Oral Mucosal Delivery. Marcel Dekker, New York.
- Salamat-Miller N, Chittchang M, Johnston TP (2005) The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev 57: 1666-1691.
- Shojaei AH (1998) Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci 1: 15-30.
- Hao J, Heng PW (2003) Buccal delivery systems. Drug Dev Ind Pharm 29: 821-832.
- Bouckaert S, Schautteet H, Lefebvre RA, Remon JP, van Clooster R (1992) Comparison of salivary miconazole concentrations after administration of a bioadhesive slow-release buccal tablet and an oral gel. Eur J Clin Pharmacol 43: 137-140.
- Nicolazzo JA, Reed BL, Finnin BC (2005) Buccal penetration enhancers--how do they really work? J Control Release 105: 1-15.
- Batchelor H (2004) Novel bioadhesive formulations in drug delivery. The Drug Delivery Companies Report Autumn/Winter, Pharma Ventures Ltd. 17-21.
- Shidhaye SS, Thakkar PV, Dand NM, Kadam VJ (2010) Buccal drug delivery of pravastatin sodium. AAPS PharmSciTech 11: 416-424.
- Shah viral H, Shelat Pragna, Shah Gaurang B (2012) Design and evaluation of thiolated chitosan based mucoadhesive and permeation enhancing bilayered buccal drug delivery system. African Journal of Pharmacy and Pharmacology 6: 491-501.
- Shah Viral H, Shelat Pragna, Shah Gaurang (2012) Design, physicochemical characterization and pharmacokinetic evaluation of bilayered buccal tablets containing statin derivative. Research Journal of Pharmaceutical, Biological and Chemical Sciences 3: 227-239.
- Sreenivas SA, Pai KV (2008) Thiolated Chitosans: Novel Polymers for mucoadhesive Drug Delivery – A Review. Tropical Journal of Pharmaceutical Research 7: 1077-1088.
- Pankaj Jadhav, Ajay Samnani, Girijesh P, Dubey BK (2012) Formulation and evaluation of simvastatin sustained release bilayer tablet using hydrophillic and or hydrophobic polymers. World Journal of Pharmacy and Pharmaceutical Sciences 1: 621-632.
- Hiremath JG, Sarfaraz MD, Hiremath D, Sarudkar SA (2009) Preparation and Physicochemical characterization of simvastatin loaded mucoadhesive bilayered tablet. Indian Journal of Novel Drug Delivery 1: 18-24.
- Taylan B, Capan Y, Guven O, Kes S, Hincal AA (1996) Design and evaluation of sustained release and buccal adhesive propronolol hydrochloride tablets. Journal of Controlled Release 38: 11–20.
- Gandhi RB, Robinson JR (1994) Oral cavity as a site for bioadhesive drug delivery. Advanced Drug Delivery Reviews 13: 43–74.
- Doijad RC, Sompur CK, Goje AJ, Maske AP, Tambolif A (2011) Development and characterization of lovastatin controlled release buccoadhesive dosage form. International Journal of Pharma and Bio Sciences 3: 133-140.
- Panchal AV, Mehta M, Shah VH, Upadhyay U (2012) Formulation and in-vitro evaluation of mucoadhesive bilayered buccal tablets of rosuvastatin calcium. International journal of pharmaceutical science and research 3: 2733-2740.
- Shah M, Chuttani K, Mishra AK, Pathak K (2011) Oral solid compritol 888 ATO nanosuspension of simvastatin: optimization and biodistribution studies. Drug Dev Ind Pharm 37: 526-537.
- Maurya SK, Bali V, Pathak K (2012) Bilayered transmucosal drug delivery system of pravastatin sodium: statistical optimization, in vitro, ex vivo, in vivo and stability assessment. Drug Deliv 19: 45-57.
- Ge Z, Zhang XX, Gan L, Gan Y (2008) Redispersible, dry emulsion of lovastatin protects against intestinal metabolism and improves bioavailability. Acta Pharmacol Sin 29: 990-997.
- Kulhari H, Pooja D, Prajapati SK, Chauhan AS (2011) Performance evaluation of PAMAM dendrimer based simvastatin formulations. Int J Pharm 405: 203-209.
- Dixit RP, Nagarsenker MS (2010) Optimized microemulsions and solid microemulsion systems of simvastatin: characterization and in vivo evaluation. J Pharm Sci 99: 4892-4902.
- Dixit RP, Nagarsenker MS (2007) Dry adsorbed emulsion of simvastatin: optimization and in vivo advantage. Pharm Dev Technol 12: 495-504.
Citation: Kumar GP, Geethika R, Anusha T, Jaweria S, Prathyusha G (2014) The Potential of Statins for Buccal Delivery. J Mol Pharm Org Process Res 2: 111. DOI: 10.4172/2329-9053.1000111
Copyright: ©2014 Kumar GP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17456
- [From(publication date): 4-2014 - Jul 04, 2025]
- Breakdown by view type
- HTML page views: 12652
- PDF downloads: 4804