The Role of Caffeine and Doxapram for Respiratory Care in Preterm Infants: A Clinical Review
Received: 22-Jan-2018 / Accepted Date: 25-Jan-2018 / Published Date: 01-Feb-2018
Abstract
This review describes the clinical role of caffeine and doxapram as respiratory stimulants for preterm infants. Based on the current evidence caffeine citrate is the preferred drug for treatment of apnea of prematurity (AOP) and for the prevention of post-extubation respiratory failure in preterm infants. It has favorable short-and long-term effects including a reduced incidence of patient ductus arteriosus, bronchopulmonary dysplasia and improvements in neurodevelopmental outcome. Caffeine citrate is safe with currently recommended dosing, but further studies are warranted regarding the safety of caffeine when used immediately after birth and with high-dosing regimens. Doxapram has also been shown to be effective in the treatment of AOP and to reduce the need for intubation. Because of concerns about serious side effects it was less frequently used in the past. Despite encouraging results from recent studies, based on the limited number of large, randomized, controlled studies, doxapram is still not recommended for routine respiratory support in the NICU. It is a third-line or rescue therapy for preterm infants with severe AOP unresponsive to caffeine and NIV.
Keywords: &aوٴeine Doxapram; Non-invasive respiratory support; Preterm infants; Apnea of prematurity; Respiratory distress syndrome; Bronchopulmonary dysplasia
Abbreviations
MX: Methylxanthines;
NIV: Non-invasive Ventilation;
iMV: Invasive Mechanical Ventilation;
NCPAP: Nasal Continuous Airway Pressure;
NIPPV: Nasal Intermittent Positive Airway Pressure;
NICU: Neonatal Intensive Care Unit;
ELGAN: Extreme Low Gestational Age Neonate;
RDS: Respiratory Distress Syndrome;
BPD: Bronchopulmonary Dysplasia:
AOP: Apnea of Prematurity;
IVH: Intraventricluar Hemorrhage;
PDA: Persistent Ductus Arteriosus;
NEC: Necrotizing Entercolitis;
DOL: Day of Life;
LD: Loading Dose;
MD: Maintenance Dose;
LISA: Less Invasive Surfactant Administration;
INSURE: Intubate, Surfactant, Extubate;
ECG: Electrocardiogram;
BP: Blood Pressure;
RR: Respiratory Rate;
BG: Blood Gases;
US: Ultrasound;
Introduction
Strategies to minimize invasive mechanical ventilation (iMV), such as use of non-invasive ventilation (NIV) in combination with novel surfactant strategies (e.g. LISA: Less Invasive Surfactant Administration, INSURE: Intubate, Surfactant, Extubate) have gained increased popularity in the Neonatal Intensive Care Unit (NICU), especially in extremely low gestational age neonates (ELGANS <28 weeks` gestation) [1-4]. ELGANs are prone to breathing difficulties from surfactant deficiency, insufficient respiratory drive and bronchopulmonary dysplasia (BPD), associated with short-and longterm morbidity. In addition to ventilator strategies, which may not always be successful and provide a non-physiological positive airway pressure, drugs like caffeine or doxapram are used for respiratory support. Caffeine belongs to the group of methylxanthines (MX) (theophylline, aminophylline, and caffeine), a well-known class of drugs used in adult and pediatric patients to treat airway obstruction and to stimulate breathing. MX have been traditionally used in the treatment of apnea of prematurity (AOP) and for weaning from iMV in preterm infants with respiratory distress syndrome (RDS) [5-8]. Caffeine is the preferred respiratory stimulant for preterm infants and has several beneficial short-and long-term effects; it is one of the most commonly prescribed medications in the NICU. According to European consensus guidelines on the management of RDS in preterm infants caffeine should be used to facilitate weaning from iMV (high quality of evidence, strong recommendation) and early caffeine has been strongly recommended for all babies at high risk of needing iMV, such as those <1.250 g birth weight, maintained on NIV [9]. Doxapram is a respiratory stimulant which is less frequently used compared to MX. It is mainly reserved as rescue-therapy if caffeine and NIV are not successful [10]. There is still a wide variety related to timing and dosage of these pharmacological therapies. The purpose of this clinical review is to evaluate the role of caffeine and doxapram in the NICU in terms of clinical use, efficacy and safety profile based on more recently published studies in the literature.
Caffeine
Although having similar short-term effects on apnea/bradycardia, caffeine (1,3,7-trimethyxanthine) has several advantages over theophylline; it has fewer side effects and a longer elimination half-life of about 100 hours that allows single daily dosing. Therapeutic plasma concentrations range from 8 to 20 μg/ml, but are not measured routinely. Caffeine is prepared synthetically as caffeine citrate and given either intravenously (IV) or enterally (PO). Standard treatment includes a loading dose (LD) of 20 mg/kg caffeine citrate (equivalent to 10 mg/kg caffeine base) and a single daily maintenance dose (MD) of 5-10 mg/kg starting 24 hours after the initial LD [11,12]. A second LD (often coupled with an increased MD) may be given 24 hours after the initial dose, if frequent apnea and bradycardia continue. Caffeine, a nonselective adenosine receptor blocker, exerts its pharmacological effects by stimulating the vagal, and vasomotory respiratory centers in the medulla and increasing the sensitivity to carbon dioxide. In addition there are number of physiologic effects on pulmonary function (e.g. improvement in lung compliance airway resistance and/or increasing diaphragmatic muscle contractility) [7,9,13]. Caffeine may also have “non-respiratory effects” such as antiinflammatory properties [14]. In 2006 a large randomized, placebo controlled trial, the CAP (Caffeine for Apnea of Prematurity); Trial was published by Schmidt and co-authors [15]. This trial demonstrated that preterm infants with birthweight 500-1250 g who were treated with a standard dose regime of caffeine citrate at a mean age of 3 days of life (DOL) had significant less BPD (36.3% vs. 46.9%, p<0.001). The higher BPD prevalence in the placebo group was explained by longer exposure to positive airway pressure (on average for one week), considered a risk factor in development of BPD. In follow-up studies of this trial caffeine treated infants had improved rates of survival without neurodevelopmental disability at 18 to 21 months (reduced incidences of cerebral palsy and cognitive delay) and improvements in motor and visual function at 5 years of age without any adverse effects [16,17]. In a recently published 11-year-follow-up of the CAP-Trial, caffeine therapy was associated with a reduced risk of motor-impairment [18]. With these reports on improved short-and long-term outcomes suggesting a lung-and neuroprotective effect [19], caffeine gained increased popularity in the NICU.
However, the time when caffeine should be initiated and the optimal dosing are still a matter of debate. In a recently published systematic review and meta-analysis (14 studies, >60.000 participants) of clinical outcomes of early versus late caffeine therapy in preterm neonates, early caffeine (initiated at 2 hours to 3 DOL) reduced the risk of the primary outcome BPD up to 30%, in both randomized controlled trials and cohort studies [20]. In cohort studies there was also a reduced incidence of PDA, brain injury, retinopathy of prematurity and postnatal steroid use, but the mortality was increased (absolute risk of mortality with early caffeine therapy 4.7% vs. 3.9%). However, the evidence for these clinical outcomes was sparse and the increased mortality was partly explained by a survival bias. Since overall rates of survival of extremely preterm infants within 24h after birth are frequently low it was possible that infants in the early caffeine group had a higher risk of mortality. In summary, the systematic review and meta-analysis suggests that early caffeine appears to be a potential therapy for BPD-prevention, but further large-scale meticulously designed trials are necessary to confirm its therapeutic advantages before routine use is recommended. A prospective, cohort study [21] in preterm infants <32 week´s gestation with RDS showed that very early caffeine administration on the 1st DOL in comparison to later treatment (2-10 DOL) was associated with a decreased need for invasive MV, lower incidence of intraventricular hemorrhage (IVH) and PDA, but had no effect on the incidence and severity of BPD. A retrospective study including preterm infants <29 week´s gestation using more stringent criteria with respect to timing of caffeine demonstrated comparable rates of BPD and PDA-ligation between early (1-2 DOL) and late caffeine (3-7 DOL) [22]. A higher incidence of these outcomes was noticed only in preterm infants with very late use (≥ 8 DOL) of caffeine. These infants had also a significant lower mean gestational age and birthweight and their risk of having poorer outcomes was not thought be related to delayed caffeine treatment. The authors of this study conclude that in infants below 29 weeks´ gestation further studies are needed before caffeine prophylaxis can be universally recommended. In a recently published update on the management of RDS, although the quality of evidence is low, early caffeine is strongly recommended as part of a strategy to minimize the need for MV [9].
The beneficial effects with standard doses of caffeine led also to the investigation of higher doses. High-dose caffeine citrate (LD 40 mg/kg, MD 20 mg/kg) when compared to a standard dose in preterm infants <32 week´s gestation with AOP was associated with significant less extubation failure and less apneas without significant side effects [23]. But, there is still some concern about the safety of caffeine citrate, especially with higher doses. Caffeine may have significant vasoconstrictive properties and has been shown to impair blood flow to the brain or gut with the potential risk of causing hypoxemia and ischemia [24,25]. In a single-center, pilot randomized trial in 74 preterm infants <30 week´s gestation a high-dose regimen of 40 mg/kg LD of caffeine citrate followed by 20 mg/kg 12h later, then 10 mg/kg at 24 and 36h (80 mg/kg total over 36h) was compared to a standard-dose regime (30 mg/kg over 3 h) . Caffeine therapy was initiated within 24 hours of life. High-dose caffeine was associated with an increased incidence of cerebellar hemorrhage (36% vs. 10%, p=0.03) with subsequent alterations in early motor performance [26]. In a secondary analysis of this cohort an increased incidence of seizures and a threefold increase in seizure duration recorded on amplitudeintegrated EEG was seen in the high-dose caffeine regime [27]. A recently published single-center retrospective study in neonates with a wide range of gestational age (22-44 weeks) found that there is a potential association between caffeine therapy and the development of necrotizing enterocolitis, especially in combination with medications or factors that cause vasoconstriction [28]. Other dose-dependent side effects or adverse drug reactions include increased diuresis, poor weight gain due to increased oxygen consumption, tachycardia, hypertension, central hyperactivity (jitterness, seizures), jaundice, feeding intolerance and exacerbation of gastroesophageal reflux [29,30].
Doxapram
Doxapram hydrochloride, an off-line drug for use in neonates, has been used for a long time as treatment of neonatal respiratory depression secondary to the maternal administration of narcotic analgesic drugs [31]. It appears to act on both, the peripheral (carotic body) chemoreceptors and the central nervous system to augment breathing efforts [10,12]. It is usually administered by continuous intravenous infusion but can be given enterally. Average MD is usually 1.0 mg/kg/h with a starting dose of 0.5 mg/kg/h, but may vary from 0.03 -5 mg/kg/h [10,12]. For respiratory support in preterm infants in the NICU doxapram may be an alternative to caffeine, but because of concerns about serious side effects it was in the past either contraindicated or used at small doses in combination with MX [32]. With the trend to use techniques for avoiding or minimizing iMV (NIV, LISA, INSURE) doxapram has been used more frequently [33], and it´s efficacy and safety has been reevaluated in recent studies. A retrospective study in preterm infants (mean gestational age 26.1 weeks) with AOP study aimed to evaluate the efficacy and predictors of the success of doxapram treatment on preventing intubation [34]. Doxapram (LD of 2.5 mg/kg, MD 2.0 mg/kg/h) was administered either by continuous intravenous infusion or enterally via a nasogastric tube and initiated at a mean postnatal age of 10 days. 77% of the infants did not need endotracheal intubation during the 48 hours of doxapram therapy and 65% of patients did not need intubation during the entire treatment course. Increased postnatal age and lower FiO2 at the start of the therapy were the main predictors of success. Potential short-and long-term side effects reported with the use of doxapram are hypertension, irritability, QT-interval prolongation, hypokalemia, gastrointestinal disturbances, nausea and vomiting, and negative effects on cerebral oxygenation and long-term mental development [12,35-38]. Most side effects appear to be transient and related to its concentration. In a retrospective study of preterm infants <1250 g birthweight from the Netherlands doxapram therapy for AOP (average dose 1.0 mg/kg/h) was not associated with an increased risk of the combined outcome death or neurodevelopmental delay at 24 month´s corrected age [39]. A recently published systematic review in infants born before 34 week´s gestation summarized all available evidence on the short and long-term effects of doxapram for AOP [40]. This review included 32 studies (among them 4 randomized controlled trials (137 infants <32 weeks gestation) with considerable heterogeneity (e.g. differences in administered dose of doxapram, duration of therapy, study design). Doxapram was found to be useful for AOP but the effect on reducing the need for MV and the incidence of BPD was not clear. Long-term, neurodevelopmental outcomes up to 18 month´s corrected age showed varying results, but were reported only in observational studies. Adverse effect (e.g. gastrointestinal side effects, agitation) were seen especially with doses >1.5 mg/kg/h. The authors concluded that no firm conclusions on the efficacy and safety of doxapram in preterm infants can be drawn [40].
Conclusion
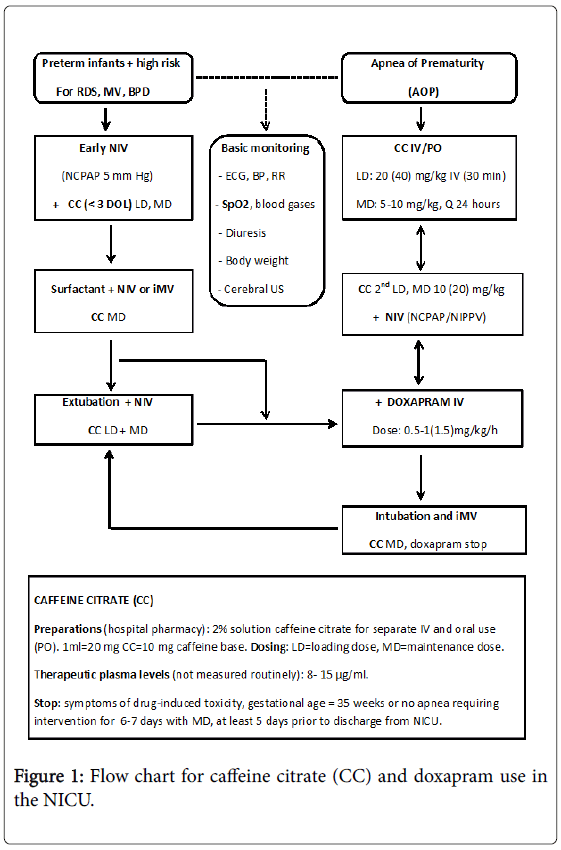
Figure 1 gives an overview of the clinical use of caffeine and doxapram in preterm infants in our NICU; the primary steps being usually a combination of caffeine citrate and NIV. Caffeine citrate is given in infants with AOP and in preterm infants at high risk for respiratory failure. Early caffeine therapy is beneficial compared to late caffeine therapy in reducing the incidence of BPD. When administered in standard doses the overall benefits seem to outweigh the potential risks. Doxapram, despite documented beneficial effects on AOP and in preventing re-intubation, still remains a third-line or rescue therapy if caffeine and NIV is not successful.
Future Directions
Caffeine citrate will keep its clinical importance for respiratory care in preterm infants. Further studies are necessary with respect to the impact of early or prophylactic and higher caffeine doses, especially in the most immature infants, before routine use can be recommended. For doxapram, despite encouraging results from recent studies, further evidence of its safety and efficacy including long-term neurodevelopmental outcome in a large multicenter, randomized controlled trial is mandatory before it can be recommended for routine respiratory support.
References
- Reiterer F, Schwaberger B, Freidl T, Schmolzer G, Pichler G, et al. (2016) Lung- protective ventilatory strategies in intubated preterm neonates with RDS. Pediatr Resp Rev 23: 89-96.
- Reiterer F, Polin R (2016) Non-invasive ventilation in preterm infants: A clinical review. Int J Pediatr Neonat Care 2: 1-4.
- Jensen EA, Kirpalani H (2016) Non-invasive respiratory support. Semin Fetal Neonatal Med 21: 133-134.
- Kuzemko JA, Paala J (1973) Apnoeic attacks in the newborn treated with aminophylline. Arch Dis Child 48: 404-406.
- Aranda JV, Gorman W, Bergsteinsson H, Gunn T (1977) Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr 90: 467-472.
- Reiterer F, Abbasi S, Stefano J, Pearlman S, Bhutani VK, et al. (1992) Early methylxanthine therapy in neonates with surfactant treated RDS: Effect on weaning and lung function. Eur Resp J 5: 156s.
- Dobson NR, Patel RM (2016) The role of caffeine in noninvasive respiratory support. Clin Perinatol 43: 733-782.
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E (2017) European consensus guidelines on the management of respiratory distress syndrome - 2016 Update. Neonatology 111: 107-125.
- Prins SA, Pans SJA, Van Weissenbruch MM, Walther FJ, Simons SHP (2013) Doxapram use for apnea of prematurity in neonatal intensive care. Int J Pediatr 2013: 1-5.
- Henderson-Smart DJ, Steer PA (2009) Caffeine versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews 2010.
- Raval DS, Reitz S, Yeh TF (1991) Apnea. In: Neonatal therapeutics (2nd edn.). Mosby Year Book, US 4: 40-53.
- Kraaijenga JV, Hutten GJ, de Jongh FH, van Kaam AH (2015) The effect of caffeine on diaphragmatic activity and tidal volume in preterm Infants. J Pediatr 167: 70-75.
- Tunc T, Aydemir G, Jaragoglu A, Cekmez F, Kuli M, et al. (2013) Toll- like receptors levels and caffeine responsiveness in rats pups during perinatal period. Regul Pept 182: 41-44.
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, et al. (2006) Caffeine therapy for apnea of prematurity. N Engl J Med 354: 2212-2221.
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, et al. (2007) Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357: 1893-1902.
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, et al. (2012) Survival without disability to ages 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307: 275-282.
- Schmidt B, Robertson RS, Anderson PJ, Asztalos EV, Costantini L, et al. (2017)Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: An 11 years follow-up of the CAP randomized clinical trial. JAMA Pediatrics 11: 564-572.
- Maitre NL, Stark AR (2012) Neuroprotection for preterm infants?: Another perspective on caffeine. JAMA 307: 304-305.
- Kua KP, Lee SW (2016) Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br J Clin Pharmacol 83: 180-191.
- Borswezka-Kornacka MK , Hozejowski R, Rutkowska M, Lauternach R (2017) Shifting the boundaries for early caffeine initiation in neonatal practice: Results of a prospective, multicenter study on very preterm infants with respiratory distress syndrome. PLOS ONE 2017: e0189152.
- Hand I, Zaghloul N, Barash L, Parris R, Aden U, et al. (2016) Timing of caffeine therapy and neonatal outcomes in preterm infants: A retrospective study. Int J Pediatr 2016: 1-6.
- Mohammed S, Nour I, Shabaan AE, Shouman B, Abdel-Hady H, et al. (2015) High versus low- dose caffeine for apnea of prematurity: A randomized controlled trial. Eur J Pediatr 174: 949-956.
- Tracy MB, Klimek J, Hinder M, Ponnampalam G, Tracy SK (2010) Does caffeine impair cerebral oxygenation and blood flow velocity in preterm infants? Acta Pediatr 99: 1319-1323.
- Lane A JP, Coombs RC, Evans DH, Levin RL (1999) Effect of caffeine on neonatal splanchnic blood flow. Arch Dis Child Fetal Neonatal Ed 80: F128–F129.
- McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE (2015) A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res 78: 198-204.
- Vesoulis ZA. McPherson C, Neil JJ, Mathur AM, Inder TE (2016) Early high-dose caffeine increases seizure burden in extremely preterm neonates: A preliminary study. J Caffeine Res 6: 101-107.
- Cox C, Hashem NG, Tebbs J, Bookstaver PB, Iskersky V (2015) Evaluation of caffeine and the development of necrotizing enterocolitis. J Neonatal Perinatal Med, pp: 339-347.
- Belen Rivas A, Arruza L, Pacheco E, Portoles A, Diz J, et al. (2016) Adverse drug reactions in neonates: A prospective study. Arch Dis Child 101: 371-376.
- Welsh C, Pan J, Belik J (2015) Caffeine impairs gastrointestinal function in newborn rats. Pediatr Res 78: 24-28.
- Gupta PK, Moore J (1973) The use of doxapram in the newborn. Int J Obstet Gynecol Br Comm 80: 1002-1006
- Yamazaki T, Kajiwara M, Itahashi K, Fujimura M (2001) Low-dose doxapram therapy for idiopathic apnea of prematurity. Pediatr Int 43: 124-127.
- Kribs A, Hartel C, Kattner E , Vochem M , Kuster H, et al. (2010) Surfactant without intubation in preterm infants with respiratory distress: First multi-center data. Klin Padiatr 222: 13-17.
- Flint R, Halbmeijer N, Messters N, van Rosmalen J, Reiss I, et al. (2017) Retrospective study shows that doxapram therapy avoided the need for endotracheal intubation in most premature neonates. Acta Paediatr 106: 733-739.
- Maillard C, Boutroy MJ, Fresson J, Barbe F, Hascooet JM (2001) QT interval lengthening in preterm infants treated with doxapram. Clin Pharmacol Ther 70: 540-545.
- Roll C, Horsch S (2004) Effect of doxapram in cerebral blood flow velocity in preterm infants. Neuropediatrics 35: 126-129.
- Fischer C, Ferdynus C, Gouyon JB, Semama DS (2013) Doxapram and hypokalemia in very preterm infants. Arch Dis Child Neonatl Ed 98: F416-418.
- Dani C, Bertini G, Pezzati M, Pratesis S, Filippi L, et al. (2006) Brain hemodynamics effects of doxapram in preterm infants. Biol Neonate 89: 69-74.
- Ten Hove CH, Vliegenthart RJ, Te Pas AB, Brouwer E, Rijeken M, et al. (2016) Long-term neurodevelopmental outcome after doxapram for apnea of prematurity. Neonatology 110: 21-26.
- Vliegenthart RJ, Ten Hove CH, Onland W, van Kaam AH (2017) Doxapram treatment for apnea of prematurity: A systematic review. Neonatology 111: 162-171.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5361
- [From(publication date): 0-2018 - Aug 04, 2025]
- Breakdown by view type
- HTML page views: 4366
- PDF downloads: 995