The Synchronicity of Chronic Lymphocytic Leukemia and Multiple Myeloma in a Bone Marrow Biopsy with Therapy Discussion
Received: 05-Feb-2018 / Accepted Date: 23-Feb-2018 / Published Date: 28-Feb-2018 DOI: 10.4172/2476-2024.1000135
Abstract
The coexistence of synchronous chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) in a bone marrow of a patient is a very rare occurrence. To our knowledge, only four publications were reported in the English literature. Herein, we describe a 75 year-old gentleman who presented with fatigue and lethargy of 8 weeks duration. He was found to be anemic, with a mild lymphocytosis, decreased renal function, normal calcium level, and conspicuous M-Spike on serum electrophoresis. Furthermore, trephine bone marrow biopsy demonstrated two distinct hematological malignancies: CLL and MM.
Introduction
Chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) are both lymph proliferative diseases affecting B-cell lymphocytes. The coexistence of CLL and MM is rare. Recent PUBMED search yielded four reported manuscripts in English literature describing 22 patients [1-4]. CLL is the most common leukemia in the United States of America. It typically presents in older age, usually in the seventh decade of life. There is a male predilection. The clinical course is commonly indolent. At times, patients can present with an advance stage, warranting immediate therapy. Approximately 10-20% of CLL patients can experience transformation to diffuse large B-cell lymphoma or its immunoblastic variant [2]. This transformation is called Richter’s syndrome.
MM is a plasma cell dyscrasia which affects the mature B-cell lymphocytes. Risk factors include advancing age (median age of 60-65 at presentation), male gender, and African-American decent. According to The WHO 2008 classification of hematological diseases identifies the following criteria to be met for multiple myeloma [5].
Case Presentation
The patient is a 75 year-old white male with a past medical history of hypertension, tophaceous gout, and myocardial infarction with prior cardiac stent placement. The patient was initially seen by his primary care provider for complaints of fatigue and lethargy of more than 8 weeks duration. His symptoms worsened with little exertion or activity. He was referred to a hematologist at Clarion Hospital after he was found to be anemic (Hgb 11.7 g/dL; red blood cell count 3.41 × 106/uL) with lymphocytosis (4,500 mm3) on initial laboratory studies.
Physical examination revealed a well-nourished male with relatively normal vital signs. His neurological examination was normal. On musculoskeletal examination, he had significant small joint upper extremity deformity with gouty tophi deposition. Lymph node exam did not reveal any significant adenopathy. Further laboratory findings revealed a repeat lymphocyte: 5,600 mm3 (600-3,400), with 63.5% lymphocytes, and a platelet count of 194,000/mm3 (Table 1).
| Symptomatic plasma cell myeloma |
| M-protein in serum or urine* |
| BM clonal plasma cells or plasmacytoma† |
| Related organ or tissue impairment heavy chain disease‡ (CRAB) |
| Asymptomatic (smoldering) myeloma |
| M-protein in serum at myeloma levels (>30 g/L) and/or ≥ 10% clonal plasma cells in BM |
| No related organ or tissue impairment end-organ damage or bone lesions [CRAB] or myeloma-related symptoms |
| CRAB indicates hypercalcemia, renal insufficiency, anemia, bone lesions. |
| * No level or serum or urine M-protein is included. M-protein in most cases is >30g/L of IgG or >25g/L of IgA or >1g/24 h of urine light chain, but some patients with symptomatic myeloma have levels lower than these. |
| † Monoclonal plasma cell usually exceeds 10% of nucleated cells in the marrow, but no minimal levels are designated because ∼ 5% of patients with symptomatic myeloma have <10% marrow plasma cells. |
| ‡ The most important criteria for symptomatic myeloma are manifestations of end-organ damage including anemia, hypercalcemia, lytic bone lesions, renal insufficiency, hyper viscosity, or recurrent infections. |
Table 1: Diagnostic criteria for plasma cell myeloma.
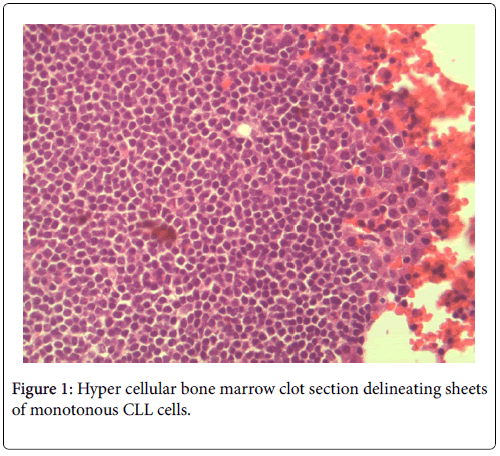
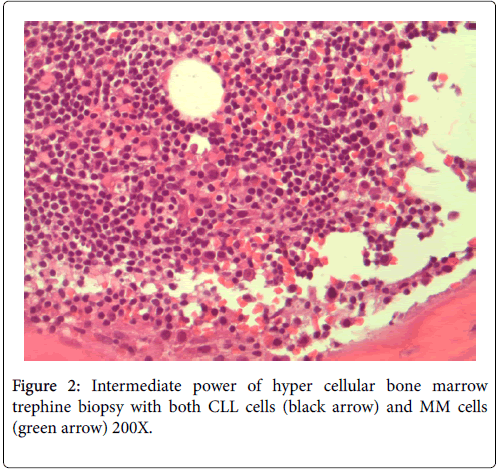
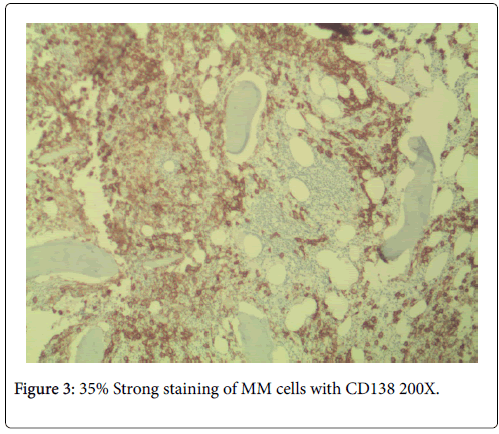
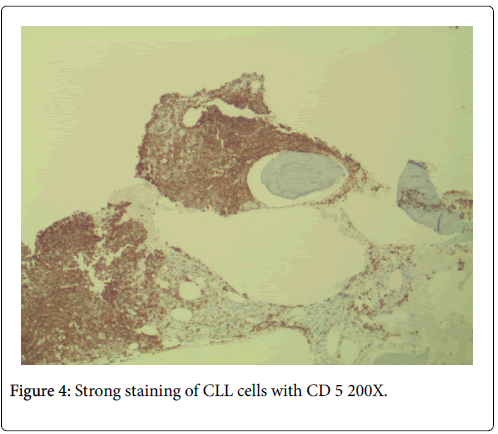
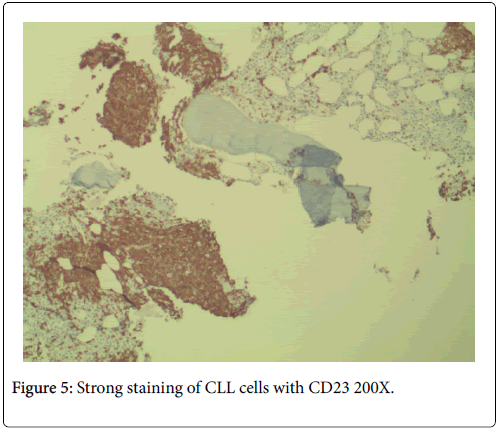
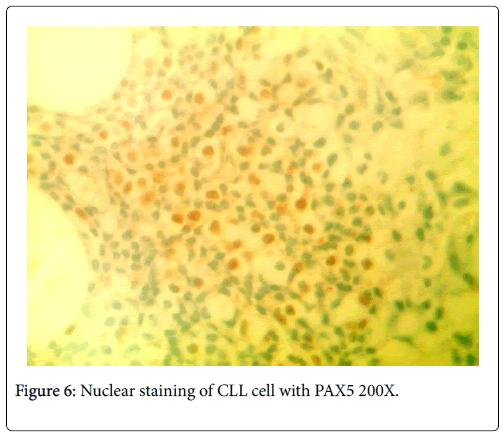
No neutropenia was seen. Immunoglobulin measurements revealed an IgG of 2040 mg/dL. There was also noted to be a marked reciprocal reduction in normal immunoglobulin production. Serum protein electrophoresis demonstrated an M-spike of 1.7 g/dL with immunofixation divulging an IgG monoclonal protein with kappa light chain specificity. Free kappa light chains were elevated at 587.5 mg/L, which yielded kappa/lambda ratio of 106.82. Skeletal survey was negative for osteoblastic or lytic lesions, though diffuse osteopenia was seen. Serum calcium level was normal at 9.2 mg/dL. BUN was elevated at 34 mg/dL, serum creatinine level was elevated at 1.73 mg/dL. Glomerular filtration rate was reduced at 39 mL/min/m2. β-2 microglobulin was 4.43 mg/L with an albumin of 3.7 g/dL. Bone marrow aspiration detected multiple myeloma as well as chronic lymphocytic leukemia (Figures 1 and 2). Bone marrow smear showed increase of plasma cells showing 35% plasma cell infiltration with CD138 stain (Figure 3). Furthermore, bone marrow smear showed lymphocytes which were CD5, CD23, and PAX 5 positive (Figures 4-6). Further cytogenetic studies revealed a multiple myeloma panel as illustrated in Table 2 below.
| Multiple Myeloma FISH Panel | |
|---|---|
| *Trisomy 4 (FGFR3) | + |
| *Trisomy 7 | + |
| *Trisomy 9 | + |
| *Trisomy/Tetrasomy 11 (CCND1) | + |
| *Trisomy/Tetrasomy 15 | + |
| *Trisomy 17 (TP53) | + |
| **Hyperdiploidy | + |
| *The extra copies of chromosomes 7, 9, 11, 15, and 17 are frequently seen in MM. | |
| **Hyperdiploidy in the absence of structural abnormalities is generally associated with favorable prognosis. | |
Table 2: cytogenetic studies revealed a multiple myeloma.
The bone marrow biopsy/clot section delineated a hyper cellular bone marrow for the stated age of seventy-five years. There were two distinct hematological malignancies present. The CLL consisted of sheets of monotonous small atypical lymphocytes. The small uniform lymphocytes harbored a high nuclear to cytoplasmic ratio. The nuclei were round and enlarged with no conspicuous cleavage or indentation. The nuclear chromatin was apparently coarse with indistinguishable nucleoli. The cytoplasm was scant with a deep basophilic hue. Overall, these sheets of cells demonstrated a striking familiar homogeneity associated with CLL.
The cells morphologically as well as in conjunction with immunohistochemistry were identified as CLL cells. CD 20 positivity was noted with CD 5 and CD 23 markers being strongly and diffusely positive. PAX-5 stain divulged a nuclear positivity which attested to a B-cell lymphocyte lineage. Essentially, no other markers were needed to identify this malignancy as CLL. The diagnosis of CLL is usually rendered on the positivity of both CD 5 and CD 23. Moreover, the pan B-cell markers are usually positive. In addition, the neoplastic plasma cells were markedly elevated to 35%, confirmed with a CD 138 stain. The multiple myeloma cells formed two patterns. The more frequent pattern was that of the malignant plasma cells forming groups juxtaposed to the CLL cell sheets. The less frequent pattern was that of the multiple myeloma cells individually infiltrating in between the CLL cells. The plasma cells were atypical and enlarged with rare binucleated forms. The plasma cells were equipped with round and large eccentrically placed nuclei. The clumpy chromatin pattern resembled that of a “cart-wheel”. No distinct nucleoli or Dutcher bodies were apparent.
The cytoplasm was basophilic and peripheral. An occasional mitosis was noted among the plasma cell population. These above morphological features in association with CD138 stain positivity and clinical morphological findings attested to a multiple myeloma diagnosis.
Discussion
Though there is no cure for multiple myeloma, treatment can help control complications while stabilizing and slowing progression of disease. With the advent of many new agents, deep remissions can be achieved with overall survival improving dramatically. Use of the International Staging System (ISS) plays a role in predicting outcomes and determining treatment strategies. Initial management of disease also includes risk stratification into 3 risk categories (i.e., standard risk, intermediate risk, and high risk) [6].
Standard risk patients include those with t (11;14) and t (6;14) by FISH analysis. Transplant ineligible patients often receive Lenalidomide (Revlimid), a biological therapy drug, which modulates the immune system to identify and attack malignant cells. Corticosteroids in the form of Dexamethasone also play a key role, harboring potent anti-myeloma activity. These medications are typically taken until progression. For those patients eligible for autologous stem cell transplantation, 4-6 cycles of Lenalidomide, Dexamethasone and Bortezomib (VRD), a targeted therapy that inhibits a cellular component necessary for protein regulation, resulting in tumor cell death. Once a partial remission is achieved, autologous stem cells are collected. These patients then receive myeloablative chemotherapy typically in the form of high-dose Melphalan, followed by reinfusion of cryopreserved autologous stem cells to facilitate hematopoietic recovery. Patients are then typically treated with maintenance Lenalidomide until disease progression.
In intermediate risk patients possessing t (4;14) or 1q+ by FISH analysis or deletion 13/hypodiploidy by conventional cytogenetics treatment also includes 4-6 cycles of Bortezomib, Lenalidomide, and Dexamethasone (VRD) followed by autologous stem cell transplantation. These patients often receive maintenance Bortezomib until further progression of disease.
Finally in high risk patients, FISH profile includes t (14;16), t (14;20), or deletion 17p13. They may also have lactate dehydrogenase (LDH) ≥ 2 times the upper limit of normal and have features consistent with primary plasma cell leukemia that suggests high risk gene expression for multiple myeloma. One option includes 4-6 cycles of Lenalidomide, Dexamethasone, and Carfilzomib (KRD). VRD is also used in this setting with other combinations of newer agents being studied in the upfront setting. The patient will undergo autologous stem cell transplantation once a partial remission or better is achieved, typically followed by maintenance Bortezomib.
In summary, three drug induction regimens have become the standard for most patients with multiple myeloma. It should be noted that there are various combinations to consider, with more than one agent now available within most drug classes [6]. When choosing a regimen, the clinician must also take into consideration such factors as pace of disease, performance status and comorbid conditions. For example, the use of cyclophosphamide (Cytoxan) in place of lenalidomide in triplet regimens (CyBorD) is a common substitution for patients who have a contraindication or intolerance to the latter [7]. Further, autologous transplantation continues to play an important role when in first remission for eligible patients. With improving supportive measures, the door to transplant remains open to an increasing number of patients. It should also be stated that Melphalan and Bortezomib combinations can also be considered for transplant ineligible patients [8].
All myeloma patients should receive complementary treatment by undergoing weight-bearing exercises to maintain a healthy bone density while being given bisphosphonates to reduce osteoclastic activity. Furthermore, influenza and pneumococcal vaccines should be given since patients with multiple myeloma are immunocompromised.
In chronic lymphocytic leukemia, since the disease process is typically indolent, newly diagnosed early stage asymptomatic patients (i.e., pt denying unintentional weight loss of ≥ 10% in the past 6 months, significant fatigue, fevers higher than 38˚C for two or more weeks, or night sweats for ≥ 1 month without evidence of infection) may benefit most from observation alone rather than immediate therapy [9]. Blood counts are collected at three-month intervals along with a clinical examination. Patients are then treated when symptoms or significant cytopenia ensue. Development of a rapid doubling time of lymphocytes in <12 months, bulky disease, autoimmune sequela or recurrent infections can also serve as indications to treat [10]. Pretreatment laboratory studies include complete blood count with differential, comprehensive metabolic panel, lactate dehydrogenase, β-2 microglobulin, and direct anti-globulin test (DAT). Evaluation of the peripheral blood with FISH analysis for del17p, del11q, trisomy 12, del13q, and TP53 is also necessary to direct appropriate treatment [9].
Once treatment is necessary, most patients are treated with systemic regimens. This said, there can be a role for involved field radiation for symptomatic or compromising localized disease in otherwise early staged patients [11]. Steroids and monoclonal antibodies can also be used for symptomatic management in such settings.
Patients with stage II or more advanced (stage 2–lymphocytosis +enlarged liver or spleen with or without lymphadenopathy; stage 3– lymphocytosis+anemia Hgb <11 g/dL with or without enlarged liver, spleen, or lymph nodes; stage 4–lymphocytosis+thrombocytopenia platelet count <100,000/microL with or without anemia or enlarged liver, spleen, or lymph nodes) are usually treated with chemotherapy based regimens.
No agreed first-line chemotherapy regimen exists for symptomatic or advanced stages of CLL, and most clinicians account for individual’s age and comorbidity before starting an initial regimen. For example, in younger patients, fludarabine (Fludara) and rituximab (Rituxan) with or without cyclophosphamide are commonly used [12]. For older patients (i.e., those aged >65 years), single agent ibrutinib (Imbruvica) is often reasonable [13]. Alternative options may include chlorambucil (Leukeran) in combination with a monoclonal antibody such as obinutuzumab (Gazyva) [14]. Bedamustine (Treanda/Bendeka) with rituximab is another popular regimen that is generally well tolerated in many treatment populations [15]. For patients with high-risk del17p or TP53 mutations, ibrutinib is recommended with consideration of allogeneic hematopoietic stem cell transplantation. For all CLL patients, influenza and pneumococcal vaccinations are recommended to reduce risk of acquiring serious infections. IVIG can also be utilized for patients with acquired hypogammaglobulinemia and recurrent infections.
Conclusion
The patient reported in this manuscript appears to have early staged CLL in conjunction with ISS stage II MM without classically defined high-risk features. At this time, it is thought that most of his symptoms, including renal insufficiency and osteopenia, are primarily related to the myeloma clone rather than that of CLL. Nevertheless, in light of both malignancies being present, further imaging is being obtained to more definitively assess the status of both entities. It can also be stated that while CLL clones can produce paraproteins, pathology coupled with excess light chain production implicate the plasma cell neoplasm in this patient. While some data are pending, the patient appears to be eligible for a triplet regimen targeting his MM. Initial thoughts are that the CyBorD regimen would be a reasonable and well tolerated option for this patient. Though treatment of his CLL does not appear necessary based on currently available studies, such a regimen may have activity against this clone as well. Bisphosphonate therapy adjusted for renal dysfunction will also be utilized as well as other standard prophylactic measures. The role of transplant in this patient is contentious at present. Future considerations do include maintenance therapy and possibly the addition of monoclonal antibody therapy.
References
- Merdin A, Yıldız J, Dal MS, Kızıl Çakar M, Kaya AH, et al. (2017) Coexistence of chronic lymphocytic leukemia and multiple myeloma, do the roots of these entities originate from the same place? Clin Case Rep 5: 1032-1033.
- Robak T (2004) Second malignancies and Richter's syndrome in patients with chronic lymphocytic leukemia. Hematology 9: 387-400.
- Chang H, Wechalekar A, Li L, Reece D (2004) Molecular cytogenetic abnormalities in patients with concurrent chronic lymphocytic leukemia and multiple myeloma shown by interphase fluorescence in situ hybridization: evidence of distinct clonal origin. Cancer Genet Cytogenet 148: 44-48.
- Brouet JC, Fermand JP, Laurent G, Grange MJ, Chevalier A, et al. (1985) The association of chronic lymphocytic leukemia and multiple myeloma: a study of eleven patients. Br J Haematol 59: 55-66.
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, et al. (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117: 5019-5032.
- Rajkumar SV (2016) Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 91: 719-734.
- Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, et al. (2009) Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 23: 1337-1341.
- Torimoto Y, Shindo M, Ikuta K, Kohgo Y (2015) Current therapeutic strategies for multiple myeloma. Int J Clin Oncol 20: 423-430.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, et al. (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111: 5446-5456.
- Pepper C, Majid A, Lin TT, Hewamana S, Pratt G, et al. (2012) Defining the prognosis of early stage chronic lymphocytic leukaemia patients. Br J Haematol 156: 499-507.
- Morrison WH, Hoppe RT, Weiss LM, Picozzi VJ Jr, Horning SJ (1989) Small lymphocytic lymphoma. J Clin Onco 7: 598-606.
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, et al. (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376: 1164-1174.
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, et al. (2015) RESONATE-2 Investigators. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 373: 2425-2437.
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, et al. (2014) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370: 1101-1110.
- Larsen JT, Shanafelt TD, Leis JF, LaPlant B, Call T, et al. (2017) Akt inhibitor MK-2206 in combination with bendamustine and rituximab in relapsed or refractory chronic lymphocytic leukemia: Results from the N1087 alliance study. Am J Hematol 92: 759-763.
Citation: Zofia T, Ikkei H, Rossetti JM (2018) The Synchronicity of Chronic Lymphocytic Leukemia and Multiple Myeloma in a Bone Marrow Biopsy with Therapy Discussion. Diagn Pathol Open 3: 135. DOI: 10.4172/2476-2024.1000135
Copyright: © 2018 Tynski Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7442
- [From(publication date): 0-2018 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 6472
- PDF downloads: 970