The Use of an Acellular Connective Tissue Matrix in Hindfoot and Ankle Fusions: Understanding the Cellular Bench Top Data with a Consecutive Patient Series: A Pilot Study
Received: 10-Aug-2018 / Accepted Date: 28-Sep-2018 / Published Date: 05-Oct-2018 DOI: 10.4172/2329-910X.1000276
Keywords: Ankle; Bone tissue; Connective tissue matrix; Fusion; Hindfoot; Placental tissue Regenerative healing
Introduction
Biomaterials are often used to support the healing and regeneration of bone tissue [1,2]. One of the key requirements for biomaterials/ scaffolds to be considered for bone tissue engineering is the biocompatibility and degradability of such biomaterials [3].
Placental connective tissue matrix (CTM) is a decellularized placental tissue matrix, which has minimal immunogenicity and retains the fundamental structure and functional characteristics of an intact excellular matrix. This means that CTM has the ability to provide a cell friendly environment, while allowing for the formation of endothelial channels and ultimately vascular penetration. Cellular migration and endothelial formation will ultimately cause a release of the appropriate growth factors for healing and allow the CTM to transition into functional tissue. CTM has been successfully used in clinical practices to promote wound healing and soft tissue reconstruction of the extremity and trunk [4-6].
In order to explore the feasibility of CTM in bone repair and regeneration applications, the biocompatibility CTM with bone-forming cells has to be established. Osteoblasts are the bone-forming cells in bone tissue and play a critical role in bone tissue development and bone regeneration [7,8]. In this study, the adhesion and proliferation of human osteoblasts on CTM were evaluated. A consecutive clinical series of hindfoot and ankle fusions was performed to understand the clinical efficacy of CTM when mixed with cancellous autograft. It was hypothesized that using CTM as a scaffold would augment the already cell friendly environment of autograft bone and accelerate healing.
Methodology
The bench top data
CTM (Interfyl, Alliqua Biomedical, Yardley PA) is provided in a particulate form for both the bench top and surgical portion of this study (Figure 1). To evaluate the effect of CTM particulates on human osteoblasts, we coated CTM particulates on ultra-low adhesive polystyrene (ULAP) surface and quantified the adhesion and proliferation of human osteoblasts on CTM coated surfaces. The expansion/proliferation on 2D surface often leads to the loss of native cell phenotypes of primary cells [9]. We evaluated if culturing human osteoblasts on CTM-coated surface maintain the characteristic of osteoblasts by measuring the alkaline phosphatase activity of osteoblasts.
Coating of ultra-low attachment polystyrene plate with CTM particulates
CTM particulates (Interfyl particulate) were re-suspended in sterile phosphate buffered saline (PBS) to make slurry at 50 mg/ml. The slurry was serially (1:2) diluted using PBS from 25 mg/ml to 1.6 mg/ml. 0.2 ml of slurry at different concentrations was transferred to each well of ultra-low attachment polystyrene 24-well plate (ULAP Corning REF 3473). The plates were air-dried in a chemical hood for 16-20 hours. The concentrations of CTM in coated wells are 2.5 mg/cm2, 1.25 mg/ cm2, 0.625 mg/cm2, 0.31 mg/cm2, and 0.15 mg/cm2 (Figure 2).
After air-drying, the wells coated with CTM were sterilized under a fluorescent lamp (Sylvania G36T5/SP Germicidal UV-C 39W) for 2 hours in a tissue culture hood (biosafety cabinet). The coated wells were gently rinsed with 1 ml of sterile H2O once before seeding cells.
Culturing human osteoblasts (HO)
Human osteoblast-femoral (HO-f) (Cat# 4610) at Passage 2 was purchased from ScienCell Research Laboratories. The cells were thawed and cultured on Poly-L-Lysine hydrobromide (PLL) (Sigma P1274) coated tissue culture treated polystyrene (TCPS) dishes according to the manufacture’s instruction. Osteoblast culturing medium (ObM) (Cat# 4601 Lot# 19526) was also purchased from ScienCell Research Laboratories.
Adhesion of human osteoblasts (HO) on CTM
When HO cells reached 85% confluence in ObM complete medium, cells were trypsinized and counted. Cells were washed once with DMEM without serum and were re-suspended in fresh DMEM without serum at 2 × 104/ml. 1 ml of HO cells were seeded in CTM-coated wells, ULAP uncoated wells, or TCPS wells at 2 × 104/well (n=3 triplicates for each coating concentration). The cells were incubated at 37°C for 1 hour. After 1 hour, the medium was removed from each well and the wells were washed twice with 1 ml PBS. To visualize the cells, 0.5 ml of DMEM+10 mg/ml of Calcein AM was added to each well and incubated at 37°C for 30 minutes. The cells were examined using 10X objective under Green Fluorescent Protein (GFP) channel (Zeiss). Eight to ten images per sample were taken and the green-fluorescent cells were counted manually and the average cell numbers from all the images were considered as the adhered cells/field for each surface.
Proliferation of human osteoblasts (HO) on CTM
When HO cells reached 85% confluence in ObM complete medium, cells were trypsinized and counted. Cells were re-suspended in fresh ObM complete medium at 2 × 104/ml. 1 ml of HO cells were seeded in CTM-coated wells, ULAP uncoated wells, or TCPS wells at 2 × 104/well (n=3 triplicates for each coating concentration). One set of CTM-coated wells were incubated with 1 ml of ObM without cells and served as blank control in Alamar Blue® assay for each individual CTM concentration. After incubation for 24, 48 or 72 hours, AlamarBlue® assay was performed with the cells in the wells. Briefly, at 24 hours, the medium was removed and 0.4 ml of ObM medium+10% AlamarBlue® (Bio-Rad) was added to each well including the blank control wells and incubated for 1 hour. One-hundred μL of medium from each well was transferred to a 96-well plate. The plate was read using TeCan plate reader at 570 nm/590 nm. The wells of 24-well plate were then washed with 1 ml of PBS and continued to be cultured in ObM medium until the 48 or 72 hour time point.
Alkaline phosphatase (ALP) activity fluorometric assay
After HO cells cultured on CTM-coated surfaces or control surfaces for 72 hours, the media were removed and cells were washed twice with PBS. Alkaline phosphatase activity fluorometric assay kit was pur-chased from BioVision (K422). 0.4 ml of ALP assay buffer was added to the cells, and 60 μl of cell lysates from each sample was used for ALP activity assay. The assay was performed following the manufacture’s protocol. The DNA content of each lysate was quantified using QuantiT ™ PicoGreen® dsDNA Assay Kit (Thermo Fisher P7589) following the manufacture’s protocol. The ALP activity of each sample was normalized to its DNA content.
Alkaline phosphatase staining by Fast Blue RR
After HO cells cultured on CTM-coated surfaces or control surfaces for 6 days, the media were removed and cells were washed twice with PBS. Cells were fixed with 4% paraformaldehyde for 5 minutes and permeabilized using 0.1% Triton × 100 for 3 minutes. Cells were washed with PBS and stained with 0.4 ml/well of Fast Blue RR solution (1/10 of capsule of Fast Blue RR salt [Sigma FBS25-10CAP] in 4.8 ml of H2O and 200 μl of Naphthol [Naphthol AS-Mx, Sigma]) at room temperature for 30 min. After Fast Blue RR staining, the cells were stained with Hoechst Dye 33258 at 1:1000 for 15 min. The cells were analyzed using Zeiss fluorescent microscope.
Results of each independent experiment were based on repetitive samples (n=3) and data were expressed as the mean ± standard deviation. One-way ANOVA with a Tukey’s multiple comparisons test was used to determine the statistical significance. Differences were considered significant at a p value of <0.05.
The clinical series
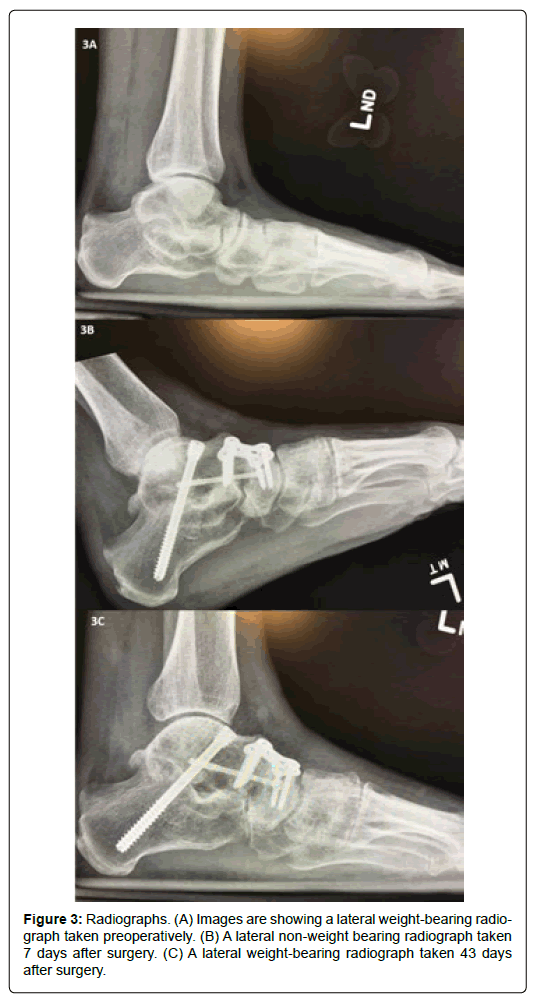
A medical record review was performed to evaluate time to fusion in patients who underwent hind-foot and ankle arthrodesis with use of autogenous bone graft mixed with 100 mg of allogeneic, decellularized, particulate human CTM from August 2016 to March 2017 by an independent physician reviewer (SCC) (Figure 3). Contributory patient demographics and co-morbidity data were recorded. These consisted of patient age (years), gender (male/female), body mass index (BMI) (kg/m2), operative limb (left/right), presence of hypertension, diabetes, Charcot arthropathy, rheumatoid arthritis, and smoking status.
For inclusion, patients were age at least 18 years old at the time of surgery, had been diagnosed with end-stage joint arthritis, exhausted all forms of conservative treatment, and elected to undergo foot and/ or ankle arthrodesis. All procedures were performed by one surgeon (SAB). Exclusion criteria included the use of a postoperative bone stimulator, or a history of an active target joint infection in the 6 months before surgery. The protocol was approved by our institutional review board and the informed consent was waived. Data was recorded into a password protected secure database. The confidentiality and privacy of individuals was ensured and maintained.
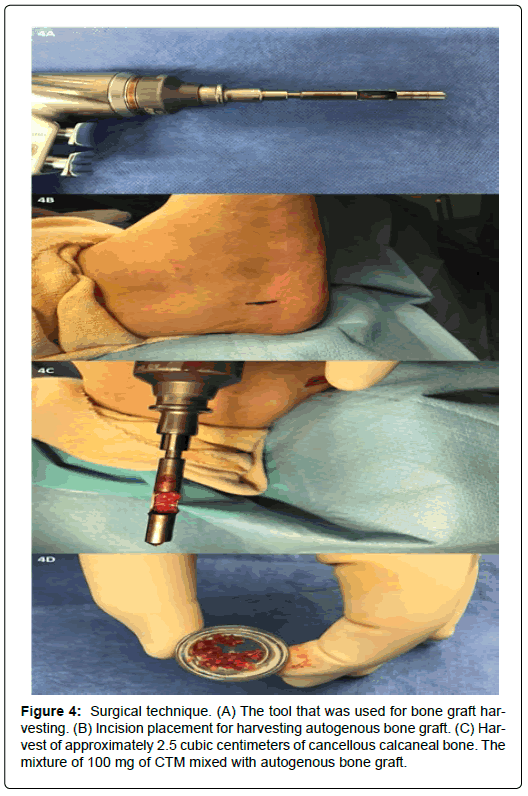
Each joint to be fused was prepared by denuding the articular surface and subchondral plate using curettage, and the subchondral bone was fenestrated using a 0.062 mm Kirschner wire. Approximately 2.5 cc of cancellous calcaneal bone was harvested from the calcaneal body of the ipsilateral limb and mixed with 100 mg of allogeneic, decellularized, particulate human CTM (Figure 4). The combined 2.5 cc/100 mg mixture was placed in each joint to be fused.
The study endpoint was fusion. Fusion was assessed by the independent surgeon and was defined as bony trabeculation on at least three cortices across the fusion site in all three radiographic views. Descriptive statistics are provided as counts and percentages and means and standard deviations.
Results
The bench top data
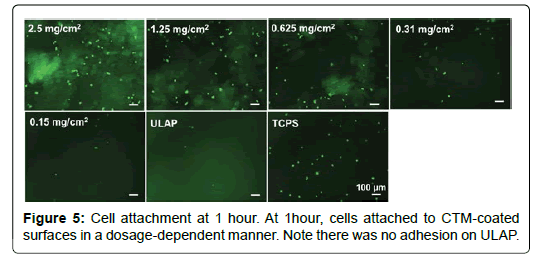
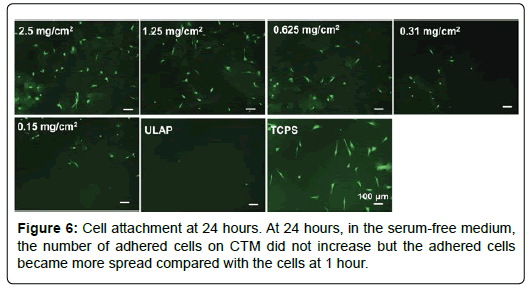
Adhesion of HO cells to CTM coated surfaces: In order to study the cell adhesion to substrates without the interference of serum, DMEM medium was used to replace the ObM complete medium. The cell adhesion at 1 hour or 24 hours was visualized by staining cells with Calcein AM and images were taken under fluorescent microscope and cell numbers were manually counted. Ultra-low attachment polystyrene was used as a negative control and tissue culture treated polystyrene surface (TCPS) was used as a positive control. As shown in Figure 5, at 1 hour, cells attached to CTM-coated surfaces in a dosagedependent manner. There was no adhesion on ULAP suggested that the adhesion observed on CTM-coated surface was due to the presence of CTM particulates. At 24 hours, in the serum-free medium, the number of adhered cells on CTM did not increase but the adhered cells became more spread compared with the cells at 1 hour (Figure 6).
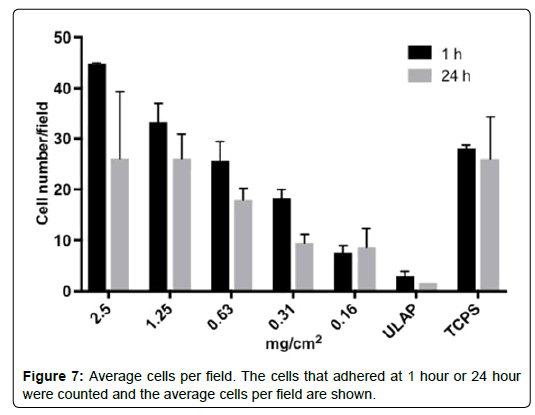
The cells that adhered at 1 hour or 24 hour were counted and the average cells per field were shown in Figure 7.
In the absence of serum, the adhesion of HO cells was CTM-dependent. With higher concentration of CTM, more cells adhered to the surface. The prolonged incubation of cells in serum-free medium did not improve the cell adhesion (1 hour vs. 24 hours), which suggested that HO cells did not thrive in serum-free medium over longer period of time.
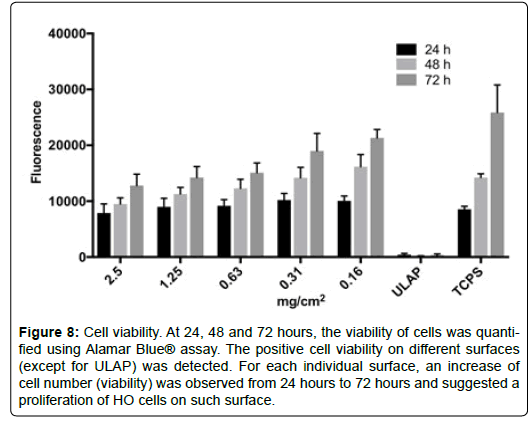
Proliferation of HO cells on CTM-coated surfaces: CTM supported the adhesion of HO cells (Figure 5). To evaluate if CTM support the proliferation of HO cells, the cell viability of HO cells in ObM complete medium was monitored over 72 hours. HO cells were seeded onto sur-faces coated with different concentrations of CTM or control surfaces. At 24, 48 and 72 hours, the viability of cells was quantified using alamarBlue ® assay (Figure 8).
Figure 8: Cell viability. At 24, 48 and 72 hours, the viability of cells was quantified using Alamar Blue® assay. The positive cell viability on different surfaces (except for ULAP) was detected. For each individual surface, an increase of cell number (viability) was observed from 24 hours to 72 hours and suggested a proliferation of HO cells on such surface.
The positive cell viability on different surfaces (except for ULAP) was detected (Figure 8). For each individual surface, an increase of cell number (viability) was observed from 24 hours to 72 hours and suggested a proliferation of HO cells on such surface. It is noted that cells seemed to grow faster on TCPS or low concentration of CTM-coated surfaces than on high concentration of CTM-coated surfaces. At high CTM concentration, most of cells were adhered to CTM particulates, which mimic a 3D substrate. On the other hand, at low CTM concentration, many cells adhere to the space between particulates, which like TCPS, was 2D substrate. It has been reported that a variety of cell showed reduced proliferation on 3D than on 2D substrates [10]. Our observation can be explained by the previous reported phenomenon. Despite the different rates of proliferation, these results demonstrate that the presence of CTM is required for the adhesion and proliferation of HO cells.
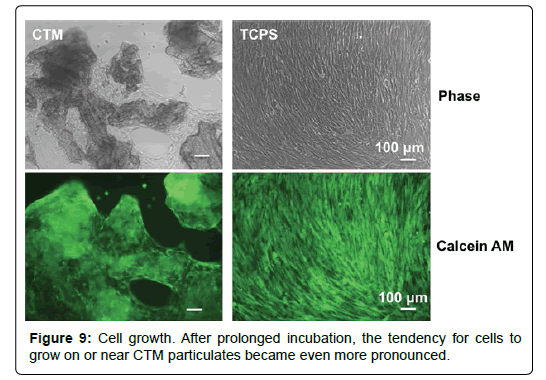
We noticed that cells tend to grow on or near CTM particulates at early time points (Figure 5). After prolonged incubation, this tendency became even more pronounced (Figure 9). This observation may suggest that CTM particulates not only support and but also attract HO cells.
Osteogenic activity of HO cells on CTM-coated surfaces: When primary cells are expanded and cultured in vitro on conventional tissue culture surfaces, the characteristic phenotypes of the primary cells tend to diminish [9,10]. The dedifferentiation of primary chondrocytes has been extensively studied and the culturing on a 2D TCPS was attributed to such phenomena [9,11]. Primary human alveolar bone cells showed significantly decreased ALP activity and mineralization capability when serially passaged on conventional tissue culture condition [11]. ALP activity is the most widely recognized biochemical marker for osteoblast activity [12,13]. In order to evaluate if the CTM maintains the primary phenotypes of HO cells during culturing, we evaluated the ALP activity of HO cells after cultured for 3 and 6 days.
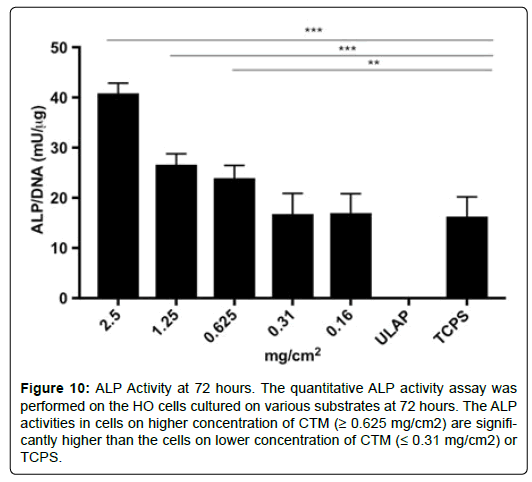
The quantitative ALP activity assay was performed on the HO cells cultured on various substrates at 72 hours (Figure 10). The ALP activities in cells on higher concentration of CTM (≥ 0.625 mg/cm2) are significantly higher than the cells on lower concentration of CTM (≤ 0.31 mg/cm2) or TCPS.
Figure 10: ALP Activity at 72 hours. The quantitative ALP activity assay was performed on the HO cells cultured on various substrates at 72 hours. The ALP activities in cells on higher concentration of CTM (≥ 0.625 mg/cm2) are significantly higher than the cells on lower concentration of CTM (≤ 0.31 mg/cm2) or TCPS.
The ALP activity of HO cells at Day 6 was also evaluated using Fast Blue RR salt staining method (Figure 11). The cells surrounding the CTM-particulates showed a more intense blue staining than cells on TCPS. In order to normalize the ALP positive cells to the whole cell population on each substrate, the samples were stained with Hoechst Dye 33258 for DNA (Figure 12).
By comparing the Fast Blue RR staining and DNA staining, one can tell that only a population (percentage) of cells on CTM-coated surface or TCPS showed positive ALP activity. Such percentage is higher on CTM-coated surface than on TCPS. Using this qualitative assay, we showed that cells cultured on CTM-coated surfaces maintained a better ALP activity, which is consistent with the results obtained from the quantitative ALP activity assay (Figure 7).
The clinical series: Fifty-nine joints (Table 1) in thirty-eight consecutive patients (aged 54.3 ± 14.6 years, 14 men) met the inclusion and exclusion criteria (Table 2). One patient was lost to follow-up and excluded from the analysis. Seven (18.9%) patients were diagnosed with diabetes mellitus, 2 (5.4%) with Rheumatoid Arthritis, and 2 (5.4%) with Charcot arthropathy. Co-morbidities are summarized in Table 3.
| Joint | Count (%) |
|---|---|
| 1st TMT | 14 (23.7) |
| 2nd TMT | 2 (3.4) |
| Ankle | 5 (8.5) |
| NC | 2 (3.4) |
| STJ | 24 (40.7) |
| TN | 12 (20.3) |
| Total | 59 (100.0) |
| Abbreviations: NC: Naviculocuneiform; STJ: Subtalar Joint; TMT: Tarsometatarsal; TN: Talonavicular. | |
Table 1: Joints.
| Patient demographics | Value |
|---|---|
| Patients | 37 (100.0) |
| Age (years) | 54.3 ± 14.6 |
| BMI (kg/m2) | 30.8 ± 6.3 |
| Gender | |
| Female | 23 (62.2) |
| Male | 14 (37.8) |
| Surgical Side | |
| Left | 14 (37.8) |
| Right | 23 (62.2) |
Table 2: Patient demographics. Data presented as mean SD and count (%).
| Co-morbidity | Count (%) |
|---|---|
| Charcot arthropathy | 2 (5.4) |
| Diabetes | 2 (5.4) |
| Hypertension | 7 (18.9) |
| Rheumatoid Arthritis | 2 (5.4) |
| Smoking History | 7 (18.9) |
Table 3: Co-morbidities. Data presented as count (%).
Indications for surgery included arthritis, hallux valgus, mal-union, Charcot arthropathy, drop foot, fracture, non-union, pes-valgus, or a combination of the aforementioned conditions. No intraoperative complications were encountered in this patient population. Postoperative complications are listed in Table 4.
| Concomitant Procedures | Count (%) |
|---|---|
| Hardware removal | 10 (27.0) |
| Tendo-Achilles lengthening | 8 (21.6) |
| Ankle scope | 5 (13.5) |
| Second metatarsal osteotomy | 2 (5.4) |
| 2nd Digit arthroplasty | 1 (2.7) |
| 2nd/3rd digit PIPJ arthroplasty | 1 (2.7) |
| 5th Digit arthroplasty | 1 (2.7) |
| Akin | 1 (2.7) |
| Evans | 1 (2.7) |
| Ex-fix removal | 1 (2.7) |
| Hallux IPJ arthrodesis | 1 (2.7) |
| Neurectomy | 1 (2.7) |
| Sural neurolysis | 1 (2.7) |
Table 4: Concomitant procedures. Data presented as count (%).
The overall mean time to fusion was 61.4 ± 68.8 days. Ninety-seven percent (57/59) of joints achieved bony union within the six months of the procedure. The two patients requiring greater than six months to achieve bony union took 14.0 and 13.3 months, respectively. The patient that required the 14 months to achieve union was diabetic with a Charcot foot, and the patient that required 13.3 months to achieve bony union was a tibio-talar-calcaneal fusion after failed total ankle arthroplasty. Removing these two outliers from the time to fusion calculation, the overall mean time to fusion improves to 49.0 ± 15.6 days, and the STJ time to fusion improves from 82.2 ± 104.4 days to 51.8 ± 17.9 days. Average time to fusion by joint is provided in Table 5 and shown in Figure 13.
| Joint | Time to fusion (days) |
|---|---|
| 1st TMT | 43.3 ± 8.9 |
| 2nd TMT | 41.5 ± 12.0 |
| Ankle | 67.8 ± 21.2 |
| NC | 38.0 ± 4.2 |
| STJ | 51.8 ± 17.9 |
| TN | 45.8 ± 9.8 |
| Total | 49.0 ± 15.6 |
| Abbreviations: NC: Naviculocuneiform; STJ: Subtalar Joint; TMT: Tarsometatarsal; TN: Talonavicular. | |
Table 5: Time to fusion. Data provided as mean ± standard deviation. The two outliers were excluded from these calculations (57 Joints).
Postoperative complications are summarized in Table 6. Ten postoperative complications were encountered in 7 patients. All complications with exception of the pulmonary embolism were considered to be minor and did not contribute to a delay of healing for any of the patients.
| Patient No. | Complications |
|---|---|
| 1 | DVT/PE, Tibial fracture |
| 2 | Loosening hardware |
| 3 | Loosening hardware |
| 4 | Broken hardware |
| 5 | Painful hardware |
| 6 | Neuritis |
| 7 | Wound dehiscence |
| Abbreviations: DVT: Deep Venous Thrombosis; PE: Pulmonary Embolism. | |
Table 6: Postoperative complications by patient.
One patient experienced pulmonary embolism and an anterior tibial crest fracture secondary to a 2.0 mm external fixation wire that pulled through the anterior tibial cortex. The patient was hospitalized and treated with appropriate anti-coagulation for the pulmonary embolism. A pin exchange was performed to stabilize the external fixator and treat the tibial crest fracture. The pulmonary embolism resolved without complication and the anterior tibial crest fracture united without issue.
Two patients demonstrated hardware loosening. In one patient, the lateral calcaneal screw loosened from an intra-medullary nail. However, anatomic alignment was maintained and healing was not interrupted. The second patient with loose hardware had no interruption of healing and did not undergo any treatment.
Discussion
The biocompatibility of CTM with human osteoblasts was evaluated in this study. The adhesion, proliferation and osteogenic activity of osteoblasts on CTM were compared with negative control (ULAP) or conventional cell culture substrate (TCPS). The benchtop data demonstrated that the adhesion of HO cells was CTM-dependent. With higher concentrations of CTM, more cells adhered to the surface. However, prolonged incubation of cells in serum-free medium did not improve cell adhesion. It was also observed that cells tend to grow on or near CTM particulates at early time points, and with prolonged incubation, this became even more prominent. A percentage of cells on CTMcoated surface showed positive ALP activity. Similarly, a percentage of cells on TCPS showed positive ALP activity, but activity was less pronounced on TCPS than the CTM-coated surface.
Based on these findings, the authors drew the following conclusions:
1. The presence of CTM particulates is required for the adhesion of human osteoblast (HO) cells.
2. CTM particulates not only support cell adhesion but also support the proliferation of HO cells.
3. In comparison with the conventional cell culture surface, HO cells on CTM-coated surface maintained a better osteogenic activity.
4. While the clinical data is a small sample size and does not utilize computed tomography (CT) scanning to assess arthrodesis, the data support our hypothesis that there may be a role for CTM to aid healing.
In the clinical series, 38 consecutive patients for a total of 59 joints underwent hindfoot or ankle arthrodesis using a combination of autogenous cancellous calcaneal bone graft mixed with 100 mg of connective tissue matrix. The clinical data demonstrated arthrodesis on at least three cortices in an average of 49.0 ± 15.6 days in 57 joints.
One may ask, why augment an autogenous bone graft with CTM? First, the bench top data support the notion that an environment rich in CTM is a tremendous substrate for cellular proliferation and ultimately, release of endogenous growth factors to support healing. Autogenous bone graft success can be affected by several factors, including patient age and health status. The benefits of CTM may help offset these factors.
Since CTM is derived from an allogenic decellularized particulate human connective tissue matrix, it retains its fundamental structural and functional characteristics, including key biochemical components of a connective tissue excellular matrix, thus providing mechanical adaption, structural support and the framework for incorporation by the recipient’s cells. The observed positive outcomes are likely the function of these characteristics. However, the identities of extracellular matrix molecules or signaling pathways involved require further investigation. The authors recommend a multi-center clinical trial with blinded computed tomography to fully assess the benefits that this pilot study has demonstrated.
Conflicts of Interest
Dr. Brigido SA serves on the surgery advisory board for celularity. He also serves as a consultant for Stryker. Bhatia M is a research consultant for celularity. Neither of the aforementioned companies had any knowledge or influence in study design, protocol, or data collection. For the remaining authors, no potential conflicts of interest exist.
Acknowledgements
No funding was received to support any of the discussed work. The preliminary clinical case series was presented at the American College of Foot and Ankle Surgeons annual scientific conference in Las Vegas, NV (2017).
References
- Hollinger JO, Brekke J, Gruskin E, Lee D (1996) Role of bone substitutes. Clin Orthop Relat Res 324: 55-65.
- Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG (1996) Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 17: 175-185.
- Frohlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, et al. (2008) Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther 3: 254-264.
- Zelen CM, Poka A, Andrews J (2013) Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: A feasibility study. Foot Ankle Int 34:1332-1339.
- Hanselman AE, Tidwell JE, Santrock RD (2014) Cryopreserved human amniotic membrane injection for plantar fasciitis: A randomized, controlled, double-blind pilot study. Foot Ankle Int 36:151-158.
- Daniels TR, Younger AS, Penner MJ, Wing KJ, Le IL, et al. (2015) Prospective randomized controlled trial of hindfoot and ankle fusions treated with rhPDGF-BB in combination with β-TCP-collagen matrix. Foot Ankle Int 36: 739-748.
- Ducy P, Schinke T, Karsenty G (2000) The osteoblast: A sophisticated fibroblast under central surveillance. Science 289: 1501-1504.
- Shapiro F (2008) Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater 15:53-76.
- Von Der Mark K, Gauss V, Von Der Mark H, Muller P (1977) Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267: 531-532.
- Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12: 207-218.
- Fernandes MH, Costa MA, Carvalho GS (1997) Mineralization in serially passaged human alveolar bone cells. J Mater Sci Mater Med 8: 61-65.
- Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, et al. (2012) Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage 20: 1170-1178.
- Sabokbar A, Millett PJ, Myer B, Rushton N (1994) A rapid, quantitative assay for measuring alkaline phosphatase activity in osteoblastic cells in vitro. Bone Miner 27: 57-67.
Citation: Brigido SA, Carrington S, Protzman NM, Mao Y, Pashuck TE, et al. (2018) The Use of an Acellular Connective Tissue Matrix in Hindfoot and Ankle Fusions: Understanding the Cellular Bench Top Data with a Consecutive Patient Series: A Pilot Study. Clin Res Foot Ankle 6: 276. DOI: 10.4172/2329-910X.1000276
Copyright: © 2018 Brigido SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6000
- [From(publication date): 0-2018 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 4849
- PDF downloads: 1151