Research Article Open Access
Thermodynamic and Hydro Dynamic Modeling Studies Using Mixed Adsorbent Prepared from Activated Charcoal and Bone Charcoal for the Removal of Copper and Cadmium
Srinivas Tadepalli1*, KSR Murthy2 and P Vijay1
1Department of Chemical Engineering, Bule Hora University, Ethiopia, Africa
2College of Engineering Studies, University of Petroleum and Energy Studies, Bidholi, Dehradun, India
- *Corresponding Author:
- Srinivas Tadepalli
Department of Chemical Engineering
Bule Hora University, Ethiopia, Africa
Tel: +251929523981
E-mail: stadepalli48@gmail.com
Received date: December 28, 2015; Accepted date: January 23, 2016; Published date: January 28, 2016
Citation: Tadepalli S, Murthy KSR, Vijay P (2016) Thermodynamic and Hydro Dynamic Modeling Studies Using Mixed Adsorbent Prepared from Activated Charcoal and Bone Charcoal for the Removal of Copper and Cadmium. Ind Chem 2:114. doi:10.4172/2469-9764.1000114
Copyright: © 2016 Tadepalli S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
and bone Charcoal as a low cost material for the removal of copper and cadmium from synthetic metal solution was studied. A number of experiments were performed in order to determine the potential capacity of the adsorbent in terms of thermo Dynamic equilibrium from the batch data and Hydro dynamic study from the column data equilibrium experiments. The Positive values of change in Enthalpy show that the process is endothermic in nature for Cd (II) and the negative values of Change in Enthalpy shows that the process is exothermic in nature for Cu (II). The standard Gibb’s free energy values are positive which means that the process is not spontaneous in nature. The negative values of ΔS show that there is decrease in randomness at the solid/solution interface during the adsorption of copper. The basic hydrodynamic parameters of the packed bed are analyzed. The influence of different parameters such as liquid velocity, particles size and void age on mass transfer in packed beds is represented. The data for mass transfer in the investigated system are shown using Sherwood number (Sh), Schmidt number (Sc), mass transfer coefficient (K) and Colburn factor (JD) as a function of Reynolds number (Re) for particles and for column. The plot between Reynolds number (Re) and the ratio of Sh/ Sc are represented at different flow rates. Therefore in the present investigation it was summarized that large flow rates and smaller bed heights rendered the minimum possible resistance for the transfer of metal ions from liquid phase to packed bed. The study revealed that mixed adsorbent prepared by blending the activated charcoal and bone charcoal in 1: 1 ratio has more potential to act as an adsorbent for the removal of Cu and Cd from aqueous solution.
Keywords
Thermo dynamic equilibrium; Hydro dynamics; Gibb’s free energy; Sherwood number; Schmidt number; Mass transfer coefficient; Colburn factor; Reynolds number; Mixed adsorbent
Introduction
Discharge of industrial waste water containing heavy metals (Cu, Cd, Cr, Zn, Hg etc) into the environment has become a serious threat to the human and aquatic life. The series of heavy metals that are made up of many elements which are chromium, Zinc, iron, lead copper which cause the environmental pollution when they exceed their toxic limit. Heavy metal pollution in air, water and soil is a global problem generated by mining and refining operations, metal processing plants and waste incineration. Heavy metals are core elements of earth’s crust which combined by metals and metalloids with atomic density greater than 4000 kg/m3 [1,2]. Some of the heavy metal ions are micro nutrients for living beings, but at higher concentration they lead to severe poisoning. The most toxic forms of these metals in their ionic species exists in the stable oxidation states like Cd2+, Pb2+, Hg2+, Ag2+ and As3+ in which they react with the bodybio-moleculesto form extremely stable bio toxic compounds which are difficult to dissociate [3-5].
In the very recent years increasing concern about the effect of toxic metals in the environment has resulted in more strict environmental regulations for industrial applications that discharge the metal bearing effluents. Removal of metal ions from waste water in an effective manner has become an important issue. Although small concentration of heavy metals is needed to all living organisms but high concentration of these metals can cause several diseases like neurological and psychological effect on human body. In the environment the heavy metals are generally more persistent than organic contaminants such as pesticides and petroleum by products. They also become mobile in soils depending on soil pH and their properties. So a portion of the total mass can leach to aquifer or can become bio available to living organism. Heavy metal poisoning can result from drinking water contamination (e.g., Lead pipes, industrial and consumer waste) in take through the food chain or high ambient air conditions near emission sources [6]. In natural environments, these elements may be sorbed by soil components or sediments dissolved in aquatic solution and/ or accumulated by living organisms such as crops, vegetables and fish and then may enter into the food chain. Therefore the sorption of heavy metals on soil components or sediments relates closely to their mobility and bio availability and plays a vital role in relieving their threat to human being and animals. Therefore the presence of ions of heavy metals in waste water even at low concentrations is a hazard to the aquatic ecosystem and raises many risks for human beings [7-11].
Distribution of heavy metal pollutants
Metal contamination of soil can occur by a variety of processes but in general it can be stated that in areas of active aerial contamination and sewage sludge disposal, it tends to show highest concentrations and contents in the upper layers of the soil profile. The distribution and mobility of an individual metal within a specific soil cannot be determined based on physical or chemical properties alone and more over biological and climatic conditions are also taken into account. Application of sewage sludge to land has become the main cause for metallic contamination which is due to the addition of excess of organic matter along with the metals. The presence of excess of organic matter not only affects the distribution of the applied metal, but also the distribution of metals already present in the soil. Soil pH also effects the distribution and mobility of metals, applied with sewage sludge. In India most of the urban areas are employed for controlled dumping of domestic refuse and such areas represent the potential reservoirs or storage for heavy metals. The areas are either used for vegetable growing purposes or reclaimed by local authorities by bull-dozing them flat, so that the effuse becomes intermixed with top soil or subsoil. Such operations invariably bring about increase in the trace element content of the soil. At this stage the enhanced levels of trace metals may produce photonic efforts or ground water pollution. When the sewage -sludge is spread over the land, the metals present are distributed up to different depths in the soil. Cu accumulation in surface layers is higher than in sewage irrigated soil. In both the cases of sewage -irrigated and non-irrigated soils, Cu concentration decreased with depth. Copper and Zinc also decreased with depth in soil profiles. Metal compounds though not a normal constituent of air are found in a great variety of forms particularly in urban areas. They occur mostly in the particulate phase with some exceptions. The sources of metallic contaminants in air are both natural (e.g., terrestrial, marine, volcanic, biogenic) and anthropogenic (e.g., Industrial and automobile combustion). Urban industrial air may contain suspended particulates of wide variety of pollutants including heavy metals such as lead, nickel, zinc, copper, cadmium etc depending upon the specific type of mining, milling or manufacturing units located in that area. Metal ions are being absorbed by the lungs ten times more efficiently than the intestines. They are of great concern as a health hazard especially in densely populated areas and work places where high levels of metal-containing vapors and particles exist. The levels show an increasing trend in different areas as follows: remote<rural<urban<industrial areas. There is evidence to show that high emission of toxic trace metals leads to an enrichment of the respective components even in remote environments such as arctic areas. The atmospheric occurrence of heavy metals depends upon the source strength, atmospheric dispersion and the deposition processes. The source strength in turn depends upon the emission factors. Emission of trace metals in the northern hemisphere (80%) is much higher than in southern hemisphere (30%). As compared to remote and unpolluted regions there is a tremendous global increase in the air concentrations of these potentially hazardous heavy metals in urban areas. A world Health Organization (WHO) guideline for air quality for some of these metals such cadmium (10-20 μg/ year), lead (0.5-1 μm/ year), and manganese (1 μg/year) mercury (1 μg/year) and vanadium (1 μg/day). Absolutely pure water does not exist in nature. Water for drinking and domestic purposes is generally supplied by river, lakes, wells and springs. Such water differ substantially from sea water and may contain organic matter from micro-organisms, salts of calcium, iron, magnesium, potassium and sodium and traces of CO2, Oxide, nitrogen, ammonia and other gases from the atmosphere. Presence of potentially hazardous heavy metals in water should be considered abnormal and this usually affects its appearance and palatability. Metals of prime environmental concern (e.g., Cadmium, copper, lead, mercury, zinc, etc.) are present in very low concentrations in sea water but increasing more due to the influence of human activities. Drinking is one of the routes of intake of heavy metals into the human body. It has also drawn special attention of health managers. The WHO has laid down the guideline values (mg/l) for heavy metals/metalloids in drinking water which are given as follows: arsenic (0.05 mg/l), Cadmium (0.005), chromium (0.05), lead (0.05), mercury (0.001) and selenium (0.01). Wide variations in metal uptake are observed in different plant species. Some plants are known to have special affinity for accumulating certain metals e.g., Astragals sp (Se), Crotalaria cobalt cola (Co), Phaseolus vulgaris roots (Zn), alga chlorculla vulgaris (Aug), Alyssum sp and sebertia accuminatic (Ni) etc. Besides the root metal may enter the plant through aerial parts including the leaf surface. High Pb content (from motor exhaust fumes) may be observed in plants growing near busy traffic areas. Minute particles less than 2 μm in diameter of some metals or metalloids such as As, Cd, Cu, Se and Zn emanating from combustion sources may get dissolved in rain water and enter the plant through leaf surface. Foliar application of some fertilizers may contribute to the bio-availability of Cu, Fe, Mn and Zn. Metals exhibit variable affinity to be absorbed by the plants. The bioaccumulation index (plant/soil ratio) for various metals is given as Cd, Zn (1 to 10), Cu, Mn, Ni (0.1 to 1.0), AS, Mn, Pb (0.01 to 0.1) and Fe, V, Ti (0.001 to 0.01) [12,13].
Toxicity of copper: Copper is one of the main constituents of heavy metal series. The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) 2011 have ranked Copper at 125th position with a total cumulative score of 805 points [1]. The excessive intake of copper results in bioaccumulation of Cu (II) in digestive organs of humans, kidney, mucosal irritation, and anemia [2,3]. The excessive intake of copper results in bioaccumulation of Cu (II) in digestive organs of humans, kidney, mucosal irritation, and anemia [2,3]. The World Health Organization (WHO) has demarcated the limit of copper (II) in drinking water as 2 mg/L [6]. The wastewater generated from most of the industries like metallurgical units, paints and pigments, alloy and manufacturing, acid mine drainage, and so forth contains huge amount of copper [4,5]. The excessive concentration of copper affects the health of living beings adversely. In past, many conventional technologies like chemical hydroxide precipitation, coagulation and flocculation, cementation, osmosis, reverse osmosis, and so forth have been practiced to remove heavy metals from liquid phase [7,8]. Most of these technologies have been found as very costly and do not work below a minimum concentration of 100 mg/L. Moreover, the usage of these technologies has led to the generation of secondary chemical sludge, thereby making its disposal undesirable. However, the removal of heavy metal ions by adsorption process has been studied in detail. Earlier, various sorts of nonconventional adsorbents like lichen, activated carbon, eucalypts leaf powder, and dead bacterium cells have been used for the removal of metal ions [9-11]. Unquestionably, the adsorption mediated by nonconventional adsorbents has contributed significantly in industrial pollution remediation.
Sources of copper pollution: Cu occurs in 0, +1, +2 oxidation states. The cupric ion (Cu2+) is the most toxic species of Cu which occurs in the form of Cu (OH)+ and Cu2 (OH)2+. In aerobic alkaline systems, CuCO3 is the main dominant soluble species. In anaerobic environment CuS will form in the form of Sulphate and chlorides. Copper also forms strong solution complexes with humic acids. Pollution due to various heavy metals is a matter of serious concern in the present scenario. The usage and importance of a particular metal gives a clear indication of pollution caused in the environmental system. Elevated environmental levels lo Cu (II) come from many sources are caused due to mining, plasticizers, Petro chemicals, metal cleaning, pulp and paper board mills, refineries, fertilizers industry which are very toxic to human and aquatic life. Copper which is widely used metal in industry is an essential trace element for human health and play an important role in carbohydrate and lipid metabolism and in the maintenance of heart and blood vessel activity. The adult human body contains 100- 150 mg of Cu (II) ion, but excess amounts in the body can be toxic. In aqueous environments, the speciation of the metal is dependent upon both the ligand concentration and pH, while the cupric ion Cu (II) is the metallic form most toxic to flora and fauna, which is also a necessary nutrient for algal growth. If allowed to enter the environment the excessive amounts of Cu (II) cause serious potential health issues such as nausea, massive gastrointestinal bleeding, respiratory difficulty, headache, dizziness, hemolytic anemia, liver and kidney failure and even to death. The World Health Organization (WHO) recommended acceptable concentration of Cu (II) in drinking water to a maximum limit of 1.5 mg/l [12-16].
Discharge limits of copper pollution: The (WHO) recommended a maximum acceptable concentration of Cu (II) in drinking water is 1.5 mg/l. Maximum soil natural concentration of copper is 2-100 ppm normal range in plants is found to be 5-30 ppm. Plant toxicity level is 30-100 ppm. According to USEPA, the Maximum Concentration Limit (MCL) of copper in water is 1.3 ppm. Environmental Protection Agency (EPA) determines the level of contaminants in drinking water at which no adverse health effects are likely to occur. These nonenforceable health goals, based solely on possible health risks and exposure over a lifetime with an aquatic margin of safety are called Maximum Contaminant level goals (MCLG). Contaminants are any physical, chemical, biological or radiological substances or matter in water. The maximum contaminant level goals for copper are 1.3 mg/l. EPA has set this level of protection based on the best available science to prevent potential health problems.
Effects of copper: Increased copper bound protein concentrations have been seen in the hepatic ailments both in adults and children. Wide variations in copper concentrations reported by different workers in normal and diseased liver samples, do not give any conclusion in this regard. The utility of hepatic copper estimations appear to be more in case of prolonged homeostasis, chronic biliary cirrhosis and Wilson’s disease. Values of copper levels in these diseases may be up to thirty times more compared to the control levels.
Copper health issues and toxicity: People who drink water containing copper in excess of the actual level may vary with short term exposure, experience gastrointestinal distress, and with longterm exposure may experience liver or kidney damage. People with Wilson’s disease should consult their personal doctor if the amount of copper in water exceeds the actual level. This health effects language is not intended to catalogue all possible health effects for copper. Rather it is intended to inform consumers of some of the possible health effects associated with copper in drinking water when the rule was finalized.
Cadmium: Cadmium is a chemical element with symbol Cd and atomic number 48. This soft, Bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it prefers oxidation state +2 in most of its compounds and like mercury it shows a low melting point compared to transition metals. Cadmium and its congeners are not always considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth’s crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Strom Iyer and Hermann, both in Germany, as an impurity in zinc carbonate. Cadmium occurs as a minor component in most zinc ores and therefore is a by-product of zinc production. It was used for a long time as a pigment and for corrosion resistant plating on steel, whereas cadmium compounds were used to stabilize plastic. The use of cadmium is generally decreasing due to its toxicity it is specifically listed in the European Restriction of Hazardous Substances [2] and the replacement of nickel-cadmium batteries with nickel-metal hydride and lithium-ion batteries. One of its few new uses is in cadmium telluride solar panels. Although cadmium has no known biological function in higher organisms, a cadmium-dependent carbonic anhydride has been found in marine diatoms [16,17].
Effects of cadmium: This metal accumulates preferentially in the liver. It is present in negligible amounts at birth, and gradually increases with age and is responsible for extensive peri-portal, interlobular fibrosis, biliary hyperplasia and other forms of liver damage. Cadmium inhibits both the content and activity of drug-metabolizing enzymes. Hepatic damage is one of the serious effects of acute cadmium toxicity. High protein died (particularly Steiner rich) protects the liver from cadmium-mediated damage. Following oral ingestion or inhalation, chromium accumulates in the liver. In human beings, chromium intoxication leads to two types of syndromes: pulmonary and gastric. In both the cases it is associated with liver damage ranging from an increase in the size of the organ to cirrhosis and organ dysfunction. The metal is bound to methionine in liver and the complex so formed is transported kidney. It concentrations is higher in the cortex region than in medulla. Tubular damage may also occur along with glomerular damage or even independently. These proteins are electrophoretic ally different from protein excreted [18]. Some of the harmful effects of Cd (II) include chronic and acute metabolic disorders such as itaiitai disease, renal damage, emphysema and testicular atrophy [14]. Because of its toxic properties and tendency for bio-accumulation in food chain, it is imperative to take effective precautions and to reduce the concentration levels of heavy metals in waste water. Cadmium is one of the heavy metals with a high potential hazard to human being and environment. It enters to water bodies through waste water from metal plating industries, Cd-Ni battery industries, mining, stabilizers and alloys. Poisoning of Cadmium in human can cause high blood pressure, kidney damage and sometimes destruction of testicular tissue and red blood cells. In small amounts cadmium is associated with hypertensive diseases and considered as carcinogenic to men [4].
Sources of cadmium pollution: The major sources of Cadmium pollution in drinking water are corrosion of galvanized pipes, erosion of natural deposits, discharge from refineries, runoff from waste batteries and paints. In the environment Cadmium exists in only one oxidation state (+2) and does not undergo oxidation-reduction reactions. In surface water and ground water, cadmium can exists as the hydrated ion or as ionic complexes with other inorganic or organic substances. Soluble forms of cadmium can migrate in water. Insoluble forms of Cadmium will settle and adsorb to sediments. Cadmium’s fate in soil depends on several factors such as pH of the soil and the availability of the organic matter. Generally, cadmium will bind strongly to organic matter and for the most part it will immobilize. Cadmium occurs in +2 oxidation states and hydroxide Cd (OH)2 and carbonate CdCO3 dominate at high pH whereas Cd2+ and aqueous sulphate species dominate at lower pH (<8). It will precipitate in the presence of phosphate, chromate, arsenate, sulphide, etc. It shows mobility at pH range of 4.5-5.5. The importance of heavy metal pollution control has increased significantly in recent decades. Toxic metal Cd (II) has become an eco-toxicological hazard of prime interest and increasing significance owing to their tendency to accumulate in the vital organs in human and animals. Cd (II) does not degrade into harmless end products in the metabolism and they rather accumulate. Cadmium is introduced into the water from cadmium-nickel batteries, smelting, metal plating, phosphate, fertilizer industries, mining, pigments, stabilizers, alloy industries and sewage sludge [12,13].
Uses of cadmium: Cadmium is used primarily for metal plating and coating operation including transportation equipment, machinery and baking enamels photography and television phosphors. It is also used in nickel- cadmium solar batteries and pigments.
Cadmium health issues and toxicity
People who drink water containing cadmium well in excess of the maximum contaminant level (MCL) for few days or weeks can cause nausea, vomiting and diarrhoea. It will suffer on muscle cramps, liver injury. Drinking of Cadmium containing water for more years could experience kidney damage and it will cause prostate cancer. The permissible level of Cd (II) according to Indian Standards 10500 in the year 1992 for drinking water, surface water and public sewers are 0.01 ppm, 2.00 ppm, and 1.00 ppm respectively [4,6]. The Permissible limits for metals in Waste water according to USEPA for some heavy metals are listed in Table 1.
| Metal | Concentration |
|---|---|
| Copper (Cu) | 1.3 mg/l or 1.3 ppm |
| Cadmium (Cd) | 0.005 mg/l or 0.005 ppm |
| Lead (Pb) | 0 ppm |
| Iron | 0.3 mg/l |
| Zinc | 5 mg/l |
Table 1: Permissible limits for metals in Waste water according to USEPA.
Available technologies for the removal of heavy metals from waste water
Many procedures have been adopted in order to remove heavy metals from aqueous streams, among the most commonly used techniques are chemical precipitation, chemical oxidation and reduction, ion exchange, filtration, electrochemical treatment, reverse osmosis (membrane technologies), evaporative recovery and solvent extraction. These classical or conventional techniques give rise to several problems such as unpredictable metal ions removal and generation of toxic sludge which are often difficult to de water and require extreme caution in their disposal. Besides that most of these methods also present some limitations whereby they are economically viable at high or moderate concentrations of metals but not at low concentrations, which means the dilute solutions containing from 1 to 100 mg/l of dissolved metals (s). Heavy metals removed by classical techniques involve expensive methodologies. These are due to high energy and frequent reagent requirements. Some of them are explained in brief with their disadvantages [19-23].
Several technologies exist for the remediation of heavy metals contaminated ground water and soil and they have some definite outcomes such as: (i) Complete or substantial destruction/ degradation of the pollutants (ii) Extraction of pollutants for further treatment or disposal (iii) Stabilization of pollutants informs less mobile or toxic (iv) Separation of non-contaminated materials and their recycling from polluted materials that require further treatment (v) Contaminant of the polluted material to restrict exposure of the wider environments. Many techniques have been employed for the treatment of wastewater containing heavy metal ions including precipitation, coagulation, In Situ reduction process, co-precipitation, evaporation, chemical coagulation/ flotation and flocculation, cementation, heavy metal removal from biosurfacants, sorption, reverse osmosis, ion exchange and adsorption. There is a long series of technologies involved in the removal of heavy metals from various types of industrial wastewater and mine drainage. The conventional heavy metal ion remedial technologies have some major technical shortcomings [24,25].
Materials and Methodology
All the chemicals including adsorbents used for the studies were purchased from Sigma Aldrich, India and have purity above 99.5%. All the reagents, buffer solutions used for the study are of analytical grade.
Methods
This section gives an overview of methods involved in the preparation of adsorbent, characterization of the prepared adsorbent, procedure carried out for adsorption in batch study. Rotary shaker (Spectra Lab instruments Private Ltd and Industrial Research (Delhi) Model HM8T) was used for agitating the solutions containing the mixed adsorbent along with the metal ion solution. Atomic Absorption Spectrophotometer (Thermo Scientific ICE 3000 series) for sample Analysis of Cu (II) and Cd(II) before and after adsorption.
Preparation of the mixed adsorbent: The mixed adsorbent (Activated Charcoal, AC+Bone charcoal, BC) was prepared in 1:1 ratio and sieve analysis (SELEC XT 264, AIMIL company ltd)was carried out in a rotary sieve shaker to determine the particle size of the mixed adsorbent. The average particle diameter of the mixed adsorbent was obtained as 572.2 nm.
Working of the particle size analyzer: The Particle size analyzer works on the principle of Dynamic Light Scattering where He- Ne Laser acts as a light source. There should be Brownian motion in the solution for the uniform distribution of the particle size. The ZetaSizer Nano series performs size measurements using a process called Dynamic Light Scattering (DLS). Dynamic Light Scattering (also known as PCS - Photon Correlation Spectroscopy) measures Brownian motion and relates this to the size of the particles. It does this by illuminating the particles with a laser and analyzing the intensity fluctuations in the scattered light.
Characterization of the mixed adsorbent: Physical Characterization such as Proximate and Ultimate analysis (ASTM Distillation, 2009). FTIR analysis, BET analyses were carried out to determine the physico-chemical properties, different functional groups available for adsorption and surface area of the adsorbent.
Proximate and ultimate analysis: Proximate analysis is a type of assay for the determination of different constituents present in the coal sample. The standard procedures are followed to determine the bulk density, average particle size diameter, moisture content, volatile content, ash content, fixed carbon and surface area. Ultimate analysis gives the composition of the sample in terms of weigh t% of Carbon, Nitrogen, Hydrogen, Oxygen and Sulfur. The carbon content determination includes the carbon present in the organic coal substance and as mineral carbonates. Hydrogen determination gives the hydrogen content in the organic materials with the coal. All nitrogen determined is assumed to be the part of the organic materials in the coal. Table 2 shows the proximate and ultimate analysis for the adsorbent. Table 3 shows the physical properties of the mixed adsorbent.
| Property | Composition of the mixed Adsorbent |
|---|---|
| Bulk Density (g/cc) | 0.74 |
| Average particle diameter | 572.2 nm |
| Moisture content | 3.43% |
| Volatile matter | 23.61% |
| Ash Content | 4.39% |
| Fixed carbon | 68.57% |
| Surface area (m2/g) | 951 |
Table 2: Proximate and Ultimate analysis of the mixed adsorbent.
| Adsorbent type | Surface area (m2/g) | Moisture (%) | Particle size (μm) | Charge |
|---|---|---|---|---|
| 100% AC | 1600 | 5 | 33.29 | neutral |
| 100% BC | 267 | 3.43 | 28.40 | -ve |
| 50% each | 951 | 4.24 | 32.44 | -ve |
Table 3: Physical Properties of the mixed adsorbent.
Batch studies
Following a systematic procedure for the removal of heavy metal ions, initially the presterilizing flasks containing heavy metal ion solution of 50 mg/l Cu (II) and Cd (II) were prepared and the mixed adsorbent of 0.25 g each (AC and BC of 1:1 ratio) was added after maintaining the desired pH. The pH was adjusted by adding 0.1M NaOH or 0.1M HCl. Adsorption process was carried out in the rotary shaker/agitator until the equilibrium. The analysis was done for the filtered samples by Atomic Absorption Spectrophotometer (AAS) to find the residual concentration in the solution. After the analysis the equilibrium time and % removal of heavy metal ions were calculated. The data obtained in the present studies were used to calculate the equilibrium metal adsorptive quantity/capacity (mg/g) by using the mass balance relationship and the percentage removal of heavy metal ions. Experiments were conducted (three repetitions) simultaneously and the average values were reported. The equilibrium metal adsorptive capacity of the metal ions (qe) and % removal were calculated by using mass balance given by
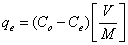 (1)
(1)
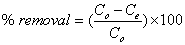 (2)
(2)
Where qe is the amount of heavy metal ion adsorbed per unit weight of adsorbent in mg/g, V is the volume of the solution treated in liters. Co, Ce is the initial and equilibrium concentration of metal ions in mg/l. M is the mass of the mixed adsorbent in grams.
Column studies
Continuous flow operation experiments were conducted in a transparent cylindrical plastic column (4 cm internal diameter and 100 cm height). A 20 mesh size stainless sieve was attached to the bottom of the column. A known quantity of the adsorbent in the ratio of 1:1 (mixed adsorbent) was added in the column to yield the desired bed height (12 cm, 24 cm, 36 cm). Cu (II) and Cd (II) solution of known concentration (100 mg/l was pumped into the column using a 40 W submersible pump at the desired flow rate (10 ml/min, 20 ml/min, 30 ml/min). Samples were collected from the exit of the column at different bed heights and at different intervals of time until the achievement of equilibrium and analyzed using Atomic Absorption Spectrophotometer (AAS). The experimental set up of the packed bed column was shown in Figure 1.
Study of bed heights (weight of the adsorbent) and volumetric flow rate: The design of the packed bed column has been studied at a bed height of 12 cm, 24 cm, 36 cm and the flow rates varying from 10, 20, 30 ml/min with an initial concentration of 100 ppm. The main design of the column involves the study of break through curves experimentally and to compare with the theoretical models such as Yoon- Nelson model, Thomas model.
Parameters and dimensions of the packed bed column: Weight of the mixed adsorbent added 50 g, 100 g, and 150 g respectively (for 12 cm -25 g each; for 24 cm -50 g each; for 36 cm -75 g each.)
Inner Diameter of the column: 4 cm
Bed height studied=12, 24, 36 cm
Total height of the column=100 cm
Adsorbent ratio=1:1 (AC+ BC)
Submersible pump used for sending the effluent into the column=40 Watts
Initial Metal Con of the metal ions Cu and Cd (Co)=100 ppm
Effect of volumetric flow rate -10, 20, 30 ml/min
Effect of Weight of the adsorbent (bed height) -12 cm, 24 cm, 36 cm
Adsorption
Adsorption refers to the selective collection and concentration of a particular type of molecules contained in a fluid phase onto a solid surface. The molecules of the adsorbate come from the fluid phase into the interface, where they remain for a period of time. In a reversible process, the molecules go back to the phase from which they came or reversibly pass into another phase while other molecules replace them at the interface. On reaching the solid surface the adsorbed molecules exchange energy with structural atoms of the surface and if sufficient time is given for adsorption, the adsorbed molecules and the surface atoms reach thermal equilibrium. At equilibrium, the number of molecules arriving at the interface in a given time is equal to the number of molecules leaving the interface to go into the fluid phase [26].
Molecular or atomic interactions occur at interfaces between gas and solid, gas and liquid, liquid and solid, two liquids and sometimes between solid phases. Interaction at an interface causes the transition of a molecule from one phase to another which is called as sorption of the molecule by the given phase. Adsorption of atoms or molecules on to a solid from a fluid phase takes place when the sorbed molecules or atoms concentrated only at the interface. Thus the substances contained in a fluid phase is said to be adsorbed on a solid phase when its concentration in the boundary region is higher than that in the bulk of the fluid phase. The substance which is adsorbed is called adsorbate (metal ions) and the phase of boundary which adsorption occurs is called adsorbent. For adsorption to take place on the surface of an adsorbent it should have the larger surface area accessible to the adsorbate, preferential ability to take up the adsorbate. Large surface areas are available from substances which are both very porous and finely divided. Adsorbents are usually highly porous materials and adsorption takes place primarily on the walls of the pores or specific sites inside the particle. The surface area available for some adsorbents may be as high as 2000 m2/g. Activated carbon; activated alumina, molecular sieve and silica gel are widely used as adsorbents. When the adsorbent reaches its saturation capacity, the adsorbed material can be removed by the industrial methods such as displacement of adsorbate, desorption of the adsorbate, combustion of the adsorbate decomposition or radioactive decay of the adsorbate. If the combustion of the adsorbate is used in the operation it is called reactivation and when the displacement or desorption of adsorbate is used; the operation is called regeneration.
All the adsorption processes are exothermic. The heat of adsorption may be defined as the decrease in the heat content of the system. Adsorption on solid surfaces may be classified on the basis of the magnitude of the energy of adsorption. Vander Waals or physical adsorption refers to a process in which energy changes are very small. When the energy changes are greater the process is called chemisorption or chemical adsorption. In case of physical adsorption, the adsorbate merely condenses in a thin film on the surface of the adsorbent. The forces which retain the adsorbate in this state are purely physical and are called as Vander Waals forces (weak). They are very weak molecular attractions and physically adsorbed substances are loosely bound. In chemical adsorption the adsorbate is much more strongly bound when compared to physical adsorption and the intermolecular forces of attraction are very strong. The heats of adsorption are in the same order of the magnitude as of the corresponding chemical reactions. The actual Chemical Combination occurs between the adsorbed molecule and the active centre on the surface of the adsorbent. Chemisorption plays an important role in Adsorption and Catalysis. In the physical adsorption the removal of adsorbed molecule can be very easy whereas in chemical adsorption the removal of adsorbate is very difficult. Physical adsorption is usually instantaneous while chemical adsorption generally requires activation energy and in some cases it may be instantaneous [27-29].
The equilibrium between the concentration or partial pressure of the adsorbate in the liquid / fluid phase and the concentration of the adsorbate held by a particular adsorbent is called isotherm of the solute and adsorbent system at a given temperature. An adsorption isotherm is a plot of volume of gas adsorbed against partial pressure at various parameters of constant temperature. There are many equations which represent the equibrium adsorption data and of these two widely used are Freundlich equation and Langmuir equation. The Langmuir equation is theoretical, but does not hold for many substances. On the other hand, Freundlich equation is empirical and holds good in many cases where Langmuir equation does not fit [12,13,30].
Stirred tank absorbers are generally used for removing pollutants and heavy metals from industrial effluents. It consists of a cylindrical tank fitted with a stirrer or air sparger. The air sparger or stirrer keeps the particles in the tank in suspension. The adsorbent is powdered carbon which is added to the solution in the tank. Used carbon is removed by sedimentation or filtration. The mode of operation may be batch or continuous [31-33]. In the continuous absorber, the solid and fluid move through the bed counter currently and come in contact with each other through the entire apparatus without periodic separation of the phases. The solid particles are fed from the top and flow down through the adsorption and regeneration sections by gravity and then returned to the top of the column by an air lift or mechanical conveyor. The fine particles use multi-stage fluidized beds, in which the fluidized solids pass through down comers from stage to stage. Adsorption may occur in two different ways according to the interactions of the adsorbent and adsorbate which are named as physical adsorption (physisorption or van der Waals) and chemical adsorption (chemisorption). The adsorption processes with nonspecific interactions are generally referred as physisorption. In chemisorption processes, electrons are shared or transferred between two phases. Since chemical bonds occur between the adsorbate and the surface of the adsorbent, new chemical compound is formed. As a result of this, interactions are very strong at the chemisorption processes with respect to the physisorption processes. Also only monolayer is observed in chemisorption and it is slower than the physisorption process. Dispersion and short range repulsive forces, Hydrogen-bonds and covalent bonds can be involved in adsorption processes. In addition, if the solid or the gas is polar in nature, there will be also electrostatic (columbic) forces comprising dipole-dipole, dipole induced dipole interactions. Due to the specific interactions between adsorbate and adsorbent, the heat of adsorption of chemisorption is higher than physisorption and is observed in the short range. However, there are different heat of adsorption ranges for physical and chemical adsorption processes 10 to 40 kJ/mol and 40 to 800 kJ/mol respectively. Adsorption of vapour on to a solid surface is a spontaneous in nature, so the overall free energy change for the process is negative. On the other hand, during the adsorption process, the adsorbing molecules lose a degree of freedom and their entropy decreases. From the thermodynamic relationship given in Equation it is obvious that for ΔG to be negative, ΔH should be negative. So, adsorption process generally becomes exothermic
ΔG=ΔH–TΔS (3)
However, an endothermic trend can also be observed in some cases. For instance, due to the lateral protein-protein interactions and conformational changes in the adsorbed protein, adsorption becomes endothermic [34,35].
Physisorption: The fundamental interacting force of physisorption is caused by weak van der Waals force. Even though the interaction energy is very weak (10 to 100 m eV) and physisorption plays an important role in adsorption system. Van der Waals forces originate from the interactions/metal binding tendencies between induced and permanent or transient electric dipoles. In contrast with chemisorption, in which the electronic structure of bonding atoms or molecules is changed and covalent or ionic bonds are formed whereas physisorption can only be observed in the environment of low temperature (thermal energy at room temperature which is equal to 26 m eV) and the absence of the relatively strong chemisorption. In practice, the type of a particular adsorption as physisorption or chemisorption depends principally on the binding energy of the adsorbent to the substrate or adsorbate [36]. The strength by which adsorbate molecules are attached/ bonded with the adsorbents determines the nature of adsorption. Normally the release of energy is in the range of 8 to 25 kJ/mole due to adsorption which is defined as physisorption and a much larger energy is required for the formation of chemical bonds which leads to chemisorption. There are always some exceptions (excluded from) these values. The prescribed value of energy differentiating physisorption and chemisorption are based on general experience. When an adsorbed molecule receives energy equal to or higher than the energy of adsorption, it will leave the surface. This phenomenon is the reverse of adsorption and is called as desorption. When the number of molecules hitting the surface and staying there is equal to the number of molecules that are leaving or evaporating the surface of the system, it is said to be in equilibrium. All the atoms or molecules adsorbed on the surface do not have similar environment since distribution of free energy on the surface is not always smooth because of the differences in the energy of the molecular orbitals of the adsorbent and also due to other internal interactions. Physisorption is due to the absence of chemical bonds and the molecule retains its gas phase electronic structure, although some disturbances are still possible. The binding energy depends on the polarizability and on the number of atoms involved of the atoms and varies between few milli eV light gases and several eV for heavy metals and large organic molecules).
Chemisorption: Chemisorption is a kind of adsorption which involves a chemical reaction between the adsorbent surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. The strong interaction between the adsorbate and the substrate (adsorbate) surface creates new types of electronic bonds [36,37]. In comparison with Physisorption, Chemisorption leaves the chemical species of the adsorbate on surface to interact. It is conventionally accepted that the energetic threshold limit separating the binding energy of physisorption from that of chemisorption is equivalent to 0.5 eV per adsorbed species. Due to specificity, the nature of chemisorption can greatly differ, depending up on the chemical identity and the surface structure of the adsorbent [36]. In Chemisorption, there is stronger perturbation of the molecular electronic structure with formation of chemical bonds along with the substrate molecules and the energies typically are of several eV.
Results and Discussion
Thermo dynamic modelling of copper and cadmium
Temperature dependence of the adsorption process is associated with several thermodynamic parameters. In order to describe the thermodynamic behaviour of the adsorption of Cu (II) and Cd (II) ions onto mixed adsorbent, the standard Gibbs free energy is calculated by using the following equation
ΔGo=-RT ln k (4)
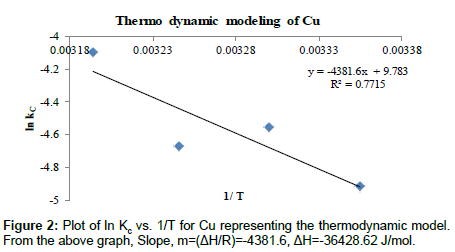
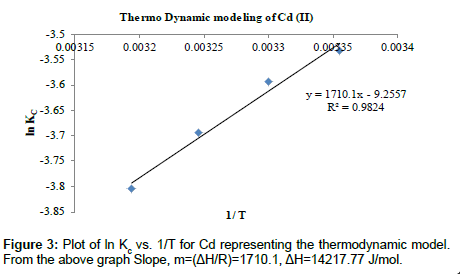
Where R is the universal gas constant (8.314 J mol-1 K-1), T is the temperature in Kelvin and k is the equilibrium or rate constant, calculated as the surface and solution metal distribution ratio. The negative values of ΔG° indicate the spontaneous nature of the adsorption process. However, the negative value of ΔG° decreases with an increase in temperature, indicating that the spontaneous nature of adsorption which is inversely proportional to the temperature. Tables 4 and 7 represent the thermo dynamic parameters for Cu (II) and Cd (II) respectively. Tables 5 and 8 describes about the various thermo Dynamic Constants Estimation for Cu (II) and Cd (II) respectively. Tables 6 and 9 represent the various Thermo Dynamic Parameter calculations such as Gibbs free energy (ΔG), Change in entropy (ΔS) and Change in enthalpy (ΔH) for Cu (II) and Cd (II) ions respectively (Figures 2 and 3).
| Temp (°C) |
Time (Min) |
Final Concentration (ppm) | Removal% | (Equilibrium Constant) kC |
|---|---|---|---|---|
| 25 | 5 | 6.55 | 86.9 | 0.131 |
| 10 | 6.03 | 87.93 | 0.1206 | |
| 15 | 5.99 | 88 | 0.1198 | |
| 20 | 5.53 | 89.2 | 0.1106 | |
| 30 | 5.14 | 89.7 | 0.1028 | |
| 40 | 4.96 | 90.07 | 0.0992 | |
| 50 | 4.77 | 90.4 | 0.0954 | |
| 60 | 4.58 | 90.83 | 0.0916 | |
| 75 | 3.07 | 92.39 | 0.0614 | |
| 90 | 2.94 | 93.46 | 0.0588 | |
| 105 | 2.17 | 95.93 | 0.0434 | |
| 120 | 1.39 | 97.21 | 0.0278 | |
| 130 | 1.38 | 97.32 | 0.0276 | |
| 140 | 1.36 | 97.35 | 0.0272 | |
| 150 | 1.36 | 97.35 | 0.0272 | |
| 160 | 1.36 | 97.35 | 0.0272 | |
| 180 | 1.36 | 97.35 | 0.0272 | |
| 200 | 1.36 | 97.35 | 0.0272 | |
| 30 | 5 | 9.46 | 87.65 | 0.1892 |
| 10 | 7.38 | 88.73 | 0.1476 | |
| 15 | 5.29 | 90.45 | 0.1058 | |
| 20 | 4.77 | 92.21 | 0.0954 | |
| 30 | 3.73 | 92.34 | 0.0746 | |
| 40 | 3.47 | 92.57 | 0.0694 | |
| 50 | 3.26 | 93.45 | 0.0652 | |
| 60 | 3.31 | 94.78 | 0.0662 | |
| 75 | 3.05 | 95.73 | 0.061 | |
| 90 | 2.69 | 96.84 | 0.0538 | |
| 105 | 2.17 | 97.74 | 0.0434 | |
| 120 | 1.13 | 98.12 | 0.0226 | |
| 130 | 1.13 | 98.13 | 0.0226 | |
| 140 | 1.12 | 98.16 | 0.0224 | |
| 150 | 1.12 | 98.17 | 0.0224 | |
| 160 | 1.12 | 98.17 | 0.0224 | |
| 180 | 1.12 | 98.17 | 0.0224 | |
| 200 | 1.12 | 98.17 | 0.0224 | |
| 35 | 5 | 8.42 | 88.94 | 0.1684 |
| 10 | 7.69 | 93.45 | 0.1538 | |
| 15 | 7.11 | 94.57 | 0.1422 | |
| 20 | 6.8 | 95.76 | 0.136 | |
| 30 | 6.44 | 96.73 | 0.1288 | |
| 40 | 6.07 | 96.93 | 0.1214 | |
| 50 | 5.81 | 98.12 | 0.1162 | |
| 60 | 4.77 | 98.25 | 0.0954 | |
| 75 | 4.3 | 98.56 | 0.086 | |
| 90 | 1.91 | 98.64 | 0.0382 | |
| 105 | 1.18 | 98.77 | 0.0236 | |
| 120 | 0.86 | 99.12 | 0.0172 | |
| 130 | 0.86 | 99.14 | 0.0172 | |
| 140 | 0.855 | 99.2 | 0.0171 | |
| 150 | 0.85 | 99.24 | 0.017 | |
| 160 | 0.85 | 99.24 | 0.017 | |
| 180 | 0.85 | 99.24 | 0.017 | |
| 200 | 0.85 | 99.24 | 0.017 | |
| 40 | 5 | 14.98 | 92.32 | 0.2996 |
| 10 | 11.75 | 95.87 | 0.235 | |
| 15 | 9.72 | 96.65 | 0.1944 | |
| 20 | 9.41 | 96.92 | 0.1882 | |
| 30 | 8.83 | 97.14 | 0.1766 | |
| 40 | 7.11 | 97.58 | 0.1422 | |
| 50 | 5.86 | 98.34 | 0.1172 | |
| 60 | 4.72 | 98.41 | 0.0944 | |
| 75 | 2.53 | 98.93 | 0.0506 | |
| 90 | 1.39 | 98.99 | 0.0278 | |
| 105 | 0.81 | 99.15 | 0.0162 | |
| 120 | 0.45 | 99.36 | 0.009 | |
| 130 | 0.447 | 99.45 | 0.00894 | |
| 140 | 0.445 | 99.67 | 0.0089 | |
| 150 | 0.44 | 99.75 | 0.0088 | |
| 160 | 0.44 | 99.75 | 0.0088 | |
| 180 | 0.44 | 99.75 | 0.0088 | |
| 200 | 0.44 | 99.75 | 0.0088 |
Table 4: Thermo Dynamic Parameters for Cu (II).
| T (°C) | T (K) | 1/T | kc | lnkc |
|---|---|---|---|---|
| 25 | 298 | 0.00336 | 0.00728 | -4.922 |
| 30 | 303 | 0.0033 | 0.01051 | -4.555 |
| 35 | 308 | 0.00325 | 0.00936 | -4.6717 |
| 40 | 313 | 0.00319 | 0.01664 | -4.096 |
Table 5: Thermo Dynamic Constants Estimation for Cu (II).
| T (K) | Kc | lnKc | ΔG=-RT ln Kc(J/mol) | ΔH (J/mol) | ΔS=(ΔH-ΔG)/T (J/mol K) |
|---|---|---|---|---|---|
| 298 | 0.00728 | -4.922 | 12194.61 | -36429 | -163.1652013 |
| 303 | 0.01051 | -4.555 | 23669.3 | -36429 | -198.3429703 |
| 308 | 0.00936 | -4.6717 | 35632.18 | -36429 | -233.9636364 |
| 313 | 0.01664 | -4.096 | 46291.1 | -36429 | -264.2802556 |
Table 6: Thermo Dynamic Parameters.
| Temp (°C) | Time(Min) | Final Concentration (ppm) | Removal% | (Equilibrium Constant)kc |
|---|---|---|---|---|
| 25 | 5 | 23.38 | 52.33 | 0.4676 |
| 10 | 23.68 | 52.54 | 0.4736 | |
| 15 | 23.61 | 52.85 | 0.4722 | |
| 20 | 23.51 | 53.27 | 0.4702 | |
| 30 | 22.6 | 54.83 | 0.452 | |
| 40 | 22 | 57.23 | 0.44 | |
| 50 | 21.75 | 58.38 | 0.435 | |
| 60 | 20.91 | 59.31 | 0.4182 | |
| 75 | 18.43 | 60.56 | 0.3686 | |
| 90 | 18.21 | 65.98 | 0.3642 | |
| 105 | 16.09 | 67.13 | 0.3218 | |
| 120 | 10.62 | 77.54 | 0.2124 | |
| 130 | 10.59 | 77.65 | 0.2118 | |
| 140 | 10.44 | 77.96 | 0.2088 | |
| 150 | 10.43 | 78.01 | 0.2086 | |
| 160 | 10.42 | 78.06 | 0.2084 | |
| 30 | 5 | 21.96 | 56.29 | 0.4392 |
| 10 | 21.44 | 57.13 | 0.4288 | |
| 15 | 20.4 | 57.65 | 0.408 | |
| 20 | 19.88 | 58.9 | 0.3976 | |
| 30 | 18.31 | 60.98 | 0.3662 | |
| 40 | 17.79 | 63.06 | 0.3558 | |
| 50 | 15.71 | 64.42 | 0.3142 | |
| 60 | 13.63 | 66.5 | 0.2726 | |
| 75 | 12.06 | 68.58 | 0.2412 | |
| 90 | 11.02 | 70.15 | 0.2204 | |
| 105 | 9.98 | 72.65 | 0.1996 | |
| 120 | 8.94 | 79.94 | 0.1788 | |
| 130 | 8.56 | 80.67 | 0.1712 | |
| 140 | 7.97 | 81.19 | 0.1594 | |
| 150 | 7.78 | 81.92 | 0.1556 | |
| 160 | 7.55 | 82.23 | 0.151 | |
| 35 | 5 | 19.88 | 60.25 | 0.3976 |
| 10 | 18.83 | 62.33 | 0.3766 | |
| 15 | 18.31 | 63.58 | 0.3662 | |
| 20 | 17.79 | 64.94 | 0.3558 | |
| 30 | 17.27 | 65.25 | 0.3454 | |
| 40 | 16.75 | 67.13 | 0.335 | |
| 50 | 15.71 | 68.58 | 0.3142 | |
| 60 | 14.67 | 70.15 | 0.2934 | |
| 75 | 13.63 | 72.75 | 0.2726 | |
| 90 | 12.06 | 73.27 | 0.2412 | |
| 105 | 10.5 | 75.56 | 0.21 | |
| 120 | 7.38 | 83.38 | 0.1476 | |
| 130 | 7.29 | 84.31 | 0.1458 | |
| 140 | 7.13 | 84.52 | 0.1426 | |
| 150 | 7.04 | 84.63 | 0.1408 | |
| 160 | 6.78 | 84.73 | 0.1356 | |
| 40 | 5 | 17.79 | 64.42 | 0.3558 |
| 10 | 16.75 | 66.5 | 0.335 | |
| 15 | 16.23 | 68.58 | 0.3246 | |
| 20 | 15.19 | 70.25 | 0.3038 | |
| 30 | 14.67 | 72.33 | 0.2934 | |
| 40 | 13.63 | 73.69 | 0.2726 | |
| 50 | 12.58 | 74.83 | 0.2516 | |
| 60 | 11.02 | 75.56 | 0.2204 | |
| 75 | 8.94 | 79.21 | 0.1788 | |
| 90 | 8.42 | 82.75 | 0.1684 | |
| 105 | 6.85 | 84.63 | 0.137 | |
| 120 | 5.81 | 87.54 | 0.1162 | |
| 130 | 5.73 | 87.75 | 0.1146 | |
| 140 | 5.61 | 87.85 | 0.1122 | |
| 150 | 5.55 | 87.96 | 0.111 | |
| 160 | 5.56 | 87.97 | 0.1112 |
Table 7: Thermo Dynamic Parameters for Cd (II).
Analysis of thermo dynamic modeling: The Positive values of change in Enthalpy show that the process is endothermic in nature for Cd (II) and the negative values of Change in Enthalpy show that the process is exothermic in nature for Cu (II). The standard Gibb’s free energy values are positive which means that the process is not spontaneous in nature. The negative values of ΔS show that there is decrease in randomness at the solid/solution interface during the adsorption of copper. Similarly for Copper and Cadmium the positive values of ΔS shows that there is an increase in randomness of the system with the decrease in temperature from 313 K to 298 K as shown in Tables 6 and 9 respectively. The poor values of R2 show that the Copper (II) ion does not fit the thermo Dynamic model while cadmium fits the system with high R2 values of 0.9824 [16,38,39].
| T (°C) | T (K) | 1/T | kc | lnkc |
|---|---|---|---|---|
| 25 | 298 | 0.00336 | 0.02923 | -3.5327 |
| 30 | 303 | 0.0033 | 0.02745 | -3.595 |
| 35 | 308 | 0.00325 | 0.02485 | -3.6949 |
| 40 | 313 | 0.00319 | 0.02224 | -3.806 |
Table 8: Thermo Dynamic Constants Estimation for Cd (II).
| T (K) | Kc | lnKc | ΔG=-RT lnKc (J/mol) | ΔH ((J/mol) | ΔS=(ΔH-ΔG)/T ((J/mol K) |
|---|---|---|---|---|---|
| 298 | 0.02923 | -3.5327 | 8752.51 | 14217.77 | 18.33979866 |
| 303 | 0.02745 | -3.595 | 9056.31 | 14217.77 | 17.03452145 |
| 308 | 0.02485 | -3.6949 | 9461.57 | 14217.77 | 15.44220779 |
| 313 | 0.02224 | -3.806 | 9904.28 | 14217.77 | 13.78111821 |
Table 9:Thermo Dynamic Parameters for Cd (II).
Hydrodynamic behavior study
In the present investigation, the hydrodynamic behavior of metal ion solution in packed bed column combined with forced convective mass transfer has been studied with respect to dimensionless numbers such as Sherwood number (Sh), Reynolds number (Re), and Schmidt number (Sc). The empirical correlations for the above mentioned dimensionless number have been shown in equations (5) and (6), respectively [40].
Sherwood number Sh=JD Re Sc1/3 (5)
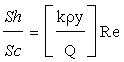 (6)
(6)
Schmidt number, 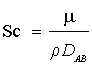 (7)
(7)
Q=Volumetric flow rate (10, 20, 30 ml/min)
Where density of metal ion solution, ρ=1000 kg/m3
Viscosity of water, μ=0.01 kg/m-s
Diffusivity of water, DAB=1.29 × 10-9 m2/s
ε, porosity of the bed=0.4 (assumed as the particles are in fine powder)
JD=Chilton-Colubrn factor
k=mass transfer coefficient (m/s)
y=mass fraction of the adsorbent varying with respect to bed heights (y for 12 cm=0.166, y for 24 cm=0.33 and y for 36 cm=0.5)
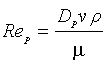 (8)
(8)
Velocity of the fluid, 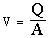 (9)
(9)
Mass flow rate of the fluid, m=Q × ρ (10)
Where Q is the volumetric flow rate of the fluid (ml/min) and ρ is the density of the metal ion solution.
Relationship between Sherwood number and Reynolds number for particles (dissolution method) where
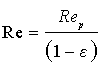 show good agreement with our experimental data.
show good agreement with our experimental data.
Rep is called Reynolds number of the particle.
Correlations to find Chilton Coulbrn factor are given [41] as
JD=5.7(Re)-1.22 for 1<Re<30(11)
JD=1.77(Re)-1.56 for 30<Re<1000(12)
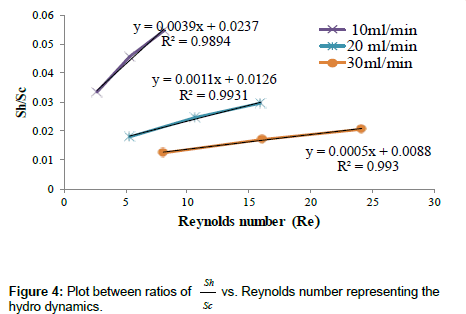
Where, JD, Κ, ρ and y are Clinton-Colbourn factor, mass transfer coefficient (m/s), density of solution, and mass fraction of the component, respectively. Finally mass transfer coefficient (k) is found from equation 6 after calculating the ratio of Sh/Sc (Sherwood number to Schmidt number). The hydro dynamic modeling data has been reported in Table 10.
| Flow rate | Bed height (Cm) | Re | Sc | Sh | Sh/ Sc | Colburn factor (JD) | Mass fraction (y) | Mass transfer Coefficient(K× 10-12) |
|---|---|---|---|---|---|---|---|---|
| 10ml/min | 12 | 2.66 | 1550 | 51.91 | 0.0334 | 1.728 | 0.166 | 12.9 |
| 24 | 5.32 | 775.2 | 35.15 | 0.0457 | 0.7417 | 0.33 | 4.33 | |
| 36 | 8.11 | 516.8 | 28.275 | 0.0547 | 0.443 | 0.5 | 2.25 | |
| 20ml/min | 12 | 5.32 | 3100.78 | 56.02 | 0.0181 | 0.7417 | 0.166 | 6.81 |
| 24 | 10.64 | 1550.38 | 38.264 | 0.02468 | 0.318 | 0.33 | 2.34 | |
| 36 | 15.96 | 1033.6 | 30.61 | 0.0296 | 0.1941 | 0.5 | 1.236 | |
| 30ml/min | 12 | 8.04 | 4651.16 | 58.48 | 0.01257 | 0.4482 | 0.166 | 4.71 |
| 24 | 16.08 | 2325.6 | 39.94 | 0.01717 | 0.1924 | 0.33 | 1.61 | |
| 36 | 24.12 | 1550.38 | 31.96 | 0.0206 | 0.1173 | 0.5 | 0.854 |
Table 10: Hydro Dynamic Modeling of Column studies.
Hydrodynamic behavior of fluid flow inside the packed bed column at various flow rates and bed heights: The hydrodynamic behavior of fluid flow inside the column has been shown in Table 10. It became evident from the above Table that the dimensionless parameters varied significantly with flow rate of the metal ion solution inside the packed bed column and bed height [42]. With the increase of flow rate there was a significant rise in the velocity of fluid inside the column, Reynolds number The Schmidt number, Sherwood number, Colubrn factor (JD) decreases in the order of bed height increase (from 12 to 36 cm) at constant flow rate. The Sherwood number decreased with the increase of flow rate from 10 ml/min to 30 ml/min. However, with the increase of bed height at fixed flow rate, there was a decrease in velocity of the metal ion solution inside the bed, Schmidt number, Sherwood number, Colubrn factor (JD) and increase in the Reynolds number was observed. The decrease in the values of these forced convective (inter phase) mass transfer parameters was due to the path resistance posed by the particles of bed, and this resistance increased with the increase of bed height and reduced when the flow rate is high. Therefore, in the present investigation it was summarized that large flow rates and smaller bed heights rendered the minimum possible resistance for the transfer of metal ions from liquid phase to packed bed. Figure 4 indicates the plot between the Reynolds number (Re) and the ratio of 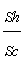 (Sherwood number to Schmidt number) at different flow rates. The trend lines are drawn along with correlation coefficient (R2) values to represent the column dynamics. From the graph it was observed that at different flow rates the regression / correlation coefficient (R2) values are very close to 1 which indicates that the column hydro dynamics are well best fitted to the adsorption system.
(Sherwood number to Schmidt number) at different flow rates. The trend lines are drawn along with correlation coefficient (R2) values to represent the column dynamics. From the graph it was observed that at different flow rates the regression / correlation coefficient (R2) values are very close to 1 which indicates that the column hydro dynamics are well best fitted to the adsorption system.
Conclusions
The potential of Activated Charcoal and bone Charcoal as a low cost material for the removal of copper and cadmium from synthetic metal solution was studied. A number of experiments were performed in order to determine the potential capacity of the adsorbent in terms of thermo Dynamic equilibrium from the batch data and Hydro dynamic study from the column data equilibrium experiments. The Positive values of change in Enthalpy show that the process is endothermic in nature for Cd (II) and the negative values of Change in Enthalpy shows that the process is exothermic in nature for Cu (II). The standard Gibb’s free energy values are positive which means that the process is not spontaneous in nature. The negative values of ΔS show that there is decrease in randomness at the solid/solution interface during the adsorption of copper. Similarly for Cadmium the positive values of ΔS for Cd (II) shows that there is an increase in randomness of the system with the decrease in temperature from 313 K to 298 K. The poor values of R2 show that the Copper (II) ion does not fit the thermo Dynamic model while cadmium fits the system with high R2 values of 0.9824. The basic hydrodynamic parameters of the packed bed are analyzed. The influence of different parameters such as liquid velocity, particles size and void age on mass transfer in packed bed is represented. The data for mass transfer in the investigated system are shown using Sherwood number (Sh), Schmidt number (Sc), mass transfer coefficient (K) and Colburn factor (JD) as a function of Reynolds number (Re) for particles and for column. The plot between Reynolds number (Re) and the ratio of Sh/ Sc are represented at different flow rates. The trend lines are drawn along with correlation coefficient (R2) values to represent the column dynamics. From the graph it was concluded that at different flow rates the regression / correlation coefficient (R2) values are very close to 1 which indicates that the column hydro dynamics are better fitted to the adsorption system. Therefore in the present investigation it was summarized that large flow rates and smaller bed heights rendered the minimum possible resistance for the transfer of metal ions from liquid phase to packed bed. The study revealed that mixed adsorbent prepared by blending the activated charcoal and bone charcoal in 1:1 ratio has more potential to act as an adsorbent for the removal of heavy metal ions (Cu and Cd) from aqueous solution.
Acknowledgements
One of the authors (Srinivas Tadepalli) is gratefully acknowledging the authorities of UPES, Dehradun especially Dr SJ Chopra, Chancellor for providing Doctoral Research Fellowship to carry out the work.
References
- (2015) Detailed data for 2011 priority list of Hazardous Substances, ATSDR, Division of toxicology and environmental medicine.
- Bayramoglu G, Arica MY, Adiguzel N (2012) Removal of Ni(II) and Cu(II) ions using native and acid treated Ni-hyperaccumulator plant Alyssum discolor from Turkish serpentine soil.Chemosphere 89: 302-309.
- Blazquez G, Martin-Lara MA, Dionisio-Ruiz E, Tenorio G, Calero M (2011) Evaluation and comparison of the biosorption process of copper ions onto olive stone and pine bark. Journal of Industrial and Engineering Chemistry 17: 824-833.
- Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater.J Environ Manage 92: 2355-2388.
- Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. Journal of Chemical Technology and Biotechnology 84: 13-28.
- Sahan T, Ceylan H, Sahiner N, Aktas N (2010) Optimization of removal conditions of copper ions from aqueous solutions by Trametes versicolor.Bioresour Technol 101: 4520-4526.
- Sari A, Tuzen M (2008) Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies.J Hazard Mater 160: 349-355.
- Sari A, Mendil D, Tuzen M, Soylak M (2008) Biosorption of Cd (II) and Cr (III) from aqueous solution bymoss (Hylocomium splendens) biomass: equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal 144: 1-9.
- Uluozlu OD, Sari A, Tuzen M, Soylak M (2008) Biosorption of Pb(II) and Cr(III) from aqueous solution by lichen (Parmelina tiliaceae) biomass.Bioresour Technol 99: 2972-2980.
- Mishra V, Balomajumder C, Agarwal VK (2012) Sorption of Zn (II) ion onto the surface of activated carbon derived from eucalyptus bark saw dust from industrial wastewater: isotherm, kinetics, mechanistic modeling, and thermodynamics. Desalination and Water Treatment 46: 1-20.
- Mishra V, Balomajumder C, Agarwal VK (2010) Zn (II) ion biosorption onto surface of eucalyptus leaf biomass: Isotherm, Kinetic, and mechanistic modeling. Clean-Soil, Air, Water 38: 1062-1073.
- Mishra V, Chandrajit B, Vijay kumar A (2013) Design and optimization of simultaneous biosorption and bioaccumulation (SBB) system: a potential method for removal of Zn (II) ion from liquid phase. Desalination and water treatment 7: 58-67.
- Mishra V, Majumder CB, Agarwal VK (2012) Simultaneous Adsorption and bioaccumulation: A study on continuous mass transfer in column reactor. Environmental Progress and Sustainable Energy 32: 605-614.
- Siva Kumar P, Palanisamy PN (2009) Packed bed column studied for the removal of Acid blue 92 and basic red 29 using non- conventional adsorbent. Indian Journal of Chemical Technology 16: 301-307.
- Ronbanchob A, Viriya M, Prasert P (2011) Kinetic and mass transfer analyses of metal biosorption by Caulerpa lentillifera. Desalination 278: 303-311.
- Thamilarasu P, Sharmila R, Karunakaran K (2011) Kinetic, equilibrium and thermodynamic studies on removal of Cr (VI) by Modified Activated Carbon prepared from Ricinus Communis Seed shell. Coromandal Journal of Science 91: 9-18.
- Priyantha N, Tennakoon DTB, Keerthiratn S, Thushara A (2000) Removal of Heavy metal ions from polluted water using environmentally friendly Materials. Journal of European sciences 48: 300-309.
- Khan MA, Ngabura M, Choong TS, Masood H, Chuah LA (2012) Biosorption and desorption of Nickel on oil cake: batch and column studies.Bioresour Technol 103: 35-42.
- Kurniawan A, Ochie V, Sisnandy A, Trilestari K, Sunardso J, et al. (2011) Performance of durian shell waste as high capacity biosorbent for Cr (VI) removal from synthetic wastewater. Journal of Ecological Engineering 37: 940-947.
- Li J, Enzan C, Haijia SU, Tianwei TAN (2011) Biosorption of Pb2+ with modified soyabean hulls as an adsorbent. Journal of Biotechnology and Bioengineering 19: 334-339.
- Ofomaja AE, Naidoo EB (2011) Biosorption of copper from aqueous solution by chemically activated pine cone: A kinetic study. Chemical Engineering Journal 175: 260-270.
- Cui P, Yun-Hai L, Xiao- Hong C, Min L, Gou-Lin H, et al. (2011) An Biosorption of Uranium (VI) from aqueous solution by dead fungal biomass of Penicillium citrinum. Journal of Chemical Engineering 10: 1- 6.
- Sina S, Stephan Z, Gabriele H, Helmut K, Harald C (2011) Biosorption of copper by Wine-relevant lactobacilli. International Journal of Food Microbiology 24: 126-131.
- Singha B, Das SK (2011) Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies.Colloids Surf B Biointerfaces 84: 221-232.
- Kun Y , Jackie H, McCandlish E, Buckley B, Patel R, et al. (2013) Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification applications. Journal of Colloids and Surfaces 425: 31-41.
- Ferrari L, Kaufmann J, Winnefeld F, Plank J (2010) Interaction of cement model systems with super plasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. Journal of Colloid Interface Science 347: 15-24.
- Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal 156: 2-10.
- Hanaor DA, Ghadiri M, Chrzanowski W, Gan Y (2014) Scalable surface area characterization by electrokinetic analysis of complex anion adsorption.Langmuir 30: 15143-15152.
- Akkaya G, Guzel F (2013) Bio removal and recovery of Cu (II) and Pb (II) from aqueous solution by a novel biosorbent watermelon (citrullus lanatus) seed hulls: Kinetic study, equilibrium isotherm, SEM and FTIR analysis. Desalination and Water Treatment 51: 7311-7322.
- Martin Lara MA, Dionisio Ruiz E, Tenorio GM, Calero G (2012) Copper biosorption by pine cone shell and thermal decomposition study the exhausted biosorbent. Journal of Industrial and Engineering Chemistry 18: 1741-1750.
- Sigworth EA, Smith SB (1972) Adsorption of inorganic compounds by activated carbon. Journal of American Water Works Association 64: 386-391.
- Schiewer S, Volesky B (1995) Modeling of the proton-metal ion exchange in biosorption.Environ Sci Technol 29: 3049-3058.
- Al-Haidary AMA, Zanganah FHH, Al-Azawi SRF, Khalil FI, Al- Dujaili AH (2011) A study on using date palm fibers and leaf base of palm as adsorbents for Pb (II) ions from its aqueous solution. Journal of water Air & Soil Pollution 214: 73-82.
- Katiyar A, Thiel SW, Guliants VV, Pinto NG (2010) Investigation of the mechanism of protein adsorption on ordered mesoporous silica using flow microcalorimetry.J Chromatogr A 1217: 1583-1588.
- Wang S, Boyjoo Y, Choueib A (2005) A comparative study of dye removal using fly ash treated by different methods.Chemosphere 60: 1401-1407.
- Bruch LW, Cole MW, Zaremba E (1997). Physical Adsorption: Forces and Phenomena. Clarendon, Oxford Press, London.
- Campuzano JC (1988) The chemical physics of solid surfaces and heterogeneous catalysis. Chemisorption Systems 5: 389-397.
- Akl Awwad M, Ahlam Farhan M (2012) Equilibrium, Kinetic and Thermodynamics of Biosorption of lead (II), Copper (II) and Cadmium (II) ions from Aqueous solutions onto Olive leaves Powder. American Journal of Chemistry 2: 238-244.
- Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell.J Hazard Mater 174: 137-143.
- Kasaini H, Mbaya RK (2009) Continuous adsorption of Pb ions in a batch reactor and packed-bed column. Hydrometallurgy 97: 111-118.
- Carlos CR, Richarrdson JF (1968) Solids movement in liquid fluidized beds-I Particle velocity distribution. Chemical Engineering Science 23: 813-824.
- Dwivedi PN, Upadhyay SN (1977) Particle-fluid mass transfer in fixed and fluidized beds – Process Design and Development. Industrial Engineering and Chemical engineering 16: 157-165.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10895
- [From(publication date):
March-2016 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 9947
- PDF downloads : 948


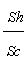 vs. Reynolds number representing the hydro dynamics.
vs. Reynolds number representing the hydro dynamics.