Research Article Open Access
Preparation of Emulsified Encapsulated Nanoparticles of Bovine Serum Albumin of Bound Glucose Oxidase and their Application in Soft Drinks/ Non-Alcoholic Beverage
Kirti Rani Sharma*
Amity Institute of Biotechnology, Amity University, Noida (U.P.), India
- Corresponding Author:
- Dr. Kirti Rani Sharma
Assistant professor, Amity Institute of Biotechnology
Amity University, Sec-125, Noida-201303, India
Tel: +919990329492
E-mail: krsharma@amity.edu
Received date: January 11, 2012; Accepted date: February 06, 2012; Published date: February 08, 2012
Citation: Sharma KR (2012) Preparation of Emulsified Encapsulated Nanoparticles of Bovine Serum Albumin of Bound Glucose Oxidase and their Application in Soft Drinks/Non-Alcoholic Beverage. J Biotechnol Biomaterial 2:126. doi:10.4172/2155-952X.1000126
Copyright: © 2012 Sharma KR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Microspheres are nanoparticles in which the glucose oxidase was encapsulated by emulsification and immobilized through covalent coupling by glutaraldehyde which is an excellent cross linking agent. Biodegradation of these emulsified encapsulated nanoparticles of bovine serum albumin of bound glucose oxidase was carried out by the proteases such as trypsin, chymotrypsin and papain. Chymotrypsin was found to be the best proteolytic enzyme among these proteases. These nanoparticles of bovine serum albumin of bound glucose oxidase were converted the glucose into gluconic acid and hydrogen peroxide in the delivery system which in turn minimized the utilization of the excess of glucose by the microbes/pathogens as a substrate for their growth. Coca-Cola has approximately half of the world market share and called as industrial player. And, then Pepsi sales stood at US $36 billion per market capitalization and thus the value sales of soft drinks/ non-alcoholic beverages will increase 27% by 2014 worldwide. Glucose is the major content of these drinks. Further, these glucose oxidase bound emulsified nanoparticles of bovine serum albumin along with chymotrypsin were used in soft drinks/non-alcoholic beverages which made the drinks free from turbidity and opalescence as well as to maintain the pH as acid regulator.
Keywords
Bovine serum albumin nanoparticles; Emulsification; Mustard oil; Glutaraldehyde; Glucose oxidase; Gluconic acid; Trypsin; Chymotrypsin; Papain
Introduction
Microspheres are spherical particles that may be in the form of nanoparticles and microcapsules in which the active gradient is dispersed in a hydrophilic or non-polymer layer. These microspheres allow the controlled release of active ingredient. Microspheres have been used as carriers in the delivery of drugs, antigens, hormones, enzymes and genes [1,2]. Besides, it has many advantages in the industrial applications. Albumin is a major plasma protein constituent as 55% of the total protein in human plasma. The exploitable features of albumin are its biodegradation into natural products, its lack of toxicity and its non-antigenicity. Albumin microspheres are easily metabolized in the body as well as the size of particles, degree of stabilization and site of metabolism are the main factors influencing the extent of metabolism. Hence, its colloidal forms have been considered as potential carriers of drugs for their site-specific localization. Albumin, being biodegradable, biocompatible and nontoxic, was chosen as a matrix material for the preparation of the microspheres [2,3]. Previously, immobilization of glucose oxidase was done on to ferrocene-modified pyrrole polymers [4], electrodeposited nickel oxide nanoparticles [5], gold particles [6], micro porous membrane of polyvinyl-diene-fluoride and poly acrylic acid [7], platinum nanoparticle [8] and silica gel [9,10]. The microsphere was prepared by the immobilization of glucose oxidase into calcium alginate gel capsules [11] and alginate microcapsules [12]. An amperiometric biosensor was made by the immobilization of glucose oxidase into redox-hydrogel [13]. Electrochemical immobilization of glucose oxidase was done in thin films of polymerized phenols [14] and onto carbon surface activated by the derivatization of reduced diazonium salts [15]. More sensitive amperiometric biosensors were made by the immobilization of glucose oxidase in chitosan matrix cross-linked with glutaraldehyde [16] and onto zinc oxide nanotubes [17]. Other well known glucose oxidase biosensor was developed by the immobilization of glucose oxidase in o-aminophenol film onto platinized glassy carbon electrode [18]. Immobilization of glucose oxidase was done onto carbon electrodes [19] as well as further glucose biosensor was developed based on the immobilization of glucose oxidase into multi wall carbon-nanotubes [20]. A novel glucose biosensor was made by the immobilization of glucose oxidase in titaniasolution gel membrane which was used for flow injection of serum glucose level [21]. Other novel modified and sensitive biosensor was developed based on the immobilization of glucose oxidase in chitosan on a glassy carbon electrode with gold-platinum alloy nanoparticles/ multiwall carbon nanotubes [22]. Albumin itself is an excellent matrix for immobilization of glucose oxidase, our work involves usage of bovine serum albumin for entrapment of biologically active materials glucose oxidase using cross linking agents and emulsifier. Microspheres were prepared using a natural polymer Bovine Serum Albumin using emulsification chemical cross-linking method.
Materials and Methods
Preparation of microspheres
Glutaraldehyde is used as cross linking agent and mustard oil is used as emulsifier which leads to give the newly formed fluorescent structure by exposing the tryptophan residues as its diene adduct [19,23]. The oil bath was prepared by adding 0.4 ml of 25% of glutaraldehyde and 2.6 ml of n-butanol in 50 ml of mustard oil along with 50 U of glucose oxidase. This solution was filled in the syringe and dispersed in prepared oil bath as well as stirred for overnight at 37°C. Next day, it was centrifuged at 5000 rpm for 10 minutes and supernatant was removed. The pellet was collected which contained the prepared emulsified nanoparticles of bovine serum albumin of bound glucose oxidase. These emulsified nanoparticles were washed five times with cold ether and two times with acetone. The emulsification with mustard oil was increased the stability of encapsulated nanoparticles as well as it was also allowed the controlled release of immobilized enzyme in the delivery system in the presence of proteolytic enzymes [10].
Preparation of test sample: Nanoparticles (2 mg) were incubated at room temperature (37°C) with 1 ml of proteolytic enzymes (trypsin, chymotrypsin and papain) containing 6, 12, 18, 24 (U) of chymotrypsin and trypsin ad papain. Assay of glucose oxidase in test sample; the enzyme assay of bound glucose oxidase in emulsified encapsulated nanoparticles of bovine serum albumin was estimated by the method as published by Worthington Mannual, New Jersey, USA.
β - D - glucose + enzyme – FAD? Enzyme – FADH2 + δ - Dgluconolactone
Enzyme - FADH2 + O2 → Enzyme – FAD + H2O2
Preparation of dye solution: o-dianisidine-HCl (1 mg) was dissolved in 1 ml methanol. Out of this 0.1 ml was taken and redissoved in 12 ml 0.1 M phosphate buffer, pH 6.0.
Preparation of reaction mixture: 2.55 ml of buffer dye solution, 0.3 ml 18% glucose solution and 0.05 ml peroxidase solution (1 mg/ ml) were mixed together. Set spectrophotometer at 460 nm and 25°C. Pipette into cuvette as follows (Table 1).
| Dianisidine-buffer mixture, pH 6.0 (oxygenated) | 2.5 ml |
| 18% Glucose | 0.3 ml |
| Peroxidase | 0.1 ml |
Table 1: Pipette into cuvette for spectrophotometer analysis.
Incubate in spectrophotometer for 3 - 5 minutes to achieve temperature equilibration and establish blank rate if any. Add 0.1 ml of appropriately diluted enzyme and record increase in A460 for 4 - 5 minutes. Calculate ΔA460 from the initial linear portion of the curve.
Calculation
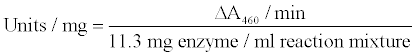
The glucose oxidase activity was checked every day (1–7 days) at different concentration of added trypsin, chymotrypsin and papain (6U, 12U, 18U & 24U). Reaction mixture of 3.0 ml was taken in a cuvette and 0.1 ml of aliquot obtained from nanoparticles in which mixture of proteolytic enzymes was added to it. Absorbance was recorded at 460 nm at 15 second intervals.
Result and Discussion
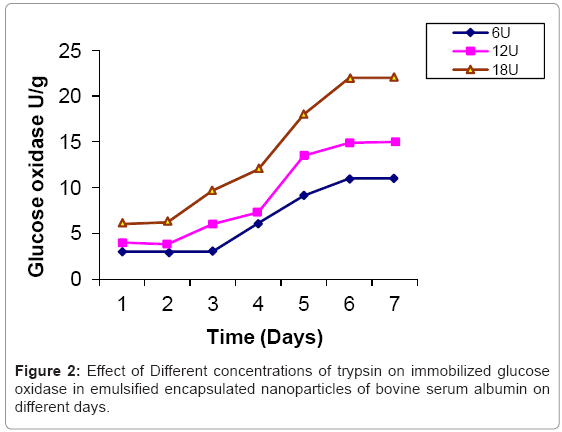
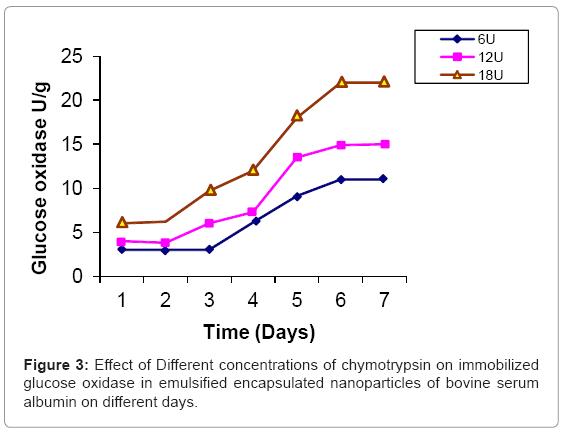
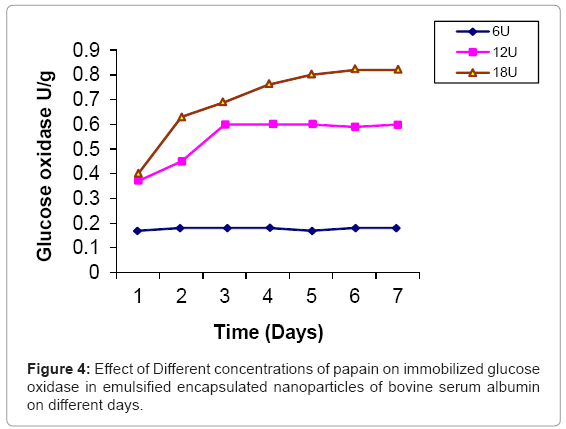
Glucose oxidase is immobilized in bovine serum albumin by encapsulation/entrapment using cross linking reagent such as glutaraldehyde and mustard oil. The nanoparticles were prepared under these conditions were spherical in shape and exhibit fluorescent at 495 nm (Figure 1). 50 U/g of Glucose oxidase was to be bound in emulsified nanoparticles of bovine serum albumin microspheres. After the encapsulation, 49 U/g of glucose oxidase was bound in prepared naoparticles and only 0.5 U/g of glucose oxidase was unbound. Thus, 99 % immobilization was done in this entrapment. The thermal stability of immobilized enzyme was increased upto 60°C as compared to free enzyme 64% whose activity was lost at 50°C in 35 hr. Storage stability was increased at 25°C in 0.05 M phosphate buffer and pH 7.4 for one month. These results were comparable to previous results [24,25]. Several researchers have reported the entrapment of biologically active compound such as insulin, & progesterone in albumin microspheres. Microspheres are commonly used in drug delivery system. Our objectives were to prepare nanoparticles using bovine serum albumin and immobilize the glucose oxidase. Glucose is very common material used in preparation of soft drinks and non-alcoholic beverages industries. Due to the presence of higher concentration of glucose makes the drink opalescent due to microbial attack. Hence removal of this excess glucose is essential before packing in the bottles. These nanoparticles were seen under phase contrast microscope with 20x magnification (Figure 1). The colour of the prepared nanoparticles was found to be off white to light brown and have unique fluorescent property. This fluorescence is due to newly formed diene type of adduct that leads to exposure o tryptophan residue (hydrocarbon that contains two carbon double bonds) after covalent coupling by using glutaraldehyde which is well known cross linking agent. Because protein contains tryptophan, tyrosine and phenylalanine among which tryptophan residue acts as a free acid and has much stronger fluorescence as compared to tyrosine and phenylalanine. The specific activity of released glucose oxidase from prepared nanoparticles were studied at different time intervals (1 to 7 days) as well as using different concentration of proteases such as trypsin, chymotrypsin and papin ( 6U, 12U, 18U & 24U) to study the biodegradability of the cross linked entrapped Glucose oxidase [26]. The enzyme activity of glucose oxidase in emulsified encapsulated nanoparticles of bovine serum albumin was estimated by the method as published in Worthington manual, New Jersey, USA [27]. Among these proteases, the chymotrypsin was found to be the best proteolytic enzymes which release the maximum activity of glucose oxidase in reaction mixture of delivery system which was 22 U/g released bound glucose oxidase after proteolysis at the fifth day (Figure 2-4) (Table 2) which confirmed entrapment of glucose oxidase in emulsified nanoparticles of bovine serum albumin under specified conditions. In the absence of proteases, the enzyme activity of glucose oxidase was not detected in the delivery system. This immobilized glucose oxidase in emulsified encapsulated nanoparticles of bovine serum albumin of bound glucose oxidase was converted the glucose into gluconic acid and hydrogen peroxide [8,28] (Figure 5). Hydrogen peroxide is completely soluble in solution. And hydrogen peroxide is also commonly present in instant coffee and has an excellent refreshing property. The gluconic acid is metabolized easily in the body after the consumption and it also makes the drink clear and free from opalescence to act as acid regulator which dissolves mineral deposits especially in alkaline solution. It is an organic compound and occurs in fruits, honey and wine. It is also used as food additive too. Even though, calcium gluconate in the form of gel is used in the treatment of burns caused by hydrofluoric acid, quinine gluonate in the treatment of malaria and gluconate injection have been used in the past to treat anemia. Coca-cola sales was reached US $24.1 billion in 2006 and but this also includes snacks and other foods. Cadbury Schweppes follows a close third to Coca-cola and Pepsi. The overall U.S. non-alcoholic beverage market including sodas, water, tea and other commercial beverages rose 1.2% in 2010 as key products. About 500 soft drinks companies operate in the U.S with the annual sales of approx. US $88 billion. Japanese companies want to expand domestic brands of soft drink/non- alcoholic beverages globally. Mexico leads world in capita volume of juice through fountain and fizzy drinks. Effect of prepared emulsified encapsulated nanoparticles of bovine serum albumin of bound glucose oxidase in the sample of cocacola, sprite, fanta and appy fizz (non-alcoholic beverages) was studied (Figure 5). A blank was taken which contained 20-25 ml fresh sample of soft drink, Test 1 was taken which contained the incubated 20-25 ml sample of soft drink at room temperature for 20 days without the mixture of prepared nanoparticles of emulsified encapsulated bovine serum albumin of bound glucose oxidase and chymotrypsin solution and Test 2 was taken that contained the incubated 20-25 ml sample at room temperature for 20 days along with the mixture of prepared nanoparticles of emulsified encapsulated bovine serum albumin of bound glucose oxidase (2 g/ml) and chymotrypsin solution (18 U/ ml). These results was showed that the test 1 was turbid due to the microbial growth which was without the prepared nanoparticles of bovine serum albumin of bound glucose oxidase and chymotrypsin but the test 2 was clear and free from turbidity as well as it is quite comparable with the blank (Figure 5a-5d). The test 2 was incubated along with the prepared nanoparticles of bovine serum albumin of bound glucose oxidase (2 g/ml) and chymoprypsin solution (18 U/ml). Hence, these results were comparable to House et al. [28] & Wang et al. [8]. Hence, removal of excess glucose is essential before packing in the bottles. This immobilized glucose oxidase in emulsified encapsulated nanoparticles of bovine serum albumin can be packed in columns and samples of non-alcoholic beverages/ soft drinks/ in vitro drugs delivery system. Thus, these emulsified encapsulated nanoparticles of bovine serum albumin microsphere of bound glucose oxidase has become a useful tool in drug delivery system and in preparation of beverages industries as a preservative to remove the excess of glucose from soft drinks/non-alcoholic beverages to make it opalescent and free from microbial/pathogenic attack which leads to stomach upset as well as food poisoning.
| Proteases | 0U/mg | 6U/mg | 12U/mg | 18U/mg | 24U/mg |
| Trypsin | 0 U/g* | 5.9 U/g* | 13.6 U/g* | 19 U/g* | 19 U/g* |
| Chymotrypsin | 0 U/g* | 11 U/g* | 15 U/g* | 22 U/g* | 22 U/g* |
| Papain | 0 U/g* | 0.18 U/g* | 0.6 U/g* | 0.82 U/g* | 0.82 U/gv |
*Activity of released glucose oxidase from encapsulated emulsified nanoparticles of bovine serum albumin was estimated by Worthington Enzyme Manual.
Table 2: Comparative study of activity of released glucose oxidase from emulsified encapsulated nanoparticles of bovine serum albumin by the different proteases.
References
- Rolland A, Wagner N, Chatelus A, Shroot BS, Chaefer H (1993) Site-specific drug deleivery to pilosebaceous structure using polymeric microspheres. Pharm Res 10: 1738-1744.
- Tulsani NB, Kumar A, Pasha Q, Kumar H, Sarma UP (2000) Immobilization of hormones for drug targeting. Artif Cells Blood Substit Immobil Biotechnol 28: 503-519.
- Thakkar H, Sharma RK, Mishra AK, Chuttani K, Murthy RR (2005) Albumin microsphere as carriers for the antiarrithitic drug celecoxib. Pharm Sci Tech 6: E65-E73.
- Foulds N, Lowe CR (1988) Immobilzation of glucose oxidase in ferrocene-modified pyrrole polymers. Anal Chem 60: 2473-2478.
- Salimi A, Sharifi E, Noorbakhsh A, Soltanian S (2007) Immobilzation of glucose oxidase on electrodeposited nickel oxide nanoparticles: Direct electron transfer and electroanalytical activity. Biosens Bioelectron 22: 3146-3153.
- Suxia Z, Nu Wang, Yaming Niu, Changqing Sun (2005) Immobilization of glucose oxidase on gold nanoparticles modified Au electrode for the construction of biosensor. Sens Actuators B Chem109: 367-374.
- Lei-Ying, ET Kang, KG Neoh (2002) Covalent immobilization of glucose oxidase on microporous membranes prepared from poly (vinyl-diene-fluoride) with grafted body of poly (acrylic acid) side chains. J Membrane Sci 208: 361-374.
- Wu H, Wang J, Kang X, Wang C, Wang D, et al. (2009) Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/ grapheme/chitosan nanoparticle film. Talanat 80: 403-406.
- Jia WZ, Wang K, Zhu ZJ, Tian HS, Xia XH (2007) One step-immobilzation of glucose oxidase in a silica matrix on a pt electrode by electrochemically induced solution gel process. Langmuir 23: 11896-11900.
- WangH, Wang X, Zang X, Qin X, Zhao Z, et al. (2009) A novel glucose biosensor based on the immobilization of glucose oxidase onto gold nanoparticles modified nanowires. Biosens Bioelectron 25: 142-146.
- Blandino A, Macias M, Cantero D (2001) Immobilzation of glucose oxidase within calcium gel capsules. Process Biochemistry 68: 601-606.
- Zhu H, Srivastava R, Brown JQ, McShane MJ (2005) Combined physical and chemical immobilization of glucose oxidase in alginate microspheres improves stability of encapsulation and activity. Bioconjug Chem 16: 1451-1458.
- Pishko MV, Michael AC, Heller A (1991) Amperometric glucose micro-electrodes prepared through immobilization og glucose oxidase in redox-hydrogels. Anal Chem 63: 2268-2272.
- Barlett PN, Tebbutt P, Tyrrell CH (1992) Electrochemical immobilization of enzymes 3. Immobilzation of glucose oxidase in thin films of electrochemically polymerized phenols. Anal Chem 64: 138-142.
- Christian Bourdillon, Michel Delmer, Christophe Damaielle, Rachid Hitmi, Jacques Moiroux, et al. (1992) Immobilization of glucose oxidase on a carbon surface derivatized by electrochemical reduction of diazonium salts. J Electroanal Chem 336: 113-123.
- Milao Y, Chia LS, Neoh KG, Tan SN (2001) Amperometric glucose biosensor based on immobilization of glucose oxidase in chitosan matrix cross linked with glutaraldehyde. Electroanaylsis 13: 347-349.
- Kong T, Chen Y, Ye Y, Zhang K, Wang Z, et al. (2009) An Amperometric glucose biosensor based on immobilization of glucose oxidase on the ZnO nanotubes. Sens Actuators B Chem 138: 344-350.
- Zhang Z, Liu H, Deng J (1996) A glucose biosensor based on immobilization of glucose oxidase in electro polymerized O-aminophenol film on platinized glassy carbon electrode. Anal Chem 68: 1632-1638.
- Palmieri A 3rd (1979) Microencapsulation and dissolution parameter of undecebovanillylamide: a potenitail coyote antidote. J Pharm Sci 68: 1561-1562.
- Manesh KM, Kim HT, Santhosh P, Gopalan AI, Pilles K. (2008) A novel glucose biosensor based on immobilization of glucose oxidase into multiwall carbon nanotubes-polyelectrolyte-loaded electrospum nanofibrous membrane. Biosens Bioelectron 23: 771-779.
- Yu J, Liu S, Ju H (1996) Glucose sensor for flow injection analysis of serum glucose based on glucose oxidase in titania-solution gel membrane. Biosens Bioelectron 19: 401-409.
- Kang X, Mai Z, Zou X, Cai P, Mo J (2007) A novel glucose biosensor based on immobilization of glucose oxidase in chitosan on a glassy carbon electrode modified with gold-platinum alloy nanoparticles/ multiwall carbon nanotubes. Anal Biochem 369: 71-79.
- Fairhurst D, Mithchnick M (1995) Submicron encapsulation of organic sunscreen. Cosmetics and Toiletries 110: 47-50.
- Kouassi GK, Irudayaraj J, McCarty G (2005) Activity of glucose oxidase functionalized onto magnetic nanoparticle. Biomagn Res Tech 3: 1.
- He C, Liu J, Xie L, Zang Q, Li C, et al. (2009) Improvement in specific activity and thermal stability after immobilization. Langmuir 25: 13456-13460.
- Bright HJ, Appleby M (1969) The pH dependence of the individual steps in the glucose oxidase reaction. J Biol Chem 244: 3625-3634.
- Worthington V (1988) Worthington Enzyme Manual: enzymes and related biochemicals. Worthington Biochemical Corporation, Free hold, New Jersey, USA.
- House JL, Anderson EM, Ward WK (2007) Immobilzation techniques to avoid enzyme loss from oxidase based biosensors: a one-year study. J Diabetes Sci Technol 1: 18-27.
- Bianco P, Haladjian, Bourdillon C (1990) Immobilization of glucose oxidase on carbon electrodes. J Electroanal Chem 293: 151-163.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16205
- [From(publication date):
February-2012 - Dec 19, 2025] - Breakdown by view type
- HTML page views : 11427
- PDF downloads : 4778