Prevalence of Methicillin- Resistant Staphylococcus aureus in Health Personnel of a Second Level Hospital in Mexico City and Its Relationship with the Rate of Nosocomial Infection
Received: 11-Jun-2013 / Accepted Date: 29-Aug-2013 / Published Date: 03-Sep-2013 DOI: 10.4172/2161-1165.1000134
Abstract
Background
MRSA infection is an important cause of skin and soft tissue, the colonization increases the risk of infection. We estimate the effect of MRSA in health workers of critical areas of a school hospital from second level in Mexico City and its relation of Nosocomial infections.
Methods
Were performed nasal cultured in Health Workers (HW) patients were taken culture according to clinical and a questionnaire was completes. Identification of MRSA was determined by susceptibility to oxacillin, and SSCmec gene was identified the PFGE was performed to look for clonality. Statistical analysis was performed.
Results
Included, 269 patients and 108 of HW, 15% and 12% were MRSA in each group. The relative risk of colonization by MRSA in healthcare workers was 2.77 times higher in intensive care and internal medicine than any other service.The odds of infection with MRSA in hospitalized patients undergoing surgery prior is 41,964 times greater than in patients admitted with other diagnosis. The frequency of SSCmecII was (24/31) in HW and patients. The PFGE indicated the presence of a MRSA clone in both patients and health workers in these critical areas.
Conclusions
The prevalence of carriers of MRSA in staff was similar to that reported in other hospitals of México. There is no direct evidence to validate that colonized health workers increases the risk of nosocomial MRSA infection in patients.
Keywords: MRSA; Nasal colonization; Nosocomial infections, Mexico city
160632Introduction
In the last two decades there has been a dramatic increase of nosocomial infections caused by strains of Methicillin- Resistant Staphylococcus aureus MRSA [1]. Currently the infections caused by this pathogen are present in hospitals in all the countries around the world [2,3]. The Society for Healthcare and Epidemiology of America identified the MRSA as one of the most important pathogens associated with infections that occur inside the United States hospital facilities [4]. Infected or colonized patients with MRSA help transmit these resistant organisms inside the hospital, especially in the intensive care units [1,5]. The nature of the environment found in the intensive care units makes this area of the hospital become an important place for the emergency and dissemination of multi-resistant pathogens like MRSA. When there is colonization with Methicillin-Resistant Staphylococcus aureus increases the risk of presenting clinical infection, which is one of the most important pathogens that generates skin infections that can go from superficial to severe skin infections associated with a high mortality rate [6,7].
Study reports made in the USA [8], indicate that 35% of the children and 60% of the adults become intermittent carriers of this microorganism.
Care providers are more susceptible to being colonized and the colonization rate increases with the intensity and frequency of contact: doctors with a 22%, ward nurses with a 48% have more contact with patients [9].
The classical resistance to methicillin is codified by the methicillin resistance determinant (mec), a segment of DNA, which measures between 30 and 50 Kb and appears in the MRSA strains, but not in sensitive strains [10].
A study made in 1996 using isolated prototype strains from diverse continents, threw the first evidence of the existence of three types of staphylococcal cassette chromosomes (SCCmec I-III). In recent years a forth type named (SCCmec IV) was described. Studies made in Mexico indicate that types II and IV are found circulating the MRSA strains [11].
In our country, the Epidemiologic Vigilance Hospital Network in Spanish called la Red Hospitalaria de Vigilancia Epidemiológica (RHOVE) notified that the mortality percentages in infected patients with Staphylococcus aureus varies between 5 and 70%, and that the mortality rates associated with this bacteria can rise (50%). Therefore, reporting that during the years 1997-2003 Staphylococcus aureus occupied third place in morbidity and fourth in mortality. Diverse vigilance infection studies associated with healthcare indicate that from 8.3% to 36% of these infections are caused by MRSA [11].
In consequence, from the epidemiologic point of view, it necessary to identify the sources and monitor the dissemination of MRSA strains during the investigation of an outbreak, as part of a vigilance program and a current control [12].
The objective of this study was to evaluate the colonization effect of MRSA on health workers and its relationship with nosocomial infections due to MRSA, using its own genotyping to determine its possible cloning origin.
Methods
From June 25th to August 15th 2009 a transversal, observational and descriptive study was applied on health workers and hospitalized patients of a second level hospital (“Dr. Manuel Gea Gonzállez”), in Mexico City within critical areas like (emergency area, intensive care unit, internal medicine, general surgery, and inhalation therapy). The health workers (n=108) finished a questionnaire and had a nasal culture, the patients used a Nosocomial Infections Epidemiologic Vigilance document and (n=269) cultures were taken according to the established clinical characteristics. The identification of Staphylococcus aureus was made according to the established microbiological guidelines [13]. Six isolations of Staphylococcus aureus were included from hospitalized patients during 2008, which were related to nosocomial infection. The purpose was to investigate the cloning relationship between these isolations and the new isolations. The study protocol was approved by the Ethics and Research Commissions at the “Dr. Manuel Gea Gonzállez” General Hospital, as well as the Public Health National Institute School in Spanish named Colegio del Instituto Nacional de Salud Pública; following the Helsinki criteria.
Determination of oxacillin susceptibility
All the isolations identified with Staphylococcus aureus were screened for oxacillin susceptibility using the (Kirby-Bauer Technique and through the CHROM Agar MRSA [14,15].
Gene SSCmec recognition
All the isolations of Staphylococcus aureus that were resistant or intermediate resistant to oxacillin, were analyzed to detect the mec complex; using a PCR Multiplex described by Oliveira and de Lencastre [16].
The amplified products of the PCR were analyzed by electrophoresis on 2% agarose gels stained in a 0.1-μg mL with ethidium bromide solution and photographed to be visually analyzed.
Pulsed field gel electrophoresis (PFGE) in MRSA isolationsc
To search for cloning ability a pulsed field gel electrophoresis was performed. All the bacteria cultures were processed according to the Blanc et al. [17] method’s description, 20U of the Smal enzyme were used to restrict DNA, which was resolved in the pulsed field gel electrophoresis CHEF-MAPPER (Bio-Rad™), under the following conditions: 3 volts/cm/1-30 , sec/17 h, followed by 6 volts/cm /1-30 sec/8h. The patterns of the PFGE were interpreted on the criteria basis established by Tenover et al. [18].
The gels were seen using Ethidium bromide and were photographed in an image analyzer Gel Doc (Bio-Rad™), using UV light at 302 nm.
Results
A total of 377 Staphylococcus aureus strains were analyzed, MRSA was identified with 15% (16/108) in health workers and 12% (33/269) in patients with related infection to MRSA, of which only eight were documented as related infection due to health care during the period of time the study was made.
The table 1 described the health professionals, who participated in this study, general characteristics; emphasizing MRSA’s prevalence both in the adult’s intensive therapy care and in the internal medicine staff members with 31.25% each one. In the respiratory therapy area it was isolated at 12.5%, and the table 2 described the characteristics of the patients that were included during the time the study was made. It is evident that general surgery is the service that had the highest MRSA presence (24.24%), although the adult’s intensive therapy and internal medicine had 21.2% and 18.18% respectively; and emergencies 15.15%. Consistent with previous surgery, showing the highest incidence of MRSA (18.2%), as well as in the upper respiratory airways infection (12.1%) (Table 3).
| Characteristic | Total participating health personnel n=108 |
Presence MRSA n=16 Prevalence 15 % |
| Sex ratio W:M Age(years) |
2.48 Women to Men 31.77 IC 95 % (30.08-33.45) |
2.2 Women to Men 29.5 IC 95 % (25.29-33.71) |
| Service | ||
| Emergencies Adult | 26 (24.1 %) | 2 (12.5 %) |
| Intensive Care Unit | 22 (20.4 %) | 5 (31.25 %) |
| Internal Medicine | 25 (23.1 %) | 5 (31.25 %) |
| General Surgery | 21 (19.4 %) | 1 (6.25 %) |
| Inhalotherapy | 6 (5.6 %) | 2 (12.5 %) |
| Other | 8 (7.4 %) | 1 (6.25 %) |
| Staff | ||
| Medical | 21 (19.6 %) | 2 (12.5 %) |
| Nursing | 69 (64.5 %) | 10 (62.5 %) |
| Inhalotherapy | 6 (5.6 %) | 2 (12.5 %) |
| Other | 11 (10.3 %) | 1 (6.25 %) |
| Not specified | 0 | 1 (6.25%) |
| Working time in the hospital area(years) |
2.3964 IC 95 % (1.5638-3.2291) |
2.7223 IC 95 % (-.0938 – 5.5383) |
Table 1: Features Health staff General Hospital "Dr. Manuel Gea Gonzalez", 2009.
| Characteristics of patients |
Total patients in the study |
Presence MRSA |
| n=269 | n= 33 Prevalence 12 % | |
| Sex, ratio W:M | 1.24 Women to Men |
1.53 Women to Men |
| Age(years) | 47.06 IC 95 % (44.76-49.35) |
48.11 IC 95 % (40.92-55.31) |
| Service | ||
| Emergencies Adult | 82 (30.5 %) | 5 (15.15 %) |
| Intensive Care Unit | 25 (9.3 %) | 7 (21.21 %) |
| Internal Medicine | 53 (19.7 %) | 6 (18.18 %) |
| General Surgery | 54 (20.1 %) | 8 (24.24 %) |
| Other | 55 (20.44 %) | 7 (21.21 %) |
Table 2: Characteristics of patients included in the study period, with isolation of MRSA, 2009.
| Diagnosis | Total diagnoses in 269patients studied (n=295) |
Presence MRSA Prevalence 12 % n = 33 |
Without presence MRSA n= 262 |
| Skin and soft Tissue infection |
62 (21.01%) | 3(9.1%) | 59(22.5%) |
| Wounds | 10 (3.4 %) | 0 | 10 (3.8 %) |
| Previous surgery | 14 (4.7 %) | 6 (18.2%) | 8 (3.05 %) |
| Surgical site infection |
12 (4.0%) | 3 (9.1 %) | 9 (3.43 %) |
| Prosthesis | 6 (2.0 %) | 2 (6.1%) | 4 (1.5 %) |
| Respiratory tractinfection |
58 (19.7%) | 4 (12.1%) | 54 (20.6 %) |
| Diabetesmellitus | 35 (11.9%) | 3 (9.1%) | 32 (12.2 %) |
| Cancer | 16 (5.4 %) | 1 (3.03) | 15 (5.7 %) |
| HIV and other immuno deficiency |
20 (6.8 %) | 0 | 20 (7.6 %) |
| Others | 51 (17.28%) | 0 | 51 (19.5 %) |
| Un specified | 11 (3.7%) | 11 (33.3) | 0 |
Table 3: Descriptive characteristics of biological products from patients diagnosed with Staphylococcus aureus General Hospital “Dr. ManuelGeaGonzalez”, 2009.
According to logistic regression analysis was found to the relative risk of colonization by MRSA in healthcare workers was 2.77 times higher in intensive care and internal medicine than any other service with a probability of nasal colonization of MRSA 21%. The odds of infection with MRSA in hospitalized patients undergoing surgery prior is 41,964 times greater than in patients admitted with other diagnosis with a probability of MRSA infection of 77.55%.
The detection of the mec complex with PCR multiplex showed the presence of mec and a 77.4% prevalence of the SSCmecII.
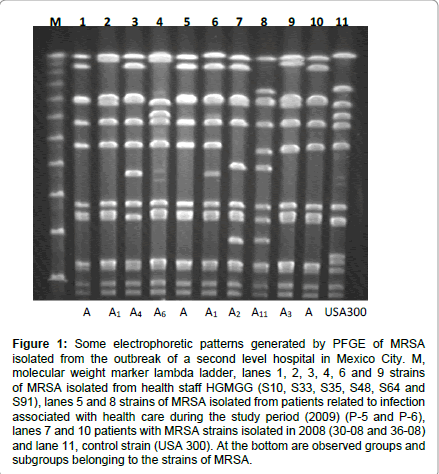
For the genotyping analysis with PFGE, 16 healthcare staff member’s isolations were included, 14 were from nosocomial infection patients, from whom 8 belonged to this study period and 6 were isolated in 2008, twelve electrophoretic patterns that were clearly distinguishable were identified (Figure 1), and they had in between 9 and 12 fragments (from 34 to 930 Kb) that can be observed depending on the pattern of the strain.
Figure 1: Some electrophoretic patterns generated by PFGE of MRSA isolated from the outbreak of a second level hospital in Mexico City. M, molecular weight marker lambda ladder, lanes 1, 2, 3, 4, 6 and 9 strains of MRSA isolated from health staff HGMGG (S10, S33, S35, S48, S64 and S91), lanes 5 and 8 strains of MRSA isolated from patients related to infection associated with health care during the study period (2009) (P-5 and P-6), lanes 7 and 10 patients with MRSA strains isolated in 2008 (30-08 and 36-08) and lane 11, control strain (USA 300). At the bottom are observed groups and subgroups belonging to the strains of MRSA.
In this figure is distinguished profile A, formed by a group of seven strains, and showed an identical pattern, including four members of staff and patients three; one of which was obtained a year earlier before the current outbreak was recognized. Similarly to other profiles 11, three of them named (A1-A3), which involved 11 strains, and they are only different from profile A in one or two bands respectively. The subtypes A3, A4, A5 and A6 show three different bands each one therefore; according to the criteria described by ten over and col [18], it tells us that all of these strains (15/31) are closely related with the outbreak. From the A7 to A11 subtype, there are 5 to 6 different bands, indicating that these strains (8/30) may be related.
Discussion
In this study the nasal colonization prevalence on health care staff members of a second level hospital school is described. This second level hospital forms part of the reference system for the National Health Institutes and General Hospitals in Mexico City. Here a general prevalence of MRSA with 15% was found on the personnel of the Emergency, Intensive Care Unit, Internal Medicine and General Surgery areas, and these results are similar to the Bisaga et al. [19]. They determined a 15% nasal colonization prevalence on the healthcare personnel that works in an Emergency Department at a third level Hospital School in Illinois, USA.
The periods of study are also similar, our study from June to August 2009 and Bisaga et al. [19], May through September 2006.
In relationship with the MRSA infections variation, Van de Griend et al. [20], described on a study made in Iowa, USA, that there was a higher rate incidence of invasive infections by MRSA on a community level during summer, and that was the reason why we took the decision to conduct this study during the summer.
Has been demonstrated that an area to approach for the infection control associated with nosocomial infections is the General Surgery Service, due to the fact that it has several critical points in its processes, and by modifying them they can have a high impact on the vigilance and infection control measures associated to healthcare with resistant microorganisms.
The increase of infections related with healthcare due to resistant microorganisms at the General Hospital “Dr. Manuel Gea González”, is considered a public health problem that requires immediate attention to control it, and the recommendations of international organisms such as the WHO [21] and the Control Disease Center [22] must be taken into account.
The odd of colonization by MRSA in health workers and the probability of MRSA nasal colonization, implies that screening should be performed regularly and under the surveillance of a vigilance epidemiologic program. Such program should include a regular sampling of the fixed working staff in these areas, as well as the outsourced personnel (doctors, interns, residents, nursing trainees), they may be considered as a source of dissemination for this types of strains as it has been proven in previous studies [23,24].
The high index of relative risk of MRSA infection in patients with surgical lights a red light for the care of these, although some studies have suggested that active surveillance from cultures can decrease nosocomial MRSA infections in surgical patients [25], other evidence suggests that the detection of MRSA without a concentrated effort to change other important factors, including basic medical practice used in infection control and patient care cohort of staff results in an effect moderate on MRSA [26] infection. The transmission and persistence of multi resistant microorganisms stays there because of factors such as: vulnerable patients to colonization with risk factors [27], selective pressure due to antimicrobials and the presence of colonized healthcare personnel [28]. When they enter into contact with the patient’s environment, they may introduce a multi resistant microorganism if they don’t take the necessary precautions to avoid infectious agent transmission.
These findings are of clinical interest, especially in the surgical and intensive therapy areas, because if an increase in the number of nosocomial infections (surgical injuries, pneumonia related to mechanical ventilation) is observed due to this bacterial agent, prevention measures must be started to sanitize with chlorhexidine and the decolonization of MRSA carrier staff members and furthermore personnel changes should be made.
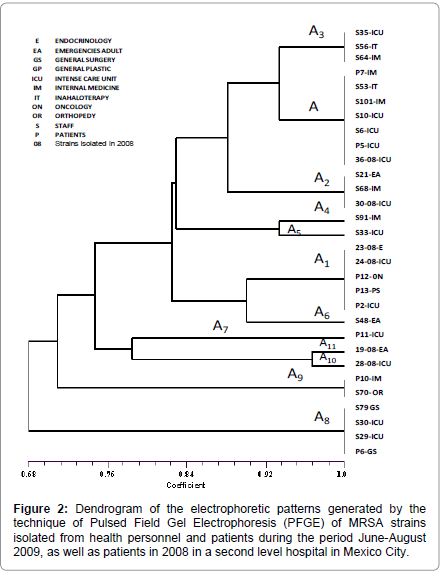
The arrangement of the observed clustering in the dendrogram (Figure 2) and the SSCmecII prevalence showed that between these strains there exists a great similarity, and it shows evidence that the outbreak was caused by only one clone. Isolated strains from healthcare staff members that work in critical areas and patients showed the clone or one of its subtypes in the electrophoretic profile. Furthermore, the evidence that isolated strains recovered a year before showed the same electrophoretic pattern of the outbreak clone, alerts us of the existence of an important epidemiologic clone that is found circulating inside the hospital since October 2008, and has continued disseminating until the study was made.
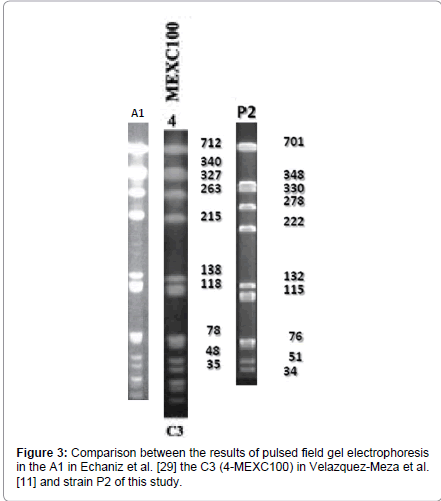
When we compared our information with similar studies in the country like the one from Echaniz-Aviles et al. [29], and the one from Velálzquez-Meza et al. [11] it has been observed that according to the molecular weight of the electrophoretic pattern performed on the three projects, the strain from Echaniz-Aviles et al. [29] (A1) and the one from Velálzquez-Meza et al. [11] (C3-4MEXC100), are very similar to the A1 pattern of this study; which proofs the existence of similar clones in other hospitals in our country (Figure 3).
We consider necessary the implementation of an active surveillance program with the purpose to reduce the percentage of infections caused by resistant pathogens, and to diminish associated costs with infections obtained inside hospitals. As well as the intervention in different procedures in order to restrict the staff member’s colonization and as a consequence avoid nosocomial infection, which is the particular case of allograft plastic surgeries and cardiac surgeries. But above all, to help diminish the mortality caused by these infections.
References
- Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK (2009) Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997-2007. JAMA 301: 727-736.
- Asensio A, Cantón R, Vaqué J, Rosselló J, Calbo F, et al. (2006) Nosocomial and community-acquired meticillin-resistant Staphylococcus aureus infections in hospitalized patients (Spain, 1993-2003). J Hosp Infect 63: 465-471.
- Pan A, Carnevale G, Catenazzi P, Colombini P, Crema L, et al. (2005) Trends in methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections: effect of the MRSA "search and isolate" strategy in a hospital in Italy with hyperendemic MRSA. Infect Control Hosp Epidemiol 26: 127-133.
- Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, et al. (2003) SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol 24: 362-386.
- Fridkin SK, Gaynes RP (1999) Antimicrobial resistance in intensive care units. Clin Chest Med 20: 303-316.
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355: 666-674.
- Delaney JA, Schneider-Lindner V, Brassard P, Suissa S (2008) Mortality after infection with methicillin-resistant Staphylococcus aureus (MRSA) diagnosed in the community. BMC Med 6: 2.
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, et al. (2008) Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis 197: 1226-1234.
- Suffoletto BP, Cannon EH, Ilkhanipour K, Yealy DM (2008) Prevalence of Staphylococcus aureus nasal colonization in emergency department personnel. Ann Emerg Med 52: 529-533.
- Elizabeth PR (2007) BiologÃa Molecular Aplicada al Control de Infecciones Intrahospitalarias. Rev Med Clin Condes 18: 330- 337.
- Velazquez-Meza ME, Aires de Sousa M, Echaniz-Aviles G, Solórzano-Santos F, Miranda-Novales G, et al. (2004) Surveillance of Methicillin-Resistant Staphylococcus aureus in a Pediatric Hospital in Mexico City during a 7-Year Period (1997 to 2003): Clonal Evolution and Impact of Infection Control. J Clin Microbiol 42: 3877-3880.
- Castellano-González MJ, Perozo-Mena AJ, Vivas-Vega RL, Ginestre-Pérez MM, Rincón-Villalobos GC (2009) Molecular and phenotypical typification of methicillin resistant Staphylococcus aureus (MRSA) strains in a university hospital. Rev Chilena Infectol 26: 39-48.
- Bannerman T (2007) Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically. In: En Murray P, Baron E, Jorgensen J, Pfaller M, Yolken R (Eds.), Manual of Clinical Microbiology. (9thedn), Volume 1. Chapter 28. ASM Press. Washington, DC, USA.
- Clinical and Laboratory Standards Institute (2008) Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement M100-S08 28.
- Pape J, Wadlin J, Nachamkin I (2006) Use of BBL CHROMagar MRSA medium for identification of methicillin-resistant Staphylococcus aureus directly from blood cultures. J Clin Microbiol 44: 2575-2576.
- Oliveira DC, de Lencastre H (2002) Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46: 2155-2161.
- Blanc DS, Struelens MJ, Deplano A, De Ryck R, Hauser PM, et al. (2001) Epidemiological validation of pulsed-field gel electrophoresis patterns for methicillin-resistant Staphylococcus aureus. J Clin Microbiol 39: 3442-3445.
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2233-2239.
- Bisaga A, Paquette K, Sabatini L, Lovell EO (2008) A prevalence study of methicillin-resistant Staphylococcus aureus colonization in emergency department health care workers. Ann Emerg Med 52: 525-528.
- Van De Griend P, Herwaldt LA, Alvis B, DeMartino M, Heilmann K, et al. (2009) Community-associated methicillin-resistant Staphylococcus aureus, Iowa, USA. Emerg Infect Dis 15: 1582-1589.
- Ducel G, Fabry J, Nicolle L (2003) Prevención de las infecciones nosocomiales. (2a edición), GuÃa práctica. Organización Mundial para la Salud. Ginebra, Suiza.
- Gorwitz RJ, Jermigan DB, Powers JH, Jernigan JA (2006) Strategies for Clinical Management of MRSA in the Community: Summary of an Experts´Meeting Convened by the Center for Disease Control and Prevention.
- Simor AE, Daneman N (2009) Staphylococcus aureus decolonization as a prevention strategy. Infect Dis Clin North Am 23: 133-151.
- Jernigan JA (2008) Methicillin-resistant Staphylococcus aureus colonization among health care personnel in the emergency department: what does it tell us? Ann Emerg Med 52: 534-536.
- Wernitz MH, Swidsinski S, Weist K, Sohr D, Witte W, et al. (2005) Effectiveness of a hospital-wide selective screening programmer for Methicillin- Resistant Staphylococcus Aureus (MRSA) carriers at hospital admission to prevent hospital-acquired MRSA infections. Clin Microbiol Infect 11: 457-465.
- Dancer SJ (2008) Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis 8: 101-113.
- Graffunder EM, Venezia RA (2002) Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 49: 999-1005.
- EchevarrÃa Zarate J, Iglesias Quilda D (2003) Estafilococo meticilino resistente, un problema actual en la emergencia de resistencia entre los Gram positivos. Rev Med Hered 14: 195-203.
- Echániz-Aviles G, Velázquez-Meza ME, Aires-de-Sousa M, MorfÃn-Otero R, RodrÃguez-Noriega E, et al. (2006) Molecular Characterization of a dominant Methicillin-Resistant Staphylococcus Aureus (MRSA) clone in a Mexican Hospital (1999-2003) Clin Microbiol Infect 12: 22-28.
Citation: Espinosa de los Monteros LE, Atonal DM, Trejo GR, Jiménez CA, Jiménez R LV, et al. (2013) Prevalence of Methicillin- Resistant Staphylococcus aureus in Health Personnel of a Second Level Hospital in Mexico City and Its Relationship with the Rate of Nosocomial Infection. Epidemiol 3:134. DOI: 10.4172/2161-1165.1000134
Copyright: © 2013 Espinosa de los Monteros LE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15510
- [From(publication date): 10-2013 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 10704
- PDF downloads: 4806