Systemically Antagonizing L-Type Calcium Channels Modifies Intensity and Pattern of Fos Expression Evoked by Electrical Intracochlear Stimulation in the Adult Rat Auditory Brainstem
Received: 13-Aug-2013 / Accepted Date: 22-Aug-2013 / Published Date: 29-Aug-2013 DOI: 10.4172/2161-119X.S3-005
Abstract
Specific sensory activity such as sustained acoustical or Electrical Intracochlear Stimulation (EIS) elicits electrical responses and molecular changes in neuronal subpopulations of the auditory brainstem. One of the first molecular changes observed is the expression of the immediate early gene Fos. Its protein functions as a monomer of the transcription factor Activator Protein-1 (AP-1) which triggers cascades of protein synthesis that play a crucial role in neuroplastic remodeling. We investigated the pattern of Fos expression in Anteroventral (AVCN) and Dorsal Cochlear Nucleus (DCN) and Central Inferior Colliculus (CIC) following 2 h of unilateral EIS at 1.6, 50, and 400 Hz in anesthetized untreated and nimodipine-treated rats. Our data indicate that with increasing stimulation frequency the population of cells expressing Fos changed in composition and size in AVCN, DCN, and CIC. In all investigated auditory regions, systemic nimodipine treatment, an antagonist of L-type calcium channels, resulted in a significant increase in the number of neurons expressing Fos upon EIS compared to non-treated rats at the same stimulation parameters. Apparently, antagonizing L-type calcium channels enhances the responsiveness of neurons, and thus their readiness to respond with functional changes to sensory activity, in the auditory brainstem with little influence on the tonotopic precision of the response.
Keywords: Adult brain plasticity, Neuroplasticity, Nimodipine, Cochlear implant, L-type Ca channels, Central auditory system, Cochlear nucleus, Colliculus inferior, Electrical intracochlear stimulation, Fos
257374Abbreviations
ABR: Auditory brainstem response; ANOVA: One-Way Analysis Of Variance; AP-1: Activating Protein-1; AVCN: Anteroventral Cochlear Nucleus; AVCNc: AVCN Contralateral to Stimulation; AVCNi: AVCN Ipsilateral to Stimulation; c: Contralateral; CI: Cochlear implant; CIC: Central Inferior Colliculus; CICc: CIC Contralateral to Stimulation; CICi: CIC Ipsilateral to Stimulation; Co: Control; DAB: 3,3'-Diaminobenzidine Tetrahydrochloride; DCN: Dorsal Cochlear Nucleus; DCNc: DCN Contralateral to Stimulation; DCNi: DCN Ipsilateral to Stimulation; EABR: Electrically Evoked Auditory Brainstem Response; EIS: Electrical Intracochlear Stimulation; Fos: Oncogene fos; Gap43: Growth Associated Protein43; h: Hour(s); Hz: Hertz;i-Ipsilateral; IEG: Immediate Early Gene; impl: Implantation; i.p: Intraperitoneal; Jun: Product of the Immediate-Early Gene Jun; LSO: Lateral Superior Olive; MNTB: Medial Nucleus of the Trapezoid Body; mRNA: Messenger Ribonucleic Acid; nimo: Nimodipine; p: P-Value in Statistical Significance Testing; ROI: Region of Interest; s.c.: Subcutaneous; VCN: Ventral Cochlear Nucleus; vs.: Versus
Introduction
Forming a complex network of interconnected pathways, central auditory neurons are affected by cochlea-born signals, distributing them at different levels of the auditory brainstem. The mode of neuronal response to sensory activity ranges from fast electrophysiological changes to long-lasting modifications of their molecular composition, including the option of differential gene expression. One of these genes induced in central auditory cells by acoustical or electrical stimulation is the immediate-early gene fos [1-8]. Its product Fos has been investigated over the last decades and is used as an important tool for studies concerning neuronal plasticity within the auditory system [9-13]. Dimerizing with Jun, Fos forms the limiting monomer of the heterodimeric Activator Protein-1 (AP-1) transcription factor [14-18]. AP-1 itself triggers different genes involved in a variety of cellular functions, e.g. the growth and plasticity associated protein 43 (Gap43) [19-24].
Following Electrical Intracochlear Stimulation (EIS) of normal hearing adult rats, tonotopic patterns of Fos expression were found throughout the auditory pathway [25-28]. From studies demonstrating a mismatch between the stimulation dependence of Fos expression and 2-deoxyglucose uptake [29,30] it became apparent that Fos indicates readiness for structural and functional changes within neurons and may participate in the molecular mechanisms of learning [31-35].
An essential link for synaptic function and plasticity are calcium conducting ion channels [36-38]. Within the auditory system, the inner hair cells mark the first stage of voltage-gated calcium channel signal processing. These channels are playing an important role in activating the primary afferent nerve fibers and are presumed to be of the L-type [39-43]. This type of calcium channel initiates activation of other ion channels, enzymes and mediates transmitter release as well as gene expression [44-51]. Furthermore, L-type calcium channels are commonly found in the cardiovascular system where they are important in regulating vascular blood flow through smooth muscles [52-56].
Pursuing the finding that L-type-calcium channels are closely linked to neuronal activity and function of the auditory system, we evaluated the impact of nimodipine, a specific L-type calcium channel antagonist, with cerebrovasodilatatory and neuronal effects in combination with Electrical Intracochlear Stimulation (EIS) [57-60]. For EIS all parameters were carefully controlled. As in previous studies, we verified tonotopic Fos expression in the major regions of the ascending auditory brainstem such as: the Anteroventral Cochlear Nucleus (AVCN), the Dorsal Cochlear Nucleus (DCN) and the Central Inferior Colliculus (CIC) [7,18,28,61,62]. Jakob and Illing demonstrated a dependency of the level of Fos expression on stimulation intensity and frequency [63]. Contrary to the expectation of attenuating neural activity and thus the nerve’s potential to plastic remodeling when applying the L-type calcium channel antagonist nimodipine, the present study provides evidence that nimodipine treatment elevates EIS evoked Fos expression in AVCN, DCN and CIC.
Methods
Animals
Thirty-one adult male and female Wistar rats, aged 6 to 9 weeks, were used. Care and use of the animals as reported here were approved by the appropriate agency (Regierungspräsidium Freiburg, permission number 37/9185.81/G-10/83).
Nimodipin application
Before subcutaneously (s.c.) nimodipine treatment rats were intraperitoneally (i.p.) anesthetized with a mixture of ketamin (50 mg/kg, KETAVET, Pharmacia GmbH, Berlin) and xylazine (5 mg/ kg, ROMPUN, Bayer Vital GmbH, Leverkusen, Germany). For EIS, anesthesia was achieved with urethane (i.p., 1.5 g/kg, Fluka AG, Buchs, Switzerland). Nimodipine (2 mg/kg, NIMOTOP S, Bayer Vital GmbH, and Leverkusen, Germany) was given in 10 subcuntaneous depots on both sides along the spine 3 days prior to either direct sacrifice (controls) or before 2 h of passive or active cochlear implants.
Controls
Controls were run for naïve and nimodipine-treated rats with and without implantation of an electrode carrier that remained passive (Table 1).
Electrical intracochlear stimulation (EIS)
The cochlea was approached using a retroauricular surgical path. The round window was exposed and an electrode carrier was inserted into the cochlea over the round window. In seven rats, non-treated (n=4) and nimodipine-treated (n=3), the electrode carrier was implanted in a ventro-basal position for 2 h without being activated.
| Group | Treatment | No. of experiments |
|---|---|---|
| 1 | --- | 3 |
| 2 | nimodipine | 3 |
| 3 | implantation | 4 |
| 4 | nimodipine + implantation | 3 |
| 5 | EIS | 9 |
| 6 | nimodipine + EIS | 9 |
The total number of 31 Wistar rats has been divided into six different experimental groups. Controls were run without (group 1) and with nimodipine (group 2). Group 3 (no nimodipine) and group 4 (with nimodipine) underwent a 2 h dummy implantation of the electrode carrier. EIS was being performed without (group 5) and in combination with nimodipine (group 6). Within the two EIS groups, three animals were used for each setup of stimulation (1.6, 50, 400 Hz).
Table 1: Types of Experiments.
EIS was always applied unilaterally (left side) for a fixed duration of 2 h. Sacrifice and transcardial perfusion followed immediately after cessation of EIS. We used a cochlear implant (Model CI24M/ CI11+11+2M) run by a Nucleus Implant Communicator, both kindly provided by Cochlear GmbH, Hannover, Germany. Bipolar stimulation consisted of biphasic pulses with a 50 μs phase width at a frequency of 1.6 Hz, 50 Hz or 400 Hz. In the nimodipine-treated group an additional dose of 0.02 mg of nimodipine was injected into the round window directly before implantation of the electrode carrier.
Audiometry
Assessing the auditory brainstem response (ABR), steel needles were placed subcutaneously at vertex and bilaterally at mastoids and a 20 Hz train of click stimuli was presented to one side through a brass pipe equipped with a conical plastic tip into the outer ear canal, while the other ear was masked by white noise at the same sound pressure level. Sound intensity was stepwise increased, attempting to elicit an ABR visualized by an averager (Multiliner E; Evolution 1.70 c; Toennies & Jäger GmbH, Höchberg, Germany). Therefore, 300 sweeps were summed in a frequency band of 0.1-3 kHz to set the mean ABR amplitude. Electrical Auditory Brainstem Responses (EABRs) were recorded to verify the effectiveness of stimulation. EABRs were visualized by the same averager, running 100 sweeps when stimulating at 1.6 Hz and running 500 sweeps for EIS at 50 Hz and 400 Hz. We aimed to obtain EABRs that at least allowed us to disinguis 4 to 5 troughs, with maximal amplitude in the range of 4 to 10 μV, matching acoustic stimuli of 60 to 95 dB above hearing threshold (cp. [28,61,63]) by adjusting the current level.
Immunocytochemistry
Following EIS, rats received a lethal dose of thiopental (i.p., 50 mg/200 g body weight of TRAPANAL, Nycomed, Konstanz, Germany) and were transcardially perfused. The fixative contained 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. Brains were removed and soaked in 30% saccharose buffer over night at 4°C before being cryo-cut into 30 μm thick frontal sections. Pre-incubation included 0.1% Triton X-100, 0.045% H2O2, 1% sodium-borohydride and 1% milk powder, each in 0.02 M sodium phosphate buffer at 7,4 pH for 30 min before a primary antibody raised in goat against Fos (1:2000; lot no. K1808/F1109, Santa Cruz Biotechnology Inc, Santa Cruz, USA) was applied to the sections. Following incubation for 48 h at 4°C a matching biotinylated secondary anti-goat antibody (1:200; Vector Laboratories Inc., Burlingame, USA) was used to visualize binding sites of the primary antibody with diaminobenzidine staining [61]. Negative controls were run in order to verify the specificity of the primary and secondary antibody [7]. Fos positive nuclei in the parabrachial area and the central gray served as positive controls.
Counting and statistics
Photographic documentation of DAB stained sections through the major auditory brainstem regions was performed with an x10 objective and a digital camera (AxioCam, Zeiss, Jena, Germany) at an 8 bit grey tone scale. When necessary, overlapping mosaic photos were taken to cover the whole cross sectional profile of a given region and then assembled to Mutiple Image Alignment (MIA) by using an image analysis program (analySIS, Soft Imaging System GmbH, Münster, Germany). Minor variability in staining intensity of tissue sections among rats were adjusted by setting background staining to a constant gray tone mean level (Adobe Photoshop CS, Adobe Systems Inc., San Jose, USA). For quantitative evaluation of the Fos expression, images were imported into analySIS. The detection threshold of grey tone values was set to 145 for AVCN, DCN and CIC. Following the definition of the Region of Interest (ROI) (Figures 1,3,5 A+B, dashed lines), detection of stained nuclei (Figures 1A+B inset, 3B lower panel of inset; 5B, inset) was done by following settings: aspect ratio (1-4), mean diameter in μm (4-14 for AVCN; 2-14 for DCN and CIC), elongation (1-5), area in μm2 (8-100), grey value minimum (0-200), and roundness (0.1-1). For DCN only, stained nuclei were separately detected as small (8-29 μm2; Figure 3B, lower panel of inset, greyish colored nuclei) and large (30-100 μm2; Figure 3B, lower panel of inset, white colored nuclei). The number of Fos positive nuclei was determined for AVCN, DCN, and CIC at each stimulation frequency of nimodipine-treated and nimodipine-untreated rats. Counts per ROI area were determined. Statistical analysis was done with Prism (GraphPad Software Inc., La Jolla, USA). Mean value and Standard Error of Mean (S.E.M.) were determined and statistical testing was carried out by applying one-way analysis of variance (ANOVA), followed by Newman-Keuls post test. Different significance levels were set at (*) for p<0.05, (**) for p<0.01 and (***) for p<0.001 (Figures 1C-E; 3C-H; 4A-D; 5C-E). Stereological corrections for counting particles in the sectioned material were not made as numerical relationships were sought for rather than absolute densities.
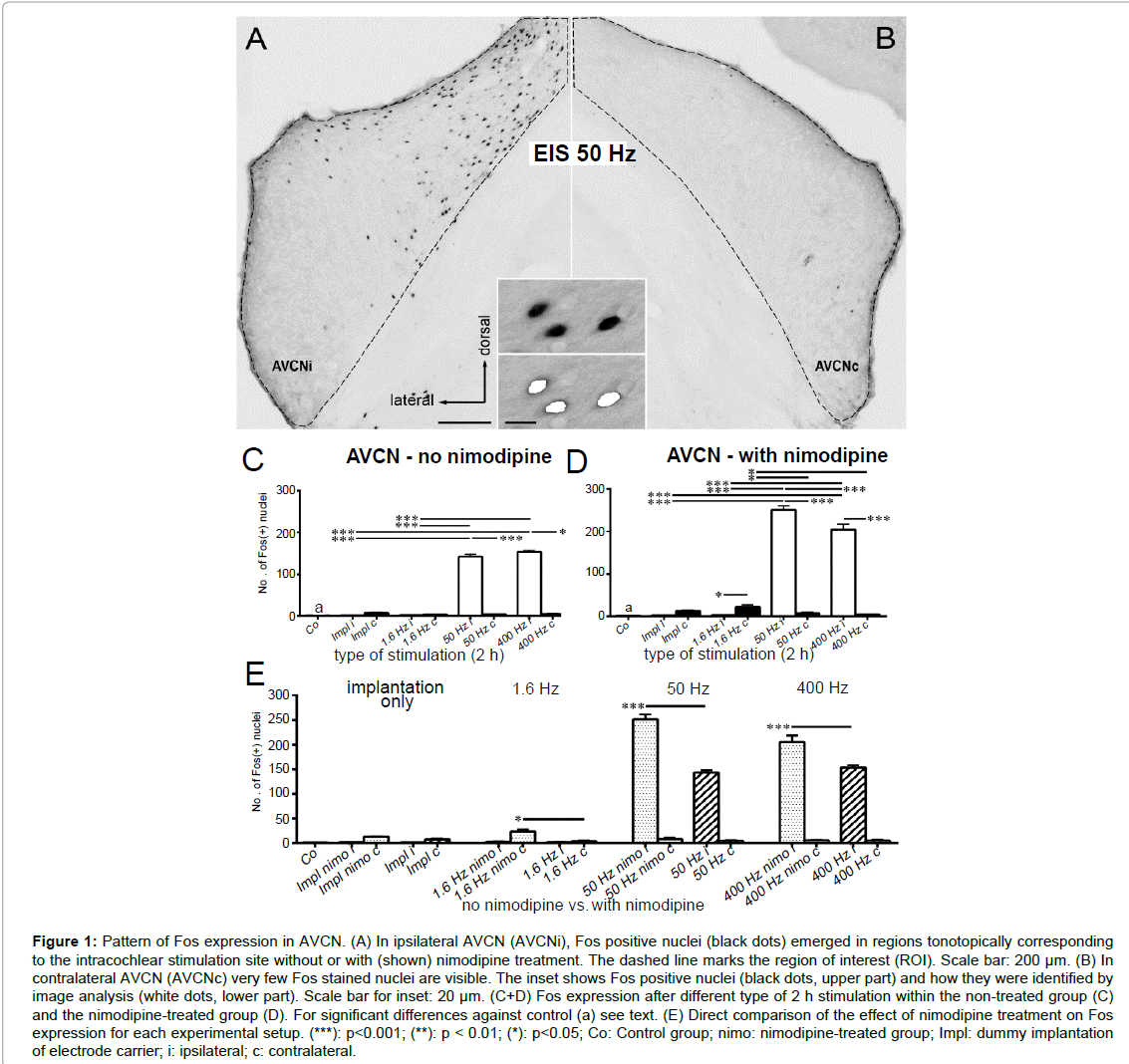
Figure 1: Pattern of Fos expression in AVCN. (A) In ipsilateral AVCN (AVCNi), Fos positive nuclei (black dots) emerged in regions tonotopically corresponding to the intracochlear stimulation site without or with (shown) nimodipine treatment. The dashed line marks the region of interest (ROI). Scale bar: 200 µm. (B) In contralateral AVCN (AVCNc) very few Fos stained nuclei are visible. The inset shows Fos positive nuclei (black dots, upper part) and how they were identified by image analysis (white dots, lower part). Scale bar for inset: 20 µm. (C+D) Fos expression after different type of 2 h stimulation within the non-treated group (C) and the nimodipine-treated group (D). For significant differences against control (a) see text. (E) Direct comparison of the effect of nimodipine treatment on Fos expression for each experimental setup. (***): p<0.001; (**): p < 0.01; (*): p<0.05; Co: Control group; nimo: nimodipine-treated group; Impl: dummy implantation of electrode carrier; i: ipsilateral; c: contralateral.
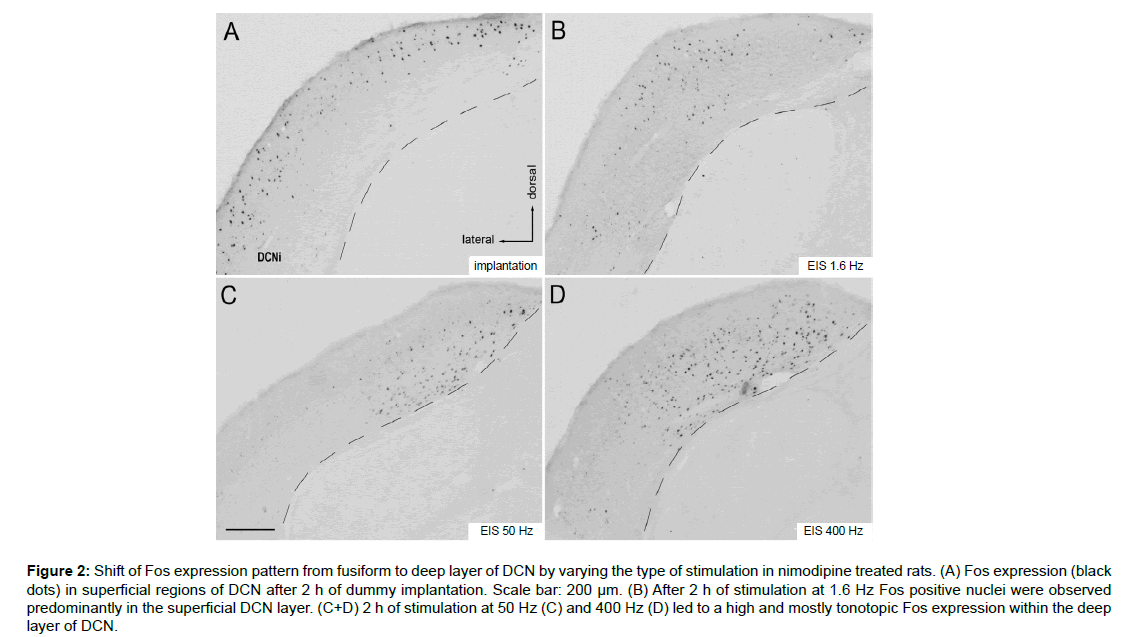
Figure 2: Shift of Fos expression pattern from fusiform to deep layer of DCN by varying the type of stimulation in nimodipine treated rats. (A) Fos expression (black dots) in superficial regions of DCN after 2 h of dummy implantation. Scale bar: 200 µm. (B) After 2 h of stimulation at 1.6 Hz Fos positive nuclei were observed predominantly in the superficial DCN layer. (C+D) 2 h of stimulation at 50 Hz (C) and 400 Hz (D) led to a high and mostly tonotopic Fos expression within the deep layer of DCN.
Results
Effect of nimodipine treatment on the ABR of mature rats
As described for the normal hearing wild-type Wistar rats, the presentation of click stimuli evoked large and clearly differentiated ABRs (cp. [62]) also in the nimodipine treated group. Consequently, nimodipine did not affect the hearing threshold.
Controls
Applying immunocytochemistry to visualize Fos in brain tissue, selected cellular nuclei turned black (Figures 1A, 3B upper panel of inset, 5A inset). Between control rats and rats treated with nimodipine 3 days prior perfusion, no significant differences in the level of Fos expression or the number of stained cells were observed in VCN, DCN, or CIC. At control level, Fos expression in VCN and DCN lay below detection level (Figures 1 C-E, 3 C-H, 4 A-D), whereas a low level of Fos positive nuclei was noticed in CIC (Figures 5C-E).
AVCN
Following EIS of untreated animals, Fos positive nuclei emerged in dorsal AVCN corresponding tonotopically to the intracochlear position of the stimulation electrodes (Figure 1A). Fos detection occurred within a Region of Interest (ROI) marking the boundaries of AVCN (Figure 1A, dashed line). Moderate (50 Hz) and high (400 Hz) intensity stimulation led to a significant increase of Fos positive nuclei on the Ipsilateral (i) side compared to control (Co) (p<0.001 for Co vs. 50 Hz i, Co vs. 400 Hz i; Figure 1C). Contralateral (c) to simulation Fos expression never exceeded control level (Figure 1B, C).
Neither 2 h of implantation of a passive electrode nor 2 h of EIS at 1.6 Hz elicited a notable number of Fos positive nuclei in the ipsilateral AVCN of untreated animals (Figure 1C). Only at 50 Hz, a significant Fos expression was observed ipsilaterally. Increasing stimulation frequency to 400 Hz Fos did not increase the number of Fos positive neurons any further (p<0.001; Figure 1C).
Following nimodipine treatment, a significant increase of Fos expression was observed in AVCNc following EIS at 1.6 Hz compared to controls (p<0.001 for Co vs. 1.6 Hz c). Additionally, Fos expression rose in AVCNi by EIS at 50 Hz and 400 Hz compared to control condition (p<0.001 for Co vs. 50 Hz i, Co vs. 400 Hz i; Figure 1D). A significant decrease of the number of Fos positive cells was elicited in AVCNc by increasing the stimulation frequency from 1.6 Hz to 50 Hz (p<0.05 for 1.6 Hz c vs. 50 Hz c; Figure 1D). Ipsilaterally, an increase was observed by 50 Hz and 400 Hz EIS (p<0.001; Figure 1D). However, for 400 Hz stimulation frequency, the number of Fos positive nuclei decreased significantly against the number at 50 Hz (p<0.001 for 50 Hz i vs. 400 Hz i; Figure 1D).
Comparing the data of the non-treated and the nimodipine-treated group, Fos expression was significantly higher in nimodipine-treated rats for all stimulation setups (p<0.001 for 50 Hz nimo i vs. 50 Hz i, 400 Hz nimo i vs. 400 Hz i; p<0.05 for 1.6 Hz nimo c vs. 1.6 Hz c; Figure 1E).
DCN
In DCN, electrode implantation itself as well as EIS frequency caused a marked effect on the layer in which Fos labeled cells emerged. Upon unilateral electrode implantation without stimulation, many cells expressed Fos throughout the superficial but not in the deep layers of DCNi (Figure 2A). With EIS of increasing frequency, Fos labeling shifted into the deep layers, with Fos labeling in the superficial layers disappearing. Deep layer responses tended to be tonotopically specific (Figures 2C and D).
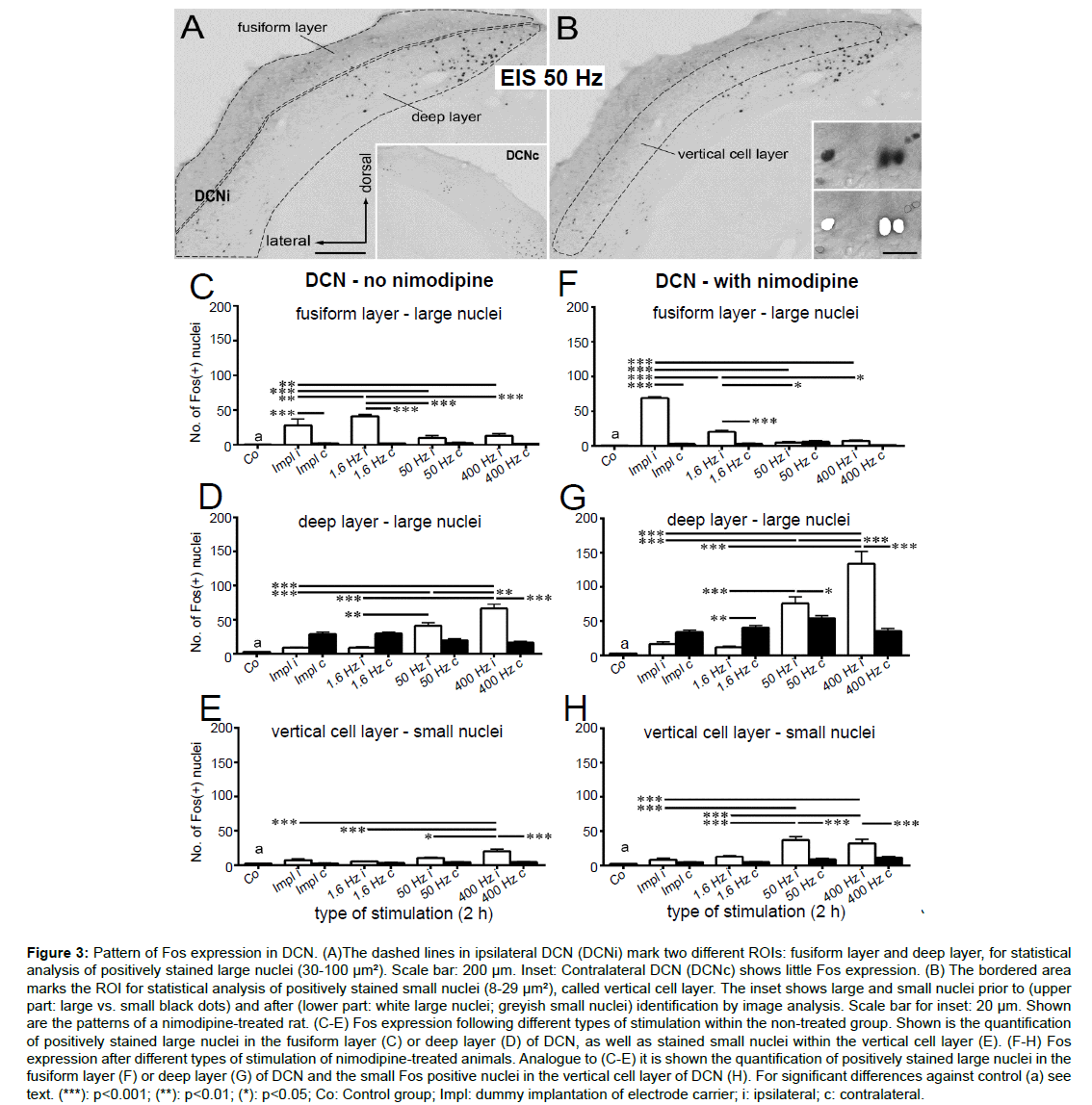
Figure 3:Pattern of Fos expression in DCN. (A)The dashed lines in ipsilateral DCN (DCNi) mark two different ROIs: fusiform layer and deep layer, for statistical analysis of positively stained large nuclei (30-100 µm2). Scale bar: 200 µm. Inset: Contralateral DCN (DCNc) shows little Fos expression. (B) The bordered area marks the ROI for statistical analysis of positively stained small nuclei (8-29 µm²), called vertical cell layer. The inset shows large and small nuclei prior to (upper part: large vs. small black dots) and after (lower part: white large nuclei; greyish small nuclei) identification by image analysis. Scale bar for inset: 20 µm. Shown are the patterns of a nimodipine-treated rat. (C-E) Fos expression following different types of stimulation within the non-treated group. Shown is the quantification of positively stained large nuclei in the fusiform layer (C) or deep layer (D) of DCN, as well as stained small nuclei within the vertical cell layer (E). (F-H) Fos expression after different types of stimulation of nimodipine-treated animals. Analogue to (C-E) it is shown the quantification of positively stained large nuclei in the fusiform layer (F) or deep layer (G) of DCN and the small Fos positive nuclei in the vertical cell layer of DCN (H). For significant differences against control (a) see text. (***): p<0.001; (**): p<0.01; (*): p<0.05; Co: Control group; Impl: dummy implantation of electrode carrier; i: ipsilateral; c: contralateral.
For DCN, detection of Fos positive cells was done in 3 different ROIs (Figures 3A,B dashed lines). In the fusiform and deep layer of DCN we detected intensely stained nuclei that were conspicuously large (30-100 μm2; Figure 3B, lower inset, white nuclei; C, D, F, G). At the same time, small nuclei (8-29 μm2; Figure 3B lower inset, grey colored nuclei; E, H) turned Fos positive which were mostly found within the vertical cell layer (Figure 3B, dashed line) of the DCN.
In the fusiform layer of normal rats, the number of large Fos positive nuclei exceeded control level by 2 h of CI implantation without stimulation and 2 h of EIS at 1.6 Hz (both ipsilateral, p<0.001; Figure 3C). Contralaterally, Fos expression remained at control level. The number of large Fos positive nuclei rose from implantation to stimulation at 1.6 Hz (p<0.01) but fell again at 50 Hz and 400 Hz when Fos expression did not exceed control level any more (p<0.001 for 1.6 Hz i vs. 50 Hz i; 1.6 Hz i vs. 400 Hz i; Figure 3C). By contrast, Fos expression in the deep layer of DCNi showed a linear increase from implantation over 1.6 Hz, 50 Hz (p<0.001 for 50 Hz i vs. impl i; p<0.01 for 50 Hz i vs. 1.6 Hz i; Figure 3D) to 400 Hz (p<0.001 for 400 Hz i vs. impl i, 400 Hz i vs. 1.6 Hz i; p<0.01 for 400 Hz i vs. 50 Hz i; Figure 3D), whereas the contralateral Fos level remained constant under the same stimulation parameters. A significant difference to control condition was observed ipsilaterally (p<0.001 for Co vs. 50 Hz i; Co vs. 400 Hz i) and contralaterally (p<0.001 for Co vs. impl c; Co vs. 1.6 Hz c; Figure 3D).
Detection of small Fos positive nuclei in vertical cell layer revealed a similar progress as for the large nuclei in the deep layer of the ipsilateral DCN of normal rats. On the stimulated side, Fos positive nuclei increased significantly from implantation over 1.6 Hz, 50 Hz to 400 Hz (p<0.001 for impl i vs. 400 Hz i, 1.6 Hz i vs. 400 Hz i; p<0.05 for 50 Hz i vs. 400 Hz i; Figure 3E). However, only at 400 Hz Fos expression was significantly higher compared to control (p<0.001 for Co vs. 400 Hz i; Figure 3E). Contralateral to the stimulated side, Fos positive nuclei were rare at any type of setup (Figure 3E).
Statistical analysis of positively stained large nuclei in the fusiform layer of DCN in nimodipine-treated rats, revealed a linear decrease on the side of stimulation (p<0.001 for impl i vs. 1.6/50/400 Hz i; p<0.05 for 1.6 Hz i vs. 50/400 Hz i; Figure 3F), whereas contralaterally the control level was maintained. The initial rise of Fos expression ipsilateral following 2 h of CI implantation dropped significantly by 2 h of EIS at 1.6 Hz (p<0.001 for impl i vs. 1.6 Hz i; Figure 3F). Matching the data obtained from the untreated group, an increase of the stimulation frequency led to a decrease of Fos expression (p<0.05 for 1.6 Hz i vs. 50 Hz i; 1.6 Hz i vs. 400 Hz i; Figure 3F).
By sharp contrast to this linear decline of the number of Fos positive cells with increasing stimulation frequency in the fusiform layer, detection of Fos positive nuclei showed a linear progression in the deep layer of the stimulated side of the DCN (Figure 3G). All stimulation frequencies resulted in a bilaterally increased Fos expression compared to control conditions (p<0.001) with the exception of ipsilateral implantation and EIS at 1.6 Hz i (p>0.05; Figure 3G). The ipsilateral increase of positively stained nuclei from implantation and 1.6 Hz to 50 Hz and further on to 400 Hz was significant (p<0.001 for impl i vs. 50/400 Hz i; 1.6 Hz i vs. 50/400 Hz i; 50 Hz i vs. 400 Hz i; Figure 3G). Contralaterally, Fos expression was detected at a level above control level (p<0.001), being significantly higher than ipsilateral at 1.6 Hz (p<0.01) but lower at 50 Hz (p<0.05) and 400 Hz (p<0.05; Figure 3G).
Parallel to the expression pattern in large Fos positive nuclei of the deep layer, a linear progression was observed for positively stained small nuclei within the vertical cell layer of DCNi with increasing stimulation frequencies (Figure 3H). On the side of stimulation, the level of Fos expression detected at 50 Hz for 2 h rose significantly against the level of unilaterally implanted as well as 1.6 Hz stimulated rats (p<0.001 for impl i vs. 50 Hz i; 1.6 Hz i vs. 50 Hz i; Figure 3H) and remained stable up to 400 Hz (p<0.001 for impl i vs. 400 Hz i; 1.6 Hz i vs. 400 Hz i; Figure 3H). A significant difference in Fos level compared to control conditions was detected ipsilaterally for all stimulation frequencies (p<0.001 for Co vs. 50 Hz i; Co vs. 400 Hz i; p<0.01 for Co vs. 1.6 Hz i; Figure 3H). On the contralateral side, Fos expression exceeded control level when stimulating with a 400 Hz frequency (p<0.05 for Co vs. 400 Hz c; Figure 3H) but remained on a constant low level for each other setup (Figure 3H).
The comparison of the data from the non-treated versus the nimodipine-treated rats allowed us to identify significant differences in Fos expression for every setup performed (Figure 4 A-D). Within the fusiform layer, differences in Fos expression could be observed in DCNi only following 2 h of implantation and 2 h of EIS at 1.6 Hz (p<0.001; Figures 4 A,B). Moderate (50 Hz) and intense (400 Hz) EIS resulted in significant differences in deep layer and vertical cell layer only (p<0.01 for deep nimo c vs. deep c; p<0.001 for all other; Figures 4C,D).
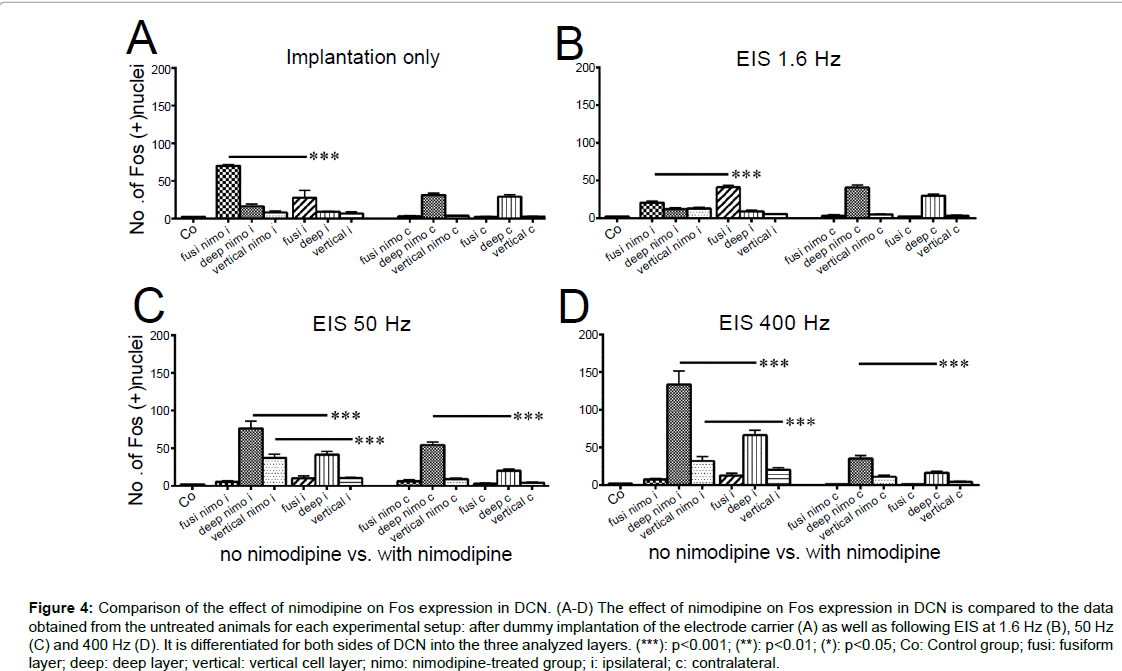
Figure 4:Comparison of the effect of nimodipine on Fos expression in DCN. (A-D) The effect of nimodipine on Fos expression in DCN is compared to the data obtained from the untreated animals for each experimental setup: after dummy implantation of the electrode carrier (A) as well as following EIS at 1.6 Hz (B), 50 Hz (C) and 400 Hz (D). It is differentiated for both sides of DCN into the three analyzed layers. (***): p<0.001; (**): p<0.01; (*): p<0.05; Co: Control group; fusi: fusiform layer; deep: deep layer; vertical: vertical cell layer; nimo: nimodipine-treated group; i: ipsilateral; c: contralateral.
Nimodipine treatment led to a significantly higher number of Fos positive nuclei in the fusiform layer after 2 h of implantation (p<0.001; Figure 4A), whereas 2 h of EIS at 1.6 Hz resulted in a significantly higher Fos expression within the non-treated group compared to the nimodipine-treated group on the side of stimulation (p<0.001; Figure 4B). Contralaterally, significant changes were not observed (p>0.05). In the deep layer of DCN, the detected level of Fos expression in nimodipine-treated rats was significantly increased against untreated after 2 h of EIS at 50 Hz and 400 Hz (p<0.001; Figure 4C,D), respectively, on both sides of the brainstem. In the vertical cell layer of the DCNi, nimodipine treatment led to a significant rise of positively stained nuclei compared to the non-treated group at stimulation frequencies of 50 Hz and 400 Hz (p<0.001; Figures 4C,D), respectively, whereas no significant changes occurred within the vertical cell layer of DCNc.
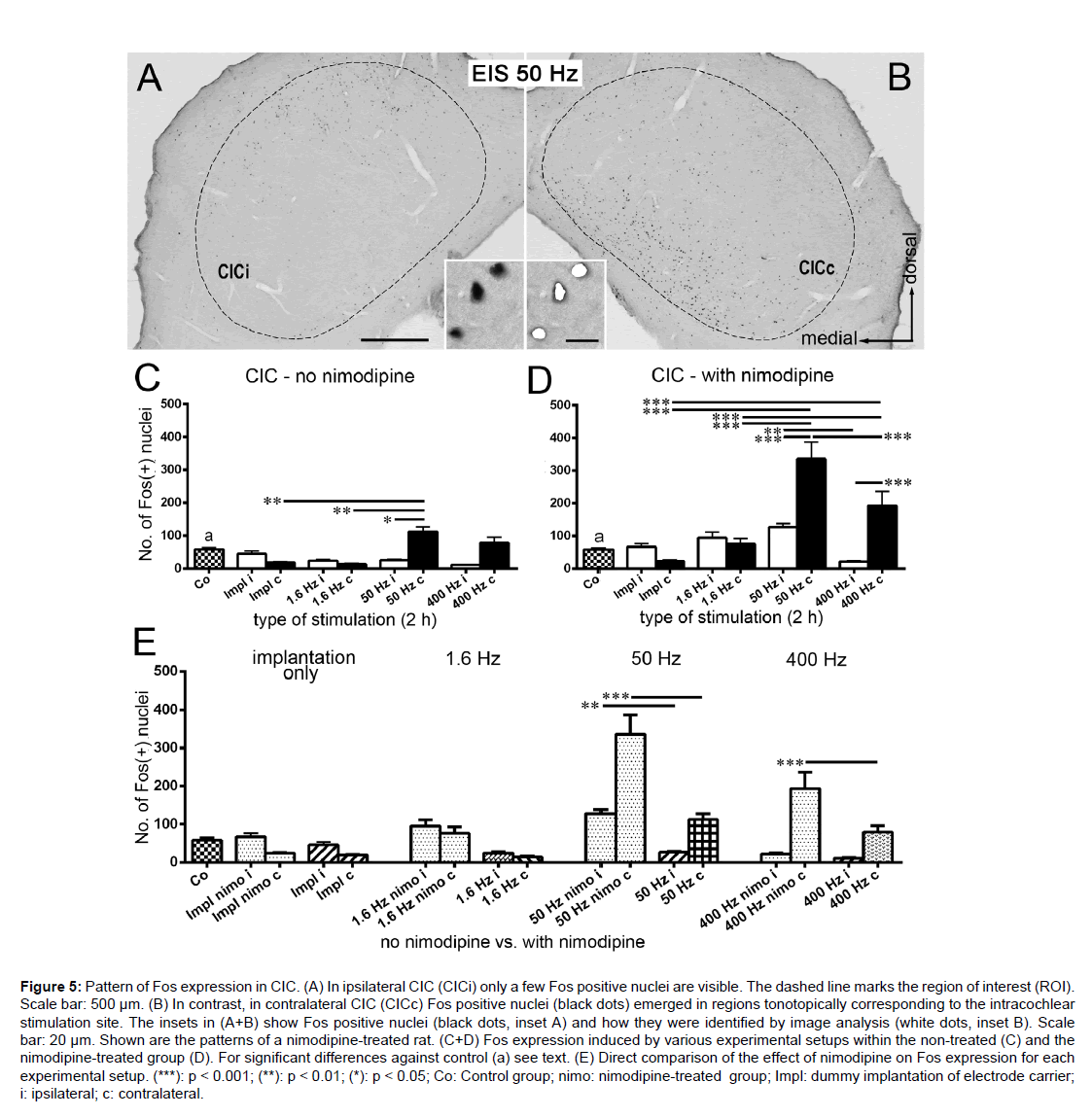
CIC
Independent of pharmacological treatment, a tonotopic Fos expression was observed in CICc after EIS (Figure 5B). In CICc of the non-treated group, there was a significant increase of Fos positive nuclei after 2 h EIS at 50 Hz against 2 h of implantation as well as 2 h of EIS at 1.6 Hz (p<0.01; Figure 5C). Additionally, a significant difference existed against the ipsilateral side (p<0.05; Figure 5C). Similar to the findings in AVCNi (Figure 1C), the Fos expression level in CICc after 50 Hz and after 400 Hz was similar. In the CICi a constantly low Fos level was observed (Figure 5C).
Figure 5:Pattern of Fos expression in CIC. (A) In ipsilateral CIC (CICi) only a few Fos positive nuclei are visible. The dashed line marks the region of interest (ROI). Scale bar: 500 µm. (B) In contrast, in contralateral CIC (CICc) Fos positive nuclei (black dots) emerged in regions tonotopically corresponding to the intracochlear stimulation site. The insets in (A+B) show Fos positive nuclei (black dots, inset A) and how they were identified by image analysis (white dots, inset B). Scale bar: 20 µm. Shown are the patterns of a nimodipine-treated rat. (C+D) Fos expression induced by various experimental setups within the non-treated (C) and the nimodipine-treated group (D). For significant differences against control (a) see text. (E) Direct comparison of the effect of nimodipine on Fos expression for each experimental setup. (***): p < 0.001; (**): p < 0.01; (*): p < 0.05; Co: Control group; nimo: nimodipine-treated group; Impl: dummy implantation of electrode carrier; i: ipsilateral; c: contralateral.
In CICc of nimodipine-treated rats Fos expression increased significantly against controls following sustained unilateral EIS at 50 Hz and 400 Hz (p<0.001 for Co vs. 50 Hz c; Co vs. 400 Hz c). In CICi, only the level after EIS at 50 Hz was significantly higher than control level (p<0.05; Figure 5D). Analogue to the response in AVCNi (Figure 1D), there was a substantial rise in the number of Fos stained nuclei from 2h of implantation over EIS at 1.6 Hz to 2 h of EIS at 50 Hz in CICc (p<0.001; Figure 5D). The further decline from 50 Hz to 400 Hz (p<0.001; Figure 5D) ended at a still significant level of Fos positive nuclei compared to control condition.
As seen in AVCN and DCN (Figures 1E, 4A-D), the comparison of the non-treated to the nimodipine-treated group revealed significant effects of blocking L-type calcium channels. Under this condition, the number of Fos positive nuclei was significantly higher in CIC at frequencies of 50 Hz (bilateral; p<0.01 for 50 Hz nimo i vs. 50 Hz i; p<0.001 for 50 Hz nimo c vs. 50 Hz c) and 400 Hz (contralateral; p<0.001; Figure 5E).
We noted that in the nimodipine-treated group, the characteristics of Fos expression in CICc showed the same progression as in the AVCNi, marked by an impressive increase of the Fos level after 50 Hz and a subsequent decrease after 400 Hz of stimulation (Figures 1D, 5D). However, this is in contrast to the temporal pattern of Fos expression within the deep layer of DCNi, where a continuous rise in Fos expression occurred with increasing stimulation frequency (Figure 3D, G).
Discussion
The major findings of this study are, first, that inhibition of L-type calcium channels by systemic application of nimodipine interferes with the effect of electrical intracochlear stimulation upon the expression of the immediate early gene fos. Second, nimodipine-dependent changes in the stimulation-evoked patterns of Fos expression match in VCN and CIC with respect to intensity and tonotopic restrictions, a parallel consistent with changes in CIC being dependent on changes in VCN. Third, this does not appear to apply for DCN. Nimodipinedependent changes in Fos expression differently affected layers of DCN that contain spatially segregated subpopulations of excitatory and inhibitory neurons.
Methodological considerations
In order to stimulate the mature rat’s auditory system under controlled experimental conditions, we chose to apply EIS rather than acoustic stimulation, giving us a near-complete control over stimulation parameters [7]. Potential differences of arousal states were largely leveled out by working on anesthetized rats. Electrode position within the cochlea was reproduced with greatest care. Recorded EABRs showed slight variations across experiments. The number of clearly distinguishable troughs indicated a good positioning of the stimulation electrodes with respect to the acoustic nerve (cp. [62]).
Diverging from previous studies where a hole was drilled into the bony cochlea in order to insert the electrode carrier, we took advantage of the round window for positioning 2 close-by ring electrodes within the cochlea [7,61]. As done by Illing and Reisch, surgical procedure was performed without cauterization of the stapedial artery and thus guaranteeing the least disturbing situation possible [64]. The choice of the stimulation frequencies (1.6 Hz, 50 Hz, 400 Hz) was motivated by the results of experiments performed by Jakob and Illing [63]. These parameters have been proven effective in inducing immediate-early gene expression within the auditory brainstem.
The nimodipine dosage of 2 mg/kg was motivated by studies from Jastreboff and Brennan and from Kay and Davies [65-66]. We chose the form of subcutaneous application in equal depots on each side along the spine in order to allow the substance be systemically effective within a fixed time window. An injection of an additional dose of 0.02 mg of nimodipine into the round window directly before implantation of the electrode carrier was carried out in order to totally block L-type calcium channels of the inner hair cells. However, this particular step showed no additional effect on Fos expression as revealed by comparisons with rats only systemically treated.
AVCN and CIC showed similar Fos expression patterns due to EIS at different stimulation frequencies
Stimulating at a low frequency failed to induce Fos in AVCNi. A peak of Fos level was reached at 50 Hz EIS and the level was being maintained even if stimulation frequency intensifies up to 400 Hz (Figure 1C). A matching progression was seen in CIC on the side opposite to EIS (Figure 5C). In both AVCNi and CICc a tonotopic Fos expression corresponded to the intracochlear position of the stimulating electrodes (Figures 1C, 5C).
The coherent pattern of Fos expressing neurons in AVCNi and CICc could be understood when focusing on the complex neural network of the central auditory system involving these spatially separated regions. Probably most important, almost all type I multipolar cells of AVCN send their excitatory axon to CICc, whereas only very few of them are directed to CICi [67]. Additionally, from AVCN excitatory spherical bushy cells project to the LSOi [68]. From LSO, inhibitory projections are sent to CICi and excitatory principal cells project to CICc [69]. Another population of AVCN excitatory neurons, globular bushy cells, project to MNTBc while glycinergic neurons of MNTB mostly send their axon to the principal cells of the LSO on the same side [68,70]. Thus, activation of AVCN neurons mediated along several paths elicits activation in CICc.
In DCN the composition of the Fos positive neuron population changed in dependence of stimulus intensity
In DCN, Fos positive nuclei showed a clear spatial pattern due to various stimulation intensities (Figures 2A-D). In concordance to the baso-ventral position of the intracochlear electrode carrier a tonotopic Fos expression became noticeable after EIS at 50 Hz on the side of stimulation (Figure 2C)
Various authors described the mammalian DCN as a laminated structure [71,72]. The most superficial layer, also referred to as the molecular layer, consists of neuropil containing few cell bodies most of which were GABA positive stellate cells [73-75]. Other presumably inhibitory interneurons such as Golgi and cartwheel cells are scattered between the molecular and the subjacent pyramidal, or fusiform layer [76-78]. Excitatory granule cells reside within the granular cell areas in the fusiform layer but also in the granule cell domain outside of DCN [79-80]. The cell bodies of the bipolar fusiform cells forms the fusiform layer, also called pyramidal cell layer by other authors [81]. The regions located deep to the somata of the fusiform cells are called the deep layer of DCN. Besides basal dendrites of fusiform cells, it contains the cell bodies and most of the dendritic arborization of the giant cells [82]. Also located in the deep layer are the vertical cells, called tuberculoventral cells by some authors [72,83].
The layering of specific cell types in DCN led us to define three ROIs for a differentiated quantitative analysis of the effects of EIS on Fos expression pattern (Figures 3A,B). In the fusiform layer there was a significant non-linear mapping into a Fos positive cell population on the side of stimulation. Two hours of implantation and 2 h of EIS at low intensity elicited Fos expression in large nuclei, whereas at higher stimulation intensity no significant expression occurred in this ROI (Figure 3C). By contrast, Fos positive large nuclei in the deep layer of DCNi showed a significant linear increase in number when increasing stimulation frequency (Figure 3D). Analogous to the large nuclei in the deep layer, there was a significant and linear mapping of Fos positive nuclei on the side of stimulation within the vertical cell layer (Figure 3E). Opposite to the side of stimulation, Fos expression in the fusiform layer and in the vertical cell layer was absent in all cases (Figures 3C, E), while in the deep layer significant expression occurred after implantation and low intensity stimulation (1.6 Hz) (Figure 3D).
Referring to Fredrich et al. we suggest that the Fos expressing neurons detected in the fusiform layer are fusiform cells, whereas Fos expression in deep DCN layer is likely to be within giant cells [84]. The ROI selecting the vertical cell layer contained small sized vertical cells (Figure 3B, lower inset, greyish small nucleus), but are likely to exclude stellate, Golgi, cartwheel, granule, fusiform and giant cells (Figures 3B, E). Both fusiform and giant cells are glutamatergic neurons, sending their axon to CICc [85-86]. Vertical cells are inhibitory inter-neurons which synapse on principal cell types, fusiform and giant cells in DCN and principal cells in AVCN [87-90].
Fusiform cells respond with changes in Fos expression pattern even to minor disturbance of the intracochlear milieu due to implantation of the electrode carrier or after low frequency stimulation of the acoustic nerve on the side of stimulation. Following EIS at moderate and higher frequencies, no changes were seen within fusiform cells in DCNi so as if the stimulation counterbalances irritations of the system due to electrode implantation. However, the inverse seemed to be true for giant cells and vertical cells in the DCN ipsilateral to stimulation. Here, increasing Fos expression was elicited by increasing stimulation frequency. In our experiments, EIS at 50 Hz was needed in order to elicit substantial Fos activation within vertical cells. This suggests that inhibition of vertical cells was more intense on fusiform than on giant cells. Also the predominance of inhibitory neurons possibly employing both glycine and GABA as neurotransmitters may explain our observations that within the fusiform layer inhibition seems to be stronger than in the deep layer with increasing EIS intensity as indicated by Fos expression [75]. We here show that with increasing stimulation frequency fusiform cells are broadly inhibited to express Fos on the side of stimulation, while giant cells in the deep layer of the DCN became more and more encouraged to do so. We conclude that within the different excitatory and inhibitory subpopulations of neurons an intensity dependent Fos expression took place across the layers of DCN (Figure 3C-E).
We hypothesize that following sustained EIS the adjustment of the predominantly inhibitory, but partly also excitatory, systems in DCN involving gene expression and possible changes of receptor density and transmitter release allowed to control signal processing. These changes occurred within different neuronal subpopulations of the DCN depending on stimulation intensity, giving us a cue to suggest an answer to the question of what function they may have in the central auditory system.
Nimodipine enhances the sensitivity of neurons to express Fos
In AVCN, Fos expression was significantly higher in nimodipinetreated rats compared to the non-treated group following EIS at equal frequencies (Figure 1E). In CIC, the nimodipine-treated rats showed significantly higher levels of Fos positive nuclei bilaterally after EIS at 50 Hz and contralaterally at 400 Hz compared to untreated rats (Figure 5E). We suggest that the correlation of these two data depends on the functional succession of these two brainstem regions in the ascending auditory system, which are both enhanced in their neuronal responsiveness to induce Fos expression due to EIS.
Comparing untreated and nimodipine-treated rats, we observed in DCN that under nimodipine treatment the Fos level in the fusiform layer was higher 2 h after implantation (Figure 4A). This increase was already counteracted by EIS when run at 1.6 z (Figure 4B). Compared to normal rats, nimodipine treatment significantly increased the number of Fos positive nuclei bilaterally in the deep layer and on the side of stimulation in the vertical layer after EIS at 50 Hz (Figure 4C) or 400 Hz (Figure 4D). Fos expression elicited by EIS is specifically elevated in AVCN, DCN and CIC.
As previously mentioned, L-type calcium channels directly regulate nerve function by activating other ion channels enzymes transmitter release or gene expression [44,45,47,49,50].
Calcium influx specifically mediated by L-type calcium channels seems to be of great importance for stimulating the transcription of the fos gene [91-93]. Zhao et al. could demonstrate that Fos expression is regulated by different types of calcium channels dependent on the pattern of electrical stimulation [94]. Their study showed that at low frequencies of up to 10 Hz of electrical stimulation, L-type as well as N-type channels contributed to Fos expression. With increasing stimulation frequency, the influence of N-type channel activity on fos transcription became less important. At 50 Hz, the fos mRNA level was entirely dependent on L-type channel activity. This study fully agrees with the results of our nimodipine experiments in which most of the differences of Fos expression occurred when EIS was applied at 50Hz and 400 Hz compared to controls.
Van der Zee et al. reported that the mean nerve conduction velocity of nimodipine-treated old rats was significantly higher than that of control animals at the same age [95]. They could show that nimodipine reduces the age-related impairment of sensory motor functions. Moreover, nimodipine was shown to accelerate the acquisition of behavioral modifications and thus learning in old rabbits [96]. Together with Fos known to be involved in the molecular machinery of learning, our experiments indicate that enhanced Fos expression induced by combining nimodipine-treatment with EIS results in an increased readiness for structural and functional changes within central auditory neurons [31,32,97,98].
Not only neurons but also astrocytes possess L-type calcium channels and thus calcium ions may be important in the signaling between the two cell types [99-101]. Blocking those channels on the plasma membranes of astrocytes with nimodipine increases the neuronal firing rate and transmitter release of neurons in the central nervous system [102]. The effect induced by nimodipine in our study might thus well be supported or mediated by the action of glial cells.
It has been reported that prophylactic use of nimodipine in vestibular schwannoma surgery seemed to have a protective influence on hearing preservation [103,104]. Nimodipine has been subject to many experimental and clinical studies demonstrating its neuroprotective potency (i.e. [105-109].
We suggest that the calcium flow through L-type calcium channels may have a neuro-protective role on neurons in the central auditory system under strong activation. When L-type calcium channels are blocked by nimodipine, the altered calcium flow increased neuronal activity as apparent by the emergence of the activity and plasticity marker Fos. Further experiments are needed to determine if this increased activity will result in neuronal death and/or plastic remodeling. Altogether, the pattern and intensity of the Fos response reveal an enhanced readiness of central auditory neurons to respond to EIS and to process these sensory signals in locally specific ways.
Acknowledgements
The authors thank T. Jakob, A. Zeber, H. Hildebrandt-Schoenfeld, M. Fredrich, and I. Hirschmüller-Ohmes for helpful discussions and R. Laszig for continuous support. Stimulation electrodes and programming software were kindly provided by Cochlear GmbH Germany and Co. KG.
References
- Saint Marie RL, Luo L, Ryan AF (1999) Spatial representation of frequency in the rat dorsal nucleus of the lateral lemniscus as revealed by acoustically induced c-fos mRNA expression. Hear Res 128: 70-74.
- Saint Marie RL, Luo L, Ryan AF (1999) Effects of stimulus frequency and intensity on c-fos mRNA expression in the adult rat auditory brainstem. J Comp Neurol 404: 258-270.
- Sato K, Houtani T, Ueyama T, Ikeda M, Yamashita T, et al. (1993) Identification of rat brainstem sites with neuronal Fos protein induced by acoustic stimulation with pure tones. Acta Otolaryngol Suppl 500: 18-22.
- Vischer MW, Häusler R, Rouiller EM (1994) Distribution of Fos-like immunoreactivity in the auditory pathway of the Sprague-Dawley rat elicited by cochlear electrical stimulation. Neurosci Res 19: 175-185.
- Zhang JS, Haenggeli CA, Tempini A, Vischer MW, Moret V, et al. (1996) Electrically induced fos-like immunoreactivity in the auditory pathway of the rat: effects of survival time, duration, and intensity of stimulation. Brain Res Bull 39: 75-82.
- Saito H, Miller JM, Pfingst BE, Altschuler RA (1999) Fos-like immunoreactivity in the auditory brainstem evoked by bipolar intracochlear electrical stimulation: effects of current level and pulse duration. Neuroscience 91: 139-161.
- Illing RB, Michler SA, Kraus KS, Laszig R (2002) Transcription factor modulation and expression in the rat auditory brainstem following electrical intracochlear stimulation. Exp Neurol 175: 226-244.
- Nakamura M, Rosahl SK, Alkahlout E, Gharabaghi A, Walter GF, et al. (2003) C-Fos immunoreactivity mapping of the auditory system after electrical stimulation of the cochlear nerve in rats. Hear Res 184: 75-81.
- Curran T, Morgan JI (1995) Fos: an immediate-early transcription factor in neurons. J Neurobiol 26: 403-412.
- Bledsoe SC Jr, Nagase S, Miller JM, Altschuler RA (1995) Deafness-induced plasticity in the mature central auditory system. Neuroreport 7: 225-229.
- Keilmann A, Herdegen T (1995) Expression of the c-fos transcription factor in the rat auditory pathway following postnatal auditory deprivation. Eur Arch Otorhinolaryngol 252: 287-291.
- Luo L, Ryan AF, Saint Marie RL (1999) Cochlear ablation alters acoustically induced c-fos mRNA expression in the adult rat auditory brainstem. J Comp Neurol 404: 271-283.
- Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P (1988) c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 55: 917-924.
- Angel P, Karin M (1991) The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129-157.
- Herdegen T, Leah JD (1998) Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev 28: 370-490.
- Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131-136.
- Rosskothen N, Hirschmüller-Ohmes I, Illing RB (2008) AP-1 activity rises by stimulation-dependent c-Fos expression in auditory neurons. Neuroreport 19: 1091-1093.
- Pennypacker KR (1995) AP-1 transcription factor complexes in CNS disorders and development. J Fla Med Assoc 82: 551-554.
- Wisdom R (1999) AP-1: one switch for many signals. Exp Cell Res 253: 180-185.
- Raivich G, Behrens A (2006) Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol 78: 347-363.
- de Groen PC, Eggen BJ, Gispen WH, Schotman P, Schrama LH (1995) Cloning and promoter analysis of the human B-50/GAP-43 gene. J Mol Neurosci 6: 109-119.
- Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20: 84-91.
- Weber JR, Skene JH (1998) The activity of a highly promiscuous AP-1 element can be confined to neurons by a tissue-selective repressive element. J Neurosci 18: 5264-5274.
- Nagase S, Miller JM, Dupont J, Lim HH, Sato K, et al. (2000) Changes in cochlear electrical stimulation induced Fos expression in the rat inferior colliculus following deafness. Hear Res 147: 242-250.
- Saito H, Miller JM, Altschuler RA (2000) Cochleotopic fos immunoreactivity in cochlea and cochlear nuclei evoked by bipolar cochlear electrical stimulation. Hear Res 145: 37-51.
- Illing RB (2001) Activity-dependent plasticity in the adult auditory brainstem. Audiol Neurootol 6: 319-345.
- Reisch A, Illing RB, Laszig R (2007) Immediate early gene expression invoked by electrical intracochlear stimulation in some but not all types of neurons in the rat auditory brainstem. Exp Neurol 208: 193-206.
- Duncan GE, Johnson KB, Breese GR (1993) Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J Neurosci 13: 3932-3943.
- Kearney JA, Frey KA, Albin RL (1997) Metabotropic glutamate agonist-induced rotation: a pharmacological, FOS immunohistochemical, and [14C]-2-deoxyglucose autoradiographic study. J Neurosci 17: 4415-4425.
- Kaczmarek L (1992) Expression of c-fos and other genes encoding transcription factors in long-term potentiation. Behav Neural Biol 57: 263-266.
- Demmer J, Dragunow M, Lawlor PA, Mason SE, Leah JD, et al. (1993) Differential expression of immediate early genes after hippocampal long-term potentiation in awake rats. Brain Res Mol Brain Res 17: 279-286.
- Cole AJ, Saffen DW, Baraban JM, Worley PF (1989) Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340: 474-476.
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, et al. (2000) Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci U S A 97: 12880-12884.
- Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, et al. (2003) Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci 23: 9116-9122.
- Konishi T, Kelsey E (1970) Effect of calcium deficiency on cochlear potentials. J Acoust Soc Am 47: 1055-1062.
- Miller RJ (1987) Multiple calcium channels and neuronal function. Science 235: 46-52.
- Person AL, Raman IM (2010) Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron 66: 550-559.
- Issa NP, Hudspeth AJ (1994) Clustering of Ca2+ channels and Ca(2+)-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci U S A 91: 7578-7582.
- Zidanic M, Fuchs PA (1995) Kinetic analysis of barium currents in chick cochlear hair cells. Biophys J 68: 1323-1336.
- Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ (1997) Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proc Natl Acad Sci USA 94: 14883-14888.
- Zhang SY, Robertson D, Yates G, Everett A (1999) Role of L-type Ca(2+) channels in transmitter release from mammalian inner hair cells I. Gross sound-evoked potentials. J Neurophysiol 82: 3307-3315.
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, et al. (2000) Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89-97.
- Hudspeth AJ (1986) The ionic channels of a vertebrate hair cell. Hear Res 22: 21-27.
- Roberts WM, Howard J, Hudspeth AJ (1988) Hair cells: transduction, tuning, and transmission in the inner ear. Annu Rev Cell Biol 4: 63-92.
- Art JJ, Wu YC, Fettiplace R (1995) The calcium-activated potassium channels of turtle hair cells. J Gen Physiol 105: 49-72.
- Pang ZP, Cao P, Xu W, Südhof TC (2010) Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J Neurosci 30: 4132-4142.
- Siegel JH, Relkin EM (1987) Antagonistic effects of perilymphatic calcium and magnesium on the activity of single cochlear afferent neurons. Hear Res 28: 131-147.
- Roberts WM, Jacobs RA, Hudspeth AJ (1990) Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci 10: 3664-3684.
- Hardingham GE, Cruzalegui FH, Chawla S, Bading H (1998) Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium 23: 131-134.
- Hardingham GE, Bading H (1998) Nuclear calcium: a key regulator of gene expression. Biometals 11: 345-358.
- van Breemen C, Leijten P, Yamamoto H, Aaronson P, Cauvin C (1986) Calcium activation of vascular smooth muscle. State of the art lecture. Hypertension 8: II89-95.
- Zhou S, Liu L, Yang X, Wu S, Chen G (2010) Paraoxon attenuates vascular smooth muscle contraction through inhibiting Ca2+ influx in the rabbit thoracic aorta. J Biomed Biotechnol 2010: 829190.
- McNeish AJ, Altayo FJ, Garland CJ (2010) Evidence both L-type and non-L-type voltage-dependent calcium channels contribute to cerebral artery vasospasm following loss of NO in the rat. Vascul Pharmacol 53: 151-159.
- Amberg GC, Earley S, Glapa SA (2010) Local regulation of arterial L-type calcium channels by reactive oxygen species. Circ Res 107: 1002-1010.
- Du H, He J, Wang S, He L (2010) Investigation of calcium antagonist-L-type calcium channel interactions by a vascular smooth muscle cell membrane chromatography method. Anal Bioanal Chem 397: 1947-1953.
- Towart R, Kazda S (1979) The cellular mechanism of action of nimodipine (BAY e 9736), a new calcium antagonist [proceedings]. Br J Pharmacol 67: 409P-410P.
- Kazda S, Towart R (1982) Nimodipine: a new calcium antagonistic drug with a preferential cerebrovascular action. Acta Neurochir (Wien) 63: 259-265.
- Hess P, Lansman JB, Tsien RW (1984) Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature 311: 538-544.
- Glossmann H, Striessnig J, Ferry DR, Goll A, Moosburger K, et al. (1987) Interaction between calcium channel ligands and calcium channels. Circ Res 61: I30-136.
- Rosskothen-Kuhl N, Illing RB (2010) Nonlinear development of the populations of neurons expressing c-Fos under sustained electrical intracochlear stimulation in the rat auditory brainstem. Brain Res 1347: 33-41.
- Rosskothen-Kuhl N, Illing RB (2012) The impact of hearing experience on signal integration in the auditory brainstem: a c-Fos study of the rat. Brain Res 1435: 40-55.
- Jakob T, Illing RB (2008) Laterality, intensity, and frequency of electrical intracochlear stimulation are differentially mapped into specific patterns of gene expression in the rat auditory brainstem. Audiol Med 6: 215-227.
- Illing RB, Reisch A (2006) Specific plasticity responses to unilaterally decreased or increased hearing intensity in the adult cochlear nucleus and beyond. Hear Res 216-217: 189-97.
- Jastreboff PJ, Brennan JF (1988) Specific Effects of Nimodipine on the Auditory System. Ann N Y Acad Sci 522: 716-718.
- Kay IS, Davies WE (1993) The effect of nimodipine on salicylate ototoxicity in the rat as revealed by the auditory evoked brain-stem response. Eur Arch Otorhinolaryngol 250: 51-54.
- Doucet JR, Ryugo DK (2006) Structural and functional classes of multipolar cells in the ventral cochlear nucleus. Anat Rec A Discov Mol Cell Evol Biol 288: 331-344.
- Malmierca MS (2003) The structure and physiology of the rat auditory system: an overview. Int Rev Neurobiol 56: 147-211.
- Saint Marie RL, Ostapoff EM, Morest DK, Wenthold RJ (1989) Glycine-immunoreactive projection of the cat lateral superior olive: possible role in midbrain ear dominance. J Comp Neurol 279: 382-396.
- Sommer I, Lingenhöhl K, Friauf E (1993) Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp Brain Res 95: 223-239.
- Osen KK (1969) Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol 136: 453-484.
- Lorente de Nó R (1981) The Primary Acoustic Nuclei. Raven Press, New York.
- Wouterlood FG, Mugnaini E, Osen KK, Dahl AL (1984) Stellate neurons in rat dorsal cochlear nucleus studies with combined Golgi impregnation and electron microscopy: synaptic connections and mutual coupling by gap junctions. J Neurocytol 13: 639-664.
- Mugnaini E (1985) GABA neurons in the superficial layers of the rat dorsal cochlear nucleus: light and electron microscopic immunocytochemistry. J Comp Neurol 235: 61-81.
- Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J (1992) An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat Embryol (Berl) 186: 443-465.
- Gates TS, Weedman DL, Pongstaporn T, Ryugo DK (1996) Immunocytochemical localization of glycine in a subset of cartwheel cells of the dorsal cochlear nucleus in rats. Hear Res 96: 157-166.
- Golding NL, Oertel D (1996) Context-dependent synaptic action of glycinergic and GABAergic inputs in the dorsal cochlear nucleus. J Neurosci 16: 2208-2219.
- Berrebi AS, Mugnaini E (1991) Distribution and targets of the cartwheel cell axon in the dorsal cochlear nucleus of the guinea pig. Anat Embryol (Berl) 183: 427-454.
- Alibardi L (2003) Ultrastructural distribution of glycinergic and GABAergic neurons and axon terminals in the rat dorsal cochlear nucleus, with emphasis on granule cell areas. J Anat 203: 31-56.
- Weedman DL, Ryugo DK (1996) Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comp Neurol 371: 311-324.
- Blackstad TW, Osen KK, Mugnaini E (1984) Pyramidal neurones of the dorsal cochlear nucleus: a Golgi and computer reconstruction study in cat. Neuroscience 13: 827-854.
- Ryugo DK, Willard FH (1985) The dorsal cochlear nucleus of the mouse: a light microscopic analysis of neurons that project to the inferior colliculus. J Comp Neurol 242: 381-396.
- Zhang S, Oertel D (1993) Tuberculoventral cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol 69: 1409-1421.
- Fredrich M, Reisch A, Illing RB (2009) Neuronal subtype identity in the rat auditory brainstem as defined by molecular profile and axonal projection. Exp Brain Res 195: 241-260.
- Alibardi L (2000) Cytology, synaptology and immunocytochemistry of commissural neurons and their putative axonal terminals in the dorsal cochlear nucleus of the rat. Ann Anat 182: 207-220.
- Beyerl BD (1978) Afferent projections to the central nucleus of the inferior colliculus in the rat. Brain Res 145: 209-223.
- Voigt HF, Young ED (1980) Evidence of inhibitory interactions between neurons in dorsal cochlear nucleus. J Neurophysiol 44: 76-96.
- Voigt HF, Young ED (1990) Cross-correlation analysis of inhibitory interactions in dorsal cochlear nucleus. J Neurophysiol 64: 1590-1610.
- Oertel D, Wu SH (1989) Morphology and physiology of cells in slice preparations of the dorsal cochlear nucleus of mice. J Comp Neurol 283: 228-247.
- Wickesberg RE, Oertel D (1990) Delayed, frequency-specific inhibition in the cochlear nuclei of mice: a mechanism for monaural echo suppression. J Neurosci 10: 1762-1768.
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H (1999) Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron 22: 789-798.
- Morgan JI, Curran T (1986) Role of ion flux in the control of c-fos expression. Nature 322: 552-555.
- Sheng M, McFadden G, Greenberg ME (1990) Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 4: 571-582.
- Zhao R, Liu L, Rittenhouse AR (2007) Ca2+ influx through both L- and N-type Ca2+ channels increases c-fos expression by electrical stimulation of sympathetic neurons. Eur J Neurosci 25: 1127-1135.
- Van der Zee CE, Schuurman T, van der Hoop RG, Traber J, Gispen WH (1990) Beneficial effect of nimodipine on peripheral nerve function in aged rats. Neurobiol Aging 11: 451-456.
- Deyo RA, Straube KT, Disterhoft JF (1989) Nimodipine facilitates associative learning in aging rabbits. Science 243: 809-811.
- Paylor R, Johnson RS, Papaioannou V, Spiegelman BM, Wehner JM (1994) Behavioral assessment of c-fos mutant mice. Brain Res 651: 275-282.
- Tischmeyer W, Grimm R (1999) Activation of immediate early genes and memory formation. Cell Mol Life Sci 55: 564-574.
- Litzinger MJ, Foster G, Brenneman DE (1986) [H3]-nitrendipine binding in non-neuronal cell cultures. Biochem Biophys Res Commun 136: 783-788.
- Salm AK, McCarthy KD (1989) Expression of beta-adrenergic receptors by astrocytes isolated from adult rat cortex. Glia 2: 346-352.
- Porter JT, McCarthy KD (1995) GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia 13: 101-112.
- Ballerini P, Ciccarelli R, Di Iorio P, Giuliani P, Caciagli F (1995) Influence of Ca2+ channel modulators on [3H]purine release from rat cultured glial cells. Neurochem Res 20: 697-704.
- Strauss C, Bischoff B, Neu M, Berg M, Fahlbusch R, et al. (2001) Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J Neurosurg 95: 771-777.
- Scheller C, Richter HP, Engelhardt M, Koenig R, Antoniadis G (2007) The influence of prophylactic vasoactive treatment on cochlear and facial nerve functions after vestibular schwannoma surgery: a prospective and open-label randomized pilot study. Neurosurgery 61: 92-98.
- Uematsu D, Greenberg JH, Hickey WF, Reivich M (1989) Nimodipine attenuates both increase in cytosolic free calcium and histologic damage following focal cerebral ischemia and reperfusion in cats. Stroke 20: 1531-1537.
- Bär PR, Traber J, Schuurman T, Gispen WH (1990) CNS and PNS effects of nimodipine. J Neural Transm Suppl 31: 55-71.
- Angelov DN, Neiss WF, Streppel M, Andermahr J, Mader K, et al. (1996) Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci 16: 1041-1048.
- Guntinas-Lichius O, Martinez-Portillo F, Lebek J, Angelov DN, Stennert E, et al. (1997) Nimodipine maintains in vivo the increase in GFAP and enhances the astroglial ensheathment of surviving motoneurons in the rat following permanent target deprivation. J Neurocytol 26: 241-248.
- Sekiya T, Yagihashi A, Asano K, Suzuki S (2002) Nimodipine ameliorates trauma-induced cochlear neuronal death. Neurol Res 24: 775-780.
Citation: Bischoff P, Rosskothen-Kuhl N, Illing RB (2013) Systemically Antagonizing L-Type Calcium Channels Modifies Intensity and Pattern of Fos Expression Evoked by Electrical Intracochlear Stimulation in the Adult Rat Auditory Brainstem. Otolaryngology S3: 005 DOI: 10.4172/2161-119X.S3-005
Copyright: © 2013 Bischoff P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14640
- [From(publication date): 1-2012 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 10025
- PDF downloads: 4615