Widespread Outbreaks of a Subtle Condition Leading to Hospitalisation and Death
Received: 22-Jul-2013 / Accepted Date: 20-Sep-2013 / Published Date: 23-Sep-2013 DOI: 10.4172/2161-1165.1000137
Abstract
Long-term cycles in healthcare costs, medical admissions, hospital bed occupancy coupled with periods of increased deaths can be discerned in western health care systems for which there has never been an adequate explanation. A new type of immune-based infectious outbreak, operating via one of the many ubiquitous and persistent viruses appears to be occurring. Given the general difficulty of reaching a definitive diagnosis in the largely elderly population presenting to both primary and secondary care in western countries has led to these outbreaks remaining largely unrecognised. It is tentatively proposed that the ubiquitous herpes virus Cytomegalovirus (CMV) could be implicated in the observed long-term patterns of deaths, hospitalisation and wider costs.
Keywords: Immune impairment; Infectious outbreak; Cytomegalovirus; Aged populations; Death; Vague symptoms
160871Introduction
Since the 1960’s emergency admissions to hospital of a mainly medical nature in all western countries have increased in a manner far in excess of that expected from demographic changes in the population [1-4]. In order to explain this gap the various studies have implicated social and behavioural factors such as health care consumerism, more conservative General Practitioner (GP) and hospital consultant behaviour, the breakdown of the family, increasing numbers of elderly living alone and failings in the processes and organisation of health and social care – although in practice it would be exceedingly difficult to quantify the contribution from each of these factors [4]. However, no one seems to have posed the obvious question; could it be that there is a genuine trend to increasing poor health? Is it a co-incidence that illnesses such as diabetes, allergies, asthma and other immune syndromes have apparently increased in parallel with these unexpected increases in medical emergency admissions? [5-10].
Elderly Populations
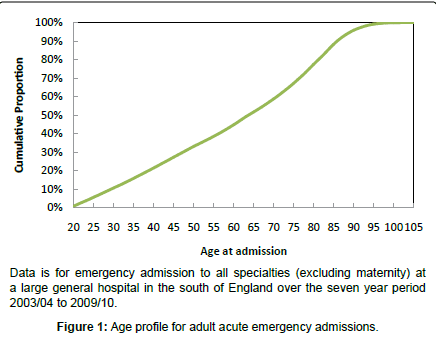
In all western countries the generally aging population has resulted in the situation where the elderly account for the bulk of all primary and secondary care encounters and this is illustrated in Figure 1 for emergency hospital admission. It can be seen that 50% of adult acute hospital admissions are for those aged over 65 years and this proportion is increasing with time. This presents a unique set of problems regarding the correct diagnosis of the cause for the presenting symptoms. In the elderly, infection often manifests as changes in cognitive function (including depression) and physical function rather than the classical signs of infection such as fever seen in the young [11]. For example, in one study conducted in Nottingham, England during 2009 over 50% of patients aged over 70 years were cognitively impaired (27% with delirium, 32% depressed, 21% apathy, 17% delusions/agitation/aggression) [12]. Chronic inflammatory conditions associated with high levels of circulating cytokines lead to muscle wastage and disability (irrespective of age), although in the elderly the natural innate inflammatory responses are enhanced and prolonged [11,13]. Assessment scores for both cognitive and physical function are therefore good predictors of emergency department attendance [14] and of infection and/or inflammation and these measures often continue to decline after the immediately apparent cause of the ‘infection’ (urinary tract infection, pneumonia, etc) has been treated [11].
In this context the presence of a subtle type of infectious immune impairment would be exceedingly difficult to discern.
An Immune-Based Syndrome
In 1981 the landmark paper by Michael Gottlieb, Joel Weisman and others described an association of unexpected infections in previously healthy young men which is now known as HIV/AIDS [15]. Are there a set of similar associations behind the seemingly inexorable rise in medical admissions seen over the past four decades? Using similar logic to that employed by Gottlieb et al a possible link has been explored in research which suggests that the increase in medical admissions (much like the cluster of unusual infections associated with HIV/AIDS) is characterised by all the features expected of an infectious outbreak rather than a more general trend which would arise if social and behavioural factors were the cause [4,16-20]:
1. The outbreaks occur at an interval of three to eight years with somewhere around five to six years being the most common.
2. At a local level (as opposed to a national level) these recurring increases occurs over a very short period (around 6 to 8 weeks) while at a national level the full spatial spread occurs over an 18 to 24 month period [21-25].
3. The onset of each ‘outbreak’ is characterised by a small but statistically significant increase in excess deaths. This immediate increase is smaller than the excess deaths which would typically characterise an influenza outbreak or similar however a cumulate increase in excess deaths over around one year can lead to total deaths in excess of a large influenza epidemic [22-25].
4. The increase is specific to aspects of medicine and mental health, i.e. admissions to the surgical specialties are largely unaffected.
5. Each outbreak leads to an approximate 10% step-like increase in medical admissions andbed occupancy with wider effects against total health care costs.
6. The increase is specific to a range of diagnoses which all have a common immune function linkage either via infection or inflammation but which are far wider in scope than those associated with HIV/AIDS [4]. This encompasses mental health issues which are dependent on immune function as an exacerbating factor and, as in AIDS, influence the trajectory of a small number of specific cancers [26].
7. Considerable regional [spatio-temporal] variation appears to be associated with each outbreak which is also suggestive of an infectious spread [4,22-25,27].
If this hypothesis is correct then wide spread disruption of health should occur and this should reflect in large scale changes in health care expenditures, especially given the generally higher costs associated with treatment of the elderly [28].
Health Care Costs
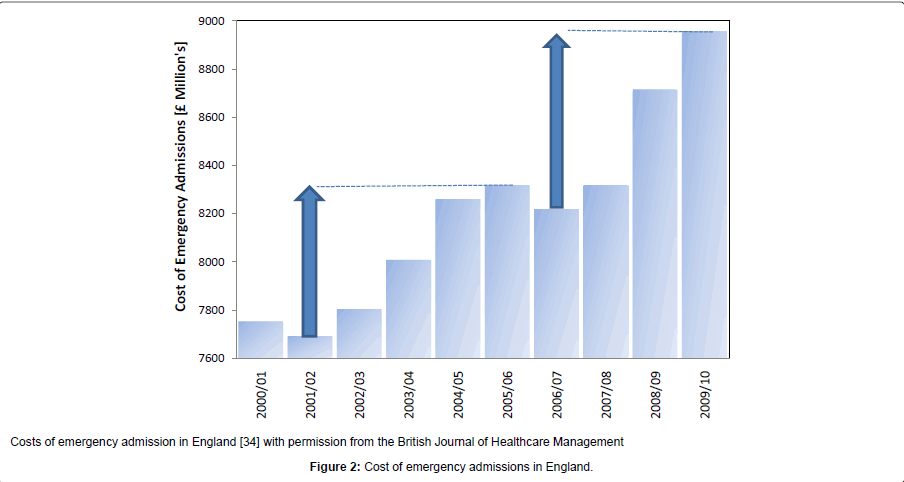
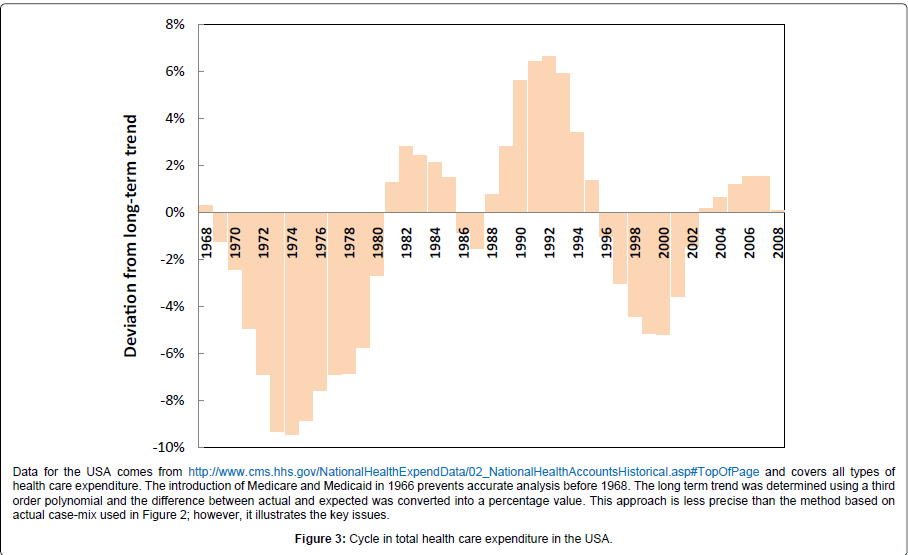
During the 1980’s the health insurance industry in the USA began to suspect that a curious cycle of profit and loss was occurring [29] and the evidence for this was formally reported in 1991 [30]. This cycle implied that the process of setting annual premiums via analysis of historical costs was subject to some form of sudden and unexplained change in the health of the population. Further research suggested that this cycle was linked to an approximate 6% [range 3% to 15%] increase in inflation-adjusted total costs [3,31] which will arise from a rapid change in the volume, case-mix and/or complexity of the health care contacts, the sum of which leads to the total cost of health insurance claims. While it is recognised that health insurance in the USA is typically more related to those of working age and generally the more affluent, the pattern of hospital admissions, occupied bed days and wider costs seen in other countries appears to mirror a cycle very similar, if not identical to, the health insurance underwriting cycle seen in the USA [3] as do total health care costs in the USA, i.e. including Medicare and Medicaid costs [32,33]. The last three of these potential infectious outbreaks occurred across the four countries of the UK (England, Scotland, Wales and Northern Ireland) toward the middle of 2002, 2007and early in 2012 [4,23]. Figure 2 explores the impact on the cost of emergency admissions in England for the 2002 and 2007 outbreaks. As can be seen the rise in 2008/09 equivalent (i.e. inflation adjusted) costs was £627 million from the trough in 2001/02 to the peak in 2005/06 or £739 million from the trough in 2006/07 to the peak in 2009/10. Both of these figures are similar to a figure of £680 million estimated using the increase in occupied beds arising from the 2002 outbreak [27]. This sudden and huge increase in (largely) medical admission and bed occupancy has been proposed to be the main contributing factor to a cycle of surplus and deficit seen within the NHS over many years [32,33] and has curious similarities to the cycle of cost increases observed in the USA [34] and to a cycle in hospital admissions and bed demand seen in Canada and Australia [3,35]. The resulting inflation adjusted cost cycle seen in the USA is explored in Figure 3 where the gap from trough to peak ranges from 4% to 12% of total health care expenditure (average around 7%) and this equates to around $180 billion in 2008 equivalent costs. This estimate is close to a figure of 6% derived from reanalysis of the annual inflation-adjusted work of Born and Santerre [31] by this author [3]. The shoulder on the side of the first trough is likely to be associated with an outbreak occurring around 1974 or1975 [3].
Clearly we are dealing with something having widespread and profound effects upon general health, including case mix and costs of health care [4].
Infectious Cycles
Outbreaks of all infectious diseases, where there is an element of acquired immunity, occur at a unique frequency particular to the infectious agent [34-42]. Certain viruses maintain a state of permanent or persistent infection in the host and this could account for the steplike change in emergency admission rates, which will arise from the pool of infected persons who could be experiencing some form of impaired or altered immunity.
Hence if the hypothesis of repeating international outbreaks is correct, then we must of necessity be looking for a ubiquitous virus known to establish a persistent infection but must additionally possess powerful immune modulating properties. In this respect, the ubiquitous herpes virus, Cytomegalovirus (CMV), could be implicated [4].
Cytomegalovirus
Up until recent years this virus has been largely regarded as only posing a risk to the developing foetus, where it is responsible for 40% of all congenital conditions [43,44] and the severely immunecompromised such as HIV/AIDS and transplant recipients [45,46]. Prevalence of this virus in Western countries increase with age with around 50% of the population CMV seropositive by age 15 to 34 and 80% by age 55 to 59 [44,47]. The multiple modes by which CMV could pose a serious problem to the seemingly immune competent has been recently reviewed along with the mechanisms by which CMV outbreaks could occur [4], however, the key point is that this virus possesses a powerful array of immune evasive and suppressing strategies against both adaptive and innate immune functions [45,48-51] leading to immuno suppression, chronic inflammation and autoimmunity [52-55] which will collectively be implicated in the observed higher all-cause mortality (hazard ratio of 1.2) for persons who are CMV seropositive [56]. The specific issues relating to mortality are discussed later. Due to the natural processes of immunoscenescence CMV has been particularly shown to be a risk factor in the elderly [57-60] where it contributes to what is known as the ‘Immune Risk Profile’ (IRP). Studies have shown that the healthy elderly are either not infected with CMV or have a significantly lower pro inflammatory state indicating that CMV is under strict immunological control [61-63].
Hence a general erosion of immune function and competence arising from life-long exposure to CMV is highly likely to lead to a host of non-specific symptoms which will be difficult to specifically attribute to CMV.
Diagnostic Uncertainty
In this respect, several studies have noted that a specific and unexplained increase in diagnoses which are described in Chapter R (signs and symptoms)of the International Classification of Disease (ICD) are associated with the increase in medical emergency admissions for the elderly [64-67] especially R00-09 (circulatory and respiratory) and R50-68 (general signs and symptoms) [68]. Re-analysis of the same data has demonstrated that particular signs and symptoms exhibit a characteristic step-like increase immediately after the 2002 and 2007 ‘outbreaks’ seen in the UK [4] and a similar ‘outbreak’ seen in Australia [69]. In the USA, a specific increase in emergency department attendances for ‘other and undefined diagnoses’ has been observed since1993 and this translated into peaks in admission via the emergency department around 1993, 1997 and 2003. The highest increase was noted in those aged over 65, African-descent and requiring three or more medications [70]. Hence the general difficulty in assigning a definitive diagnosis in the elderly is consistent with the specific step increase in admissions for signs and symptoms and this may have masked the true underlying aetiology.
In general practice it is known that less than 20% of the most frequent diagnoses account for 80% of consultations, hence, just 20% of consultations relate to the 80% of less frequent and hence less familiar diagnoses. In general, 50% of diagnoses remain a description of symptoms, 40% are named syndromes and only 10% are a confirmed diagnosis [71]. This diagnostic ambiguity will almost certainly also apply in the acute context.
In addition to the more general ‘signs and symptoms’, there are an additional set of more specific diagnoses that appear to be implicated in a series of step-like increases in emergency admission and in the widest sense all such diagnoses have a common link with infection or inflammation, i.e. inflammatory immune responses and the consequences of these [4]. The point of relevance is that a combination of diagnostic ambiguity and the generally elderly age of patients would make it exceedingly difficult to detect a general immune-based syndrome.
For example, an elderly patient presenting with pneumonia would be diagnosed as one of the multiple forms of ‘pneumonia’ although the exact reason that the patient has pneumonia rather than remaining healthy may well be related to a CMV induced recent deterioration in the IRP or other measures of inflammation and immune competence and CMV re-activation in the lungs leading to a more specific diagnosis of CMV pneumonitis [72]. Is the possibility that urinary retention can arise from CMV infection widely appreciated? [73]. Would an elderly patient presenting with depression be prescribed anti-depressants if the doctors were aware that active CMV infection is associated with depression in the elderly [74] and that disturbances in immune function are now increasingly recognised as a primary cause of depression [75,76]. Would a link between cirrhosis of the liver and CMV be explored [77]? Indeed how many clinicians would be aware that CMV has been directly linked to breast cancer [78,79], Ross syndrome [80], chronic periodontitis [81], pre-eclampsia [82], inflammatory bowel disease [83]. These are but a small sample of a growing body of medical case studies involving CMV in a diverse range of more complex illnesses which are discussed further in a later section. Indeed a recent reviews of severe CMV infection in apparently immune competent patients has concluded that severe life-threatening complications may not be as rare as previously thought [84-86]. In critically ill patients CMV infection (without bacterial infection) gave relative risk of 4 (sepsis, respiratory failure, death), 5 (pneumonia, acute respiratory distress syndrome, multi organ failure), 6 (diarrhoea) and 11 (septic shock) [87]. The likelihood is that we are treating symptoms rather than root causes.
The following sections will investigate the common themes relating to the clinical aspects of CMV infections and how these may overlap with the observed infectious-like outbreaks.
CMV Strains
CMV displays genetic polymorphisms in multiple genes [88-90] with possible clinical significance. It has been recently proposed that this diverse range of polymorphisms may confer advantage in the ability to exploit a wide range of temporary through to permanent immune impairments present in all supposedly ‘immune competent’ persons [4].
CMV is able to rapidly mutate both in vivo and in vitro. Endothelial cell and leukocyte (polymorphonuclear& monocyte) trophism is shared by all clinical isolates but is missing in laboratory adapted strains [91] while long-term exposure to a range of anti-virals produces resistant strains [92].
The prevalence of different CMV strains appears to vary widely between countries. In Japan a mutation at codon 605 (D to E) is present in 92% of CMV infected children while this particular strain has a low prevalence in Western countries [93]. In India, Glycoprotein GB3 is the most frequent compared to GB1 in China and Hungary [94] while GB1 also occurred more frequently in a 1998 study in Iowa, USA [95]. While in Baltimore, USA in young women who had recently acquired CMV infection some 75% had a unique strain or a strain shared with only one other woman, 25% shared a common strain while 4% had multiple strains [96].
These different strains do have different properties in vivo and in vitro and the kinetics of cytolysis of CMV infected fibroblasts depends on the strain [97]. The ability of different CMV strains to produce active infection of endothelial cells (the most common clinical location for CMV infection and disease) depends on mutations in the UL133- UL138 locus of the CMV genome [98]. Strain specific interactions between CMV encoded proteins and epigenetic mechanisms (histone acetylation and deacetylation) have been observed [99] and posttraumatic stress disorder (PSTD) patients have specific epigenetic modifications accompanied by higher levels of CMV antibodies [100].
Infection of infants showed clustering of disease types according to CMV Glycoprotein B genotypes [94] and another study has suggested that there is a 5-fold reduction in the risk of sequalae in congenitally infected infants for Glycoprotein GN-1 and GN-3a genotypes, however GN-4 genotypes appear to increase risk by 8-fold [101]. However in organ transplant recipients Immediate Early 1 (IE1) variants showed no discernable relationships [88]. In this respect Glycoprotein N appears to be more to do with CMV neutralisation by antibodies rather than more active CMV-manipulation of the immune system [102]. However in the latter study mixed strain infections demonstrated associations and outcomes that single-strain infections did not [88].
Multiple genotype infections are relatively common and up to six different genotypes have been detected in a single adult [89,90]. The role of different strains of CMV is important and infection of mice with multiple CMV strains leads to active infection of the salivary ducts (a potential source of salivary duct cancer) with the more pathogenic strains [103]. In lung transplant recipients single strain infections are generally asymptomatic while mixed strain infections produce symptomatic infection [89].
Introduction of a new strain is a feasible reason for these outbreaks.
Mixed Infection
In just the same way that infection with multiple CMV strains leads to more clinically serious outcomes so also does multiple infection with other viruses, bacteria and fungi. For example, in the era prior to antivirals in bone marrow transplant patients with CMV pneumonia who exhibit lymphocytosis progress to recovery while those who had mixed bacterial or fungal infection with peripheral lymphopenia died [104]. In the critically ill patient those with a mixed CMV/bacterial infection (which occurred most often in the winter, with 4-times higher length of stay and peaked in 50 to 60 year olds)had very high risk ratios of 7 (death), 10 (diarrhoea, multi-organ failure)and 220 (septic shock) [87]. Patients with mixed Hepatitis C Virus (HCV) and CMV infection who do not spontaneously clear the HCV infection generally have higher levels of CMV IgG and IgM and have double the level of CMV DNA detection in serum [105]. A case of double encephalitis is described in a patient with dual infection with HSV and CMV [106]. Acute respiratory infections (RSV, rhinovirus, enterovirus) are statistically more frequent in children infected with CMV [107].
In critically ill patients reactivation of HSV then leads to a cascade of events leading to CMV re-activation [108] and presumably vice versa and of other herpesviruses such as Human Herpesvirus 6 (HHV- 6) which can lead to further complications [109]. Such a cascade of intertwined effects is also observed in the ability of latent HHV-6, HSV and CMV infection to induce Chlamydial persistence via imbalanced oxidative stress [110]. Such cascades may form part of the proposed time-dependant shifts in GP referral for different dermatological conditions that appear to occur after these outbreaks [111].
It would appear that synergistic interactions with other resident infections are capable of enhancing the clinical consequences of a CMV-based outbreak.
Genetic Factors
As expected, genetic factors are also important. Those with a variety of schizophrenia (including neurophysiology) susceptibility genes have heightened susceptibility to CMV and several other pathogens [112]. Early life environment (epigenetic factors?) [99] Has likewise been shown to be important in the susceptibility of individuals to acquire CMV infection [113]. There is evidence for the epigenetic regulation of cytomegalovirus gene expression [114] and CMV in turn modifies the epigenetic processes [115].
Hence both genetic and epigenetic factors will be involved in the differential sensitivity of individuals to a variety of CMV-mediated disease processes.
Gender
Men are generally known to be more susceptible to infection while women have a far greater risk of illness caused by an overactive immune system [116], hence at the same level of treatment immunemediated chronic inflammatory diseases have a greater effect against women [117]. While such generalisations are useful it is of interest to note that there are distinct gender differences in the types of immune response determining susceptibility to CMV-mediated coronary artery disease with men mounting a far more inflammatory-based response centred around elevated levels of CRP while that in women is more subtle [118]. CMV antigenemia in cancer patients is higher in women [119] and women are known to have higher release of interferon and interleukin 2 in response to CMV infection [120]. In older women CMV antibody levels are associated with increased risk of diabetes, CVD, frailty and mortality (HR 2.8) [60].
The literature is conclusive that CMV seropositivity is higher in females [4,43,44,121] and it is a notable feature of the infectious outbreaks that emergency admission and death is higher in females [4,23]. Even more curiously each outbreak appears to initially elevate the gender ratio at birth (male: female) which is the opposite to what would normally be expected, i.e. the outbreak appears to have a particular effects which extends to the female foetus [122]. CMV DNA is detected in 15% of >20 week stillborn and manifests as thrombotic vasculopathy in 60% of cases [123]. Congenital CMV mortality in the US is highly race specific with odds ratio of: Native American 2.3, African American 1.9, White American 1.0, Hispanic 0.96, Asian 0.5 [124]. Male/female differences are therefore likely and in congenital CMV infection females have double the risk of brain abnormalities [125]. Hence the observation of undulations in the gender ratio initiated by each outbreak is feasible.
Hence each outbreak is associated with generally higher admissions in women and a smaller and more transient increased loss of the female foetus. Gender specific responses to CMV appear to be a likely explanation. Further research is needed to disentangle if this effect is specific to particular conditions as appears to be the case [126].
Death
It has been noted that each outbreak appears to be associated with a generalised increase in deaths which lasts for around 12 to 18 months following the onset of the outbreak [23-25]. This has been especially apparent during the most recent outbreak occurring in early 2012 in England, although slightly earlier in Scotland [24] with approximately 40,000 excess deaths since the onset of the outbreak [23]. Several large population studies have noted that those who have CMV infection show higher mortality than their non-CMV infected counterparts, especially in those with elevated CMV antibody levels who are also characterised by an exaggerated inflammatory response.
One study in elderly Latinos over a nine year period showed a fully-adjusted 35% increase in mortality due to Cardiovascular Disease (CVD) and a 19% increase in all-cause mortality for those in the highest quartile of CMV IgM titre. The effect appeared to be driven by inflammation with links to the levels of interleukin-6 (IL-6) and Tumor Necrosis Factor (TNF) [127].
Another study representative of the population of the USA over an 18 year period demonstrated that individuals who were CMV seropositive and with elevated levels of C-reactive protein (CRP) showed a 30% increase in all-cause and CVD-related mortality compared to CMV seropositive but low CRP subjects [56].
In the EPIC-Norfolk [England] study which ran from 1993 to 2011, i.e. the study spanned four outbreaks in 1993, 1996, 2002 and 2007, CMV infection was associated with a 16% increase in all-cause mortality (+6% cardiovascular disease, +13% cancer and +23% other causes of which 12% were respiratory, 16% gastrointestinal and 21% central nervous system leaving 60% across other body systems) [128]. While CMV infection alone was associated with a 16% increase in allcause mortality it was noted that those with the highest levels of CMVantibodies had a 26% increase in all-cause mortality during the study period. CMV infection of the heart has been known for some time to be associated with fatal myocarditis [129].
The fact that all-cause mortality reaches across a wide range of body systems and diagnoses in the above study is consistent with the observed increase in emergency admission for a variety of medical conditions [4] and increased deaths [25] which appear to be associated with these outbreaks. For example, in end stage renal disease (ESRD) CMV aggravates the contraction of CD4+ naïve T cells and increases the number of differentiated CD4+ and CD8+ memory cells [130,131]. In HIV disease CMV-specific CD8+ T cell responses were lower during recent HIV infection, higher during chronic untreated infection and higher still during long-term antiretroviral treatment [46]. It would appear that the ability of CMV to manipulate multiple innate and adaptive immune functions lies behind this increased all-cause mortality [45,48,51,53-55,132] and the emergence in diverse diseases including Alzheimer’s and type 2 diabetes in the elderly [133,134]. In fact there are now increasing reports of cases where CMV viremia has been the direct cause of death in a variety of clinical contexts [25,86]. Unsurprisingly the absence of CMV-mediated inflammatory responses are a characteristic of longevity in 85+ year olds [59,61-63].
How do we link the above to the approximate 3% increase in deaths which occur during the first one to two years of these outbreaks; CMVinduced inflammatory responses are obviously part of wider all-cause mortality and the introduction of a new strain of CMV (which may only lead to a small increase in the proportion of CMV seropositive) into the population (see previous section) would therefore lead to a much smaller increase in deaths. There is some evidence to suggest that the proportion of CMV seropositive individuals does indeed fluctuate over time [4] and the next step will be to ensure that future population studies take this possibility into account. Indeed the most recent 2012 outbreak in the UK has led to higher levels of increased death than in previous outbreaks and re-analysis of clinical samples collected before and during this period is probably a useful endeavour [4].
In England each outbreak leads approximately to an additional 420,000 admissions [4] and this would imply serious infection leading to hospitalisation in just 5% of adults over the age of 65, of which only 10% actually die. This figure would be achievable and is consistent with the generally erosive effects of CMV against health rather than a virus leading specifically to high mortality- which is not a desirable outcome from a viral infectious point of view.
Cancer
In recent years CMV has been increasingly implicated as both an oncomodulatory and oncogenic agent [52,78,135-141]. A recent study of the trends in new diagnoses of cancers between 1999 and 2007 in the USA has shown that the majority of cancers show roughly linear or demographic-based trends over time but that a minority of cancers show undulations that appear to roughly coincide with the outbreaks of the proposed infectious agent [26]. This group of cancers appeared to include those affecting large numbers of people such as those of the urinary tract, digestive system, skin, breast and genital system which are tissues all known to harbour CMV infection [4,142].
A review of diagnoses for hospital admissions where the patient eventually died in the period following the 2007 outbreak in England showed a high proportion of cancer patients [26]. In this respect low level of CMV infection in Glioblastomamultiforme (an aggressive brain tumour) is known to be associated with patient survival [143] and CMV viremia in cancer patients is known to vary with cancer location, type and ethnicity of the patient [119].
Hence the evidence points toward a recurring series of some form of infectious outbreak with effects against a range of cancers where CMV seems to be a common theme.
Cognitive Function
CMV is becoming increasingly recognised as a psychotrophic agent. This is unsurprising given the bi-directional relationships between depression and other illness behaviours and immune function [75,76]. In schizophrenia CMV seropositivity is known to be associated with visual search, working memory and psychomotor speed aspects of cognitive function while both CMV and HSV1 are associated with error making, a cognitive flexibility and executive function [144]. In healthy adults CMV appears to influence the degree of novelty seeking behaviour [145]. In the elderly active as opposed to dormant CMV infection has been associated with depression [74]. More recently CMV has been implicated as part of the link between stress, ageing and immunity [146]. In Alzheimers Disease (AD) CMV antibody levels are associated with extent of Neuro Fibrillary Tangles (NFT) and interferon-γ is only found in the Cerebrospinal Fluid (CSF) of CMV seropositive subjects [133].
Hence the observed increase in admissions relating to self-harm and other mental health issues during the proposed outbreaks is consistent with a role for CMV [4].
Inflammation
Numerous studies have demonstrated that it is the ability of CMV to provoke an inflammatory response in particular individuals rather than CMV infection per se that is the key to understanding why CMV leads to illness in some people more so than others. Various inflammatory markers are therefore elevated and it is this which could lead to the wide variety of illnesses observed in these outbreaks. Hence elevated CRP (in a dose dependant manner) is known to be associated with use of antidepressants and risk of hospitalization. Odds ratio 2.7 for antidepressant usage or 2.3 for hospitalization in those with CRP >10 mg/l of serum [147]. See above section.
Emergency admission to hospital for potentially preventable and other conditions is highest in the most deprived quintile (as is the proportion who are CMV seropositive [43,44,121]), those with one or more mental health conditions (previous section) and for those with four or more multi-morbidities [148]. CMV-mediated inflammation is almost certainly part of these risk factors.
Role of the Thymus
The thymus is central in immune homeostasis and generates self-tolerant niave T cells as well as self-antigen specific natural T-regulatory cells. It is a unique site where the endocrine and immune systems interact [149]. As such thymus function is regulated by both somatotrope Growth Hormone (GH) and Insulin-like Growth Factor 1 (IGF-1) which is central in insulin specific auto reactive T-cell selection [150]. The production of thymulin, an anti-inflammatory hormone, also occurs in thymic epithelial cells. Production of this hormone is strongly influenced by the neuroendocrine system [151]. A recent review of the action of CMV in disease has implicated thymic function as a specific variable of interest [4]. Thymic output reaches a maximum around age one and thereafter declines with age in a generally exponential manner with a 10-fold reduction at around age 45 and a further 10-fold reduction by age 80 [152], however, for individuals of the same age thymic output varies by a factor of 10-fold [153] due to a variety of adverse environmental factors [154]. This age related decline is enhanced bythymectomy [155], end stage renal disease [130,131] or in Severe Combined Immuno Deficiency (SCID) where bone marrow transplantation effectively restores thymic output [156]. In humans, ageing and loss of thymic function is often accompanied by impaired zinc status. In aged mice zinc supplementation has been show to increase thymic output and reduces the expression of stem cell factor, a thymo- suppressive cytokine [157].
Given the role of CMV in provoking inflammation in certain individuals it is of interest to note that thymopoiesis in elderly humans has been correlated with a shift to neutrophilic and inflammatory status [158]. A more recent study by the same group has demonstrated that both thymic output and levels of CRP are independent predictors of all-cause mortality [159]. Clearance of CMV viremia following double umbilical cord blood transplantation for hematologic malignancies in adults has been shown to rely on T cell neogenesisvia the thymus [160].
The epithelial cells of the thymus medulla secrete Macrophage- Derived Chemokines (MDC) that chemotactically attracts the immature thymocytes. These MDC are probably responsible for the maturation of the thymocytes during their migration from the cortex into the medulla.
Infection of the thymus with particular agents can therefore disrupt thymic function. Hence in mice infection with Francisellatularensis leads to severe thymic atrophy and CD4 and CD8 depletion [161]. Persistent infection of murine thymic epithelial cells with Coxsackievirus B4 decreases the production of IGF-2, a hormone involved in selftolerance toward pancreatic islet β cells [162], while persistent infection of human thymic epithelial cells with the same virus increases epithelial cell proliferation and production of inflammatory cytokines [163]. It is of interest to note that in vitro infection of human thymic epithelial and monocyte cells by measles, while causing a transient increase in thymic output leads to a measure of epithelial apotosis and a decrease in the size of the thymic cortex [164].
In line with the wider preference of CMV for epithelial cells [165] a number of studies have demonstrated specific effects of CMV against the epithelial layer in the thymus where an active and persistent infection produces distinct multinucleated cells [166]. Such infected cells show a significant reduction in the production of cells reactive with monoclonal antibodies specific for mesoderm-derived compounds and a reduction in IL-1 related antigen production [167].
Further to the theme of epithelial cells, individuals with inflammatory skin diseases typically show depressed thymic function. In particular those with atopic dermatitis had high variability in thymic output over time [168]. Unsurprisingly CMV is often associated with these conditions and increased GP referral to dermatology typically occurs during the proposed outbreaks [111].
Hence it appears highly likely that in those individuals where CMV has infected the thymus the resulting disruption of thymic functions could lead to the enhanced inflammatory responses which have been observed in some members of CMV seropositive populations.
Sub-Acute Infection
One of the big questions in CMV pathology is how the virus appears to exert its effects in the absence of a clinical level of infection. The first hint at an answer to this question comes in the observation that CMV is often associated with cancerous tissue [52,78,135-141], i.e. it is simply taking site-specific advantage of one of a variety of exploitable immune or physiological impairments [4].
Perhaps the biggest development in this area is the discovery that in patients with Inflammatory Bowel Disease (IBD) CMV can re-activate in the gut mucosa without causing detectable changes in serum viral (DNA) load or traditional antibody assays [169]. These ‘hidden’ reactivations could, however be detected using an assay for recently activated ‘effector’ CD8 T cells.
Hence site-specific CMV infections in the vasopressin producing (AVP) part of the anterior hypothalamus [170], anterior pituitary gland [171], pancreatic islets [172,173] and a host of other cell types and tissues can all induce a variety of diseases or modify the course of another disease without typical CMV clinical symptoms. See section on Acute Admission for further detail.
Evidence is therefore accumulating for a wide range of infection types from site-specific infections through to more clinically observable ‘typical’ infections.
Acute Admission
There is now a large body of case reports for patients admitted to hospital with a wide variety of symptoms where CMV is a causative or exacerbating factor. One review identified 207 case reports up to 2007 with gastrointestinal, respiratory and central nervous system infections being most common [86]. Table 1 [174-181] gives a limited selection of such case reports which illustrate some of the principles highlighted above. For those patients admitted to intensive care or burns units CMV is a well-recognised risk factor with high rates of re-activation or first time infection leading to serious viremia, other complications and even death [84,182-184]. Indeed CMV involvement in patients with sepsis or septic shock is highly likely [108,185,186] and should be investigated as a possibility.
| Diagnosis | Description |
| CMV colitis [175] | A 77-year-old man with vomiting and diarrhea 2 weeks after initial systemic chemotherapy consisting of 5-fluorouracil, leucovorin and irinotecan for a recurrent colorectal cancer. Multiple punched-out ulcers in the transverse colon, positive CMV antigen detected by indirect enzyme antibody method, although immunohistological examination of tissues biopsied at colonoscopy was negative. The symptoms ceased under ganciclovir and octreotide treatment, and the patient recovered gradually. |
| Superior mesenteric venous thrombosis [176] | 40-year-old Caucasian man with a 5-day history of fever. Serological test and pp65 antigen detection of CMV, suggesting acute infection. On the sixth day the patient complained of acute, progressive abdominal pain. Abdominal computed tomography revealed acute superior mesenteric venous thrombosis. Diffuse edema and ischemic lesions of the small bowel and its associated mesentery with a 50-cm-long segmental infarction of the proximal jejunum. The patient had an uneventful recovery and was discharged on the 11th postoperative day. |
| Coombs-negative hemolyticanemia [177] | 44-year-old Caucasian man with acute CMV infection. A 30-day history of fever and progressive asthenia. Fifteen days earlier, the patient was hospitalized, where acute CMV infection was diagnosed (positive CMV IgM, negative CMV IgG, CMV viremia, 12,698 copies/mL). C-reactive protein, 30.9 mg/L. No antiviral treatment was started because the patient was immuno competent. Fever persisted and the patient complained of progressive asthenia. At next admission the patient appeared pale and asthenic. Temperature of 38°C, heart rate of 100 beats/min, moderate hepatosplenomegaly. The blood examinations showed acute hemolyticanemia. Recovery at day 30 following ganciclovir. |
| Platypnea and orthodeoxia associated with Pneumocystis jiroveciand CMVpneumonia [178] | 75-year-old Caucasian woman with chronic renal failure due to vasculitis admitted with fever and respiratory failure due to Pneumocystis jiroveci and CMV pneumonia. Severe platypnea and orthodeoxia were major features of her illness with no history of respiratory, liver or cardiac disease. Further investigation with contrast echocardiography revealed no intracardiac or intrapulmonary shunts. As both lung bases were predominantly affected and no obvious explanation was found, platypnea and orthodeoxia were attributed to significant areas of low or zero ventilation/perfusion (V/Q) ratio. |
| Fever with splenic infarcts [179] | 36-year-old Caucasian woman with acute cytomegalovirus infection presenting with spontaneous splenic infarcts. Trans-esophageal echocardiography did not show any vegetations or mural thrombi. The patient was also found to be heterozygous for the Factor V Leiden mutation. The fever spontaneously resolved. |
| Ross syndrome [80] | 40-year-old woman who developed Ross syndrome (impairment of sweating and thermoregulation, tonic pupils, and hyporeflexia) associated with CMV infection. Her serum CMV IgM and IgG antibody titer levels were elevated. Along with clinical improvement, a gradual decrease of her elevated CMV IgM antibody titer level was seen with a continued increase in her CMV IgG level. The CMV IgM was also positive in the cerebrospinal fluid. |
| Hepatitis and Guillain- Barre Syndrome (GBS) [180] | 19-year-old Chinese girl, fatigue with pain and numbness of the limbs, with abnormal liver function. GBS based on history, clinical findings and auxiliary examinations. On day 13 of admission, her liver function was still abnormal. CMV hepatitis was diagnosed on positive serum CMV IgG and IgM. The case was improved with intravenous immunoglobulin therapy, without the use of antiviral therapy. |
| Encephalitis associated with thyoma and immunoglobulin deficiency [181] | Elderly woman with absent serum immuno globulins but normal T lymphocyte levels (which were unresponsive to CMV antigen). Died within 6 months of onset of symptoms. |
| CMV viremia and pneumonitis following tocilizumab therapy for Rheumatoid Arthritis (RA) [182] | A 41-year-old man who had a diagnosis of nonerosive RA. Fever, a productive cough with white sputum, and wheezing developed ≈3 weeks after his second infusion of tocilizumab which resulted in RA symptom resolution. After 1 week, persistent fever led to hospitalization. Worsening shortness of breath, nausea, and vomiting developed. Transferred to the Cleveland Clinic because of hypotension and intravenous dye- induced renal failure. Patient recovered with anti-viral therapy. |
Table 1: CMV-related acute admissions in immune competent adults.
Hence we can propose that typical CMV viremia (usually with fever, diarrhea, etc) is more to do with the balance of latency and reactivation in circulating fibroblasts and undifferentiated myeloid cells [187] while specific ‘hidden’ infection in a much wider range of cell types and tissues [188] regulates the expression and exacerbation of a wider range of conditions and diagnoses.
Given the ability of CMV to increase all-cause mortality across a very wide range of conditions and diagnoses and the wide range of conditions associated with increased hospital admission it would seem that CMV is therefore a good match with the observed increases in death and hospital admission (for a wide range of otherwise apparently unrelated conditions) observed in these outbreaks.
Having established a clinical basis for the proposed infectious outbreak the issue of spatio-temporal spread is crucial in demonstrating that a genuine infectious spread lies behind the phenomena.
Spatio-Temporal Spread
As with any persistent infection CMV is in a state of a continuous ‘epidemic’ as the virus is spread via contact with body fluids in a variety of ways [44,121]. As a consequence CMV mini-epidemics have been documented in households, neonatal intensive care units, geriatric units and renal transplantation units [189-192].
If we are dealing with the introduction of a new strain then the levels of CMV infected persons should change over time and a review of the available, but rather limited literature on this topic, suggests that this is indeed possible but requires further research [4]. However, study of the 2002, 2007 and 2012 outbreaks in the UK has demonstrated that there is a very definite spatial spread associated with each event. This spatial spread has been documented using deaths, emergency admissions, and changes in costs, occupied beds, associated increase in GP referrals and attendances at the emergency department due to the wider inflammatory effects against health [4,22-24,27,35,193-195].
The point of initiation in the UK appears to be specifically in Scotland, i.e. the most northern part of the UK [24]. Given the proposed role for vitamin D status in the role of CMV infection and thymic function [4] this may simply be a reflection of more northern latitude, however the exact reason remains to be quantified. Full spread across the whole of the UK appears to take around 18 to 24 months and it is this moderately slow spread that generates the appearance of longterm cycles in admissions, costs, etc when measured at national rather than local level. This would also explain the different shape of the time trends measured in different countries or states [4].
Hence we have clear evidence for an infectious-like spread but the missing link is to demonstrate that this is indeed due to the introduction of a new strain of CMV or indeed of some other virus which may then dispose the population to the reactivation of existing CMV strains.
Conclusions
It would seem that we do indeed have a phenomena contributing to the unexplained rise in medical (and especially elderly) admissions and that this phenomena is capable to causing large undulations in health service contacts and total health care costs. CMV is likely to be implicated at multiple levels, as direct agent, indirect risk factor and via opportunistic immune assault, as witnessed in patients in the intensive care unit [183,184]. Evidence has been presented to show that CMV is capable of active (but generally subclinical) infection of a wide range of cell types and tissues and is more than capable of directly and indirectly causing the wide range of diagnoses observed to associated with increased health service contacts and deaths associated with the infectious outbreaks. Is it possible that the real need for the elderly is targeted anti-viral and/or immune restoring therapy to maintain optimum health and well-being [196] and/or CMV growth inhibitors such as Vitamin A, monolaurin and lactoferrin [197], rather than the reactive treatment of presenting symptoms which they currently receive? The role of the thymus requires far greater attention as does the differences in immune response between the two genders. Widespread disruption in western populations appears to be related to country- and/or racial-specific distribution of different CMV strains. It would seem that very large sums of expenditure and the alleviation of widespread poor health may rest on the answer to these questions.
References
- Jones R (2009) Trends in emergency admissions. British Journal of Healthcare Management 15: 188-196.
- Jones R (2009) Emergency admissions and purchaser financial risk. British Journal of Healthcare Management 15: 344-350.
- Jones R (2010) The Nature of health care costs and financial risk in commissioning. British Journal of Healthcare Management 16: 424-430.
- Jones R (2013) Could cytomegalovirus be causing widespread outbreaks of chronic poor health? In Hypotheses in Clinical Medicine. M Shoja et al. Nova Science Publishers Inc, New York.
- Khot A, Burn R, Evans N, Lenney C, Lenney W (1984) Seasonal variation and time trends in childhood asthma in England and Wales 1975-81. Br Med J (Clin Res Ed) 289: 235-237.
- Hyams KC (1998) Developing case definitions for symptom-based conditions: the problem of specificity. Epidemiol Rev 20: 148-156.
- Anderson HR, Gupta R, Strachan DP, Limb ES (2007) 50 years of asthma: UK trends from 1955 to 2004. Thorax 62: 85-90.
- Moorman JE, Rudd RA, Johnson CA, King M, Minor P, et al. (2007) National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ 56: 1-54.
- González EL, Johansson S, Wallander MA, RodrÃguez LA (2009) Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health 63: 332-336.
- Keil T, Kulig M, Simpson A, Custovic A, Wickman M, et al. (2006) European cohort studies on asthma and atopic diseases: 1. Comparison of study designs a GA2LEN initiative. Allergy 61: 221-228.
- High K, Bradley S, Loeb M, Palmer R, Quagliarello V, et al. (2005) A new paradigm for clinical investigation of infectious syndromes in older adults: assessing functional status as a risk factor and outcome measure. J Am Geriatr Soc 53: 528-535.
- Goldberg SE, Whittamore KH, Harwood RH, Bradshaw LE, Gladman JR, et al. (2012) The prevalence of mental health problems among older adults admitted as an emergency to a general hospital. Age Ageing 41: 80-86.
- Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR et al. (2009) Aging promotes neutrophil-induced mortality by augmenting 1L-17 production during viral infection. Cell Host and Microbe 6: 446-456.
- Walker L, Jamrozik K, Wingfield D (2005) The Sherbrooke Questionnaire predicts use of emergency services. Age Ageing 34: 233-237.
- Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, et al. (1981) Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 305: 1425-1431.
- Jones RP (2010) Unexpected, periodic and permanent increase in medical inpatient care: man-made or new disease? Med Hypotheses 74: 978-983.
- Jones RP (2010) Can time-related patterns in diagnosis for hospital admission help identify common root causes for disease expression? Med Hypotheses 75: 148-154.
- Jones RP (2010) The case for recurring outbreaks of a new type of infectious disease across all parts of the United Kingdom. Med Hypotheses 75: 452-457.
- Jones R (2009) Additional studies on the three to six year pattern in medical emergency admissions. Healthcare Analysis & Forecasting, Camberley, UK.
- Jones R (2010) Emergency preparedness. British Journal of Healthcare Management 16: 94-95.
- Jones R (2010) Trends in programme budget expenditure. British Journal of Healthcare Management 16: 518-526.
- Jones R (2012) Increasing GP referrals: collective jump or infectious push? British Journal of Healthcare Management 18: 488-497.
- Jones R (2013) An unexplained increase in deaths in England & Wales during 2012. British Journal of Healthcare Management 19: 248-253.
- Jones R (2013) A recurring series of infectious-like events leading to excess deaths, emergency department attendances and medical admissions in Scotland. Biomedicine International.
- Jones R (2012) Diagnoses, deaths and infectious outbreaks. British Journal of Healthcare Management 18: 539-548.
- Jones R (2012) Financial risk in commissioning: cancer costs. British Journal of Healthcare Management 18: 315-324.
- Jones R (2012) Volatile inpatient costs and implications to CCG financial stability. British Journal of Healthcare Management 18: 251-258.
- Forget EL, Roos LL, Deber RB, Walld R (2008) Variations in Lifetime Healthcare Costs across a Population. Healthc Policy 4: e148-167.
- Kipp R, Cookson J, Mattie E (2003) Health insurance underwriting cycle: Effect on health plan premiums and profitability. Milliman, USA.
- Gabel J, Formisano R, Lohr B, DiCarlo S (1991) Tracing the cycle of health insurance. Health Aff (Millwood) 10: 48-61.
- Born P, Santerre R (2008) Unravelling the health insurance underwriting cycle. Journal of Insurance Regulation 26: 65-84.
- Jones R (2010) Cyclic factors behind NHS deficits and surpluses. British Journal of Healthcare Management 16: 48-50.
- Jones R (2010) Do NHS cost pressures follow long-term patterns? British Journal of Healthcare Management 16: 192-194.
- Jones R (2012) Time to re-evaluate financial risk in GP commissioning. British Journal of Healthcare Management 18: 39-48.
- Jones R (2011) Bed occupancy: the impact on hospital planning. British Journal of Healthcare Management 17: 307-313.
- Anderson RM, Grenfell BT, May RM (1984) Oscillatory fluctuations in the incidence of infectious disease and the impact of vaccination: time series analysis. J Hyg (Lond) 93: 587-608.
- Dushoff J (1996) Incorporating immunological ideas in epidemiological models. J Theor Biol 180: 181-187.
- Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7: 369-374.
- Fleming DM, Norbury CA, Crombie DL (1991) Annual and seasonal variation in the incidence of common diseases. Occas Pap R Coll Gen Pract: 1-24.
- Grassly NC, Fraser C (2006) Seasonal infectious disease epidemiology. Proc Biol Sci 273: 2541-2550.
- Grassly NC, Fraser C, Garnett GP (2005) Host immunity and synchronized epidemics of syphilis across the United States. Nature 433: 417-421.
- Koelle K, Pascual M (2004) Disentangling extrinsic from intrinsic factors in disease dynamics: a nonlinear time series approach with an application to cholera. Am Nat 163: 901-913.
- Bate SL, Dollard SC, Cannon MJ (2010) Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis 50: 1439-1447.
- Hyde TB, Schmid DS, Cannon MJ (2010) Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 20: 311-326.
- Boeckh M, Geballe AP (2011) Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 121: 1673-1680.
- Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, et al. (2010) Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 5: e8886.
- Seale H, MacIntyre CR, Gidding HF, Backhouse JL, Dwyer DE, et al. (2006) National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol 13: 1181-1184.
- Miller-Kittrell M, Sparer TE (2009) Feeling manipulated: cytomegalovirus immune manipulation. Virol J 6: 4.
- Chang WL, Barry PA (2010) Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc Natl Acad Sci U S A 107: 22647-22652.
- Fornara O, Odeberg J, Khan Z, Stragliotto G, Peredo I, et al. (2013) Human cytomegalovirus particles directly suppress CD4 T-lymphocyte activation and proliferation. Immunobiology 218: 1034-1040.
- Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A (2009) Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J Virol 83: 9618-9629.
- Söderberg-Nauclér C (2006) Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med 259: 219-246.
- Varani S, Frascaroli G, Landini M, Soderberg-Naucler C (2009) Human cytomegalovirus targets different subsets of antigen-presenting cells with pathological consequences for host immunity: implications for immunosuppression, chronic inflammation and autoimmunity. Rev Med Virol 19: 131-145.
- Varani S, Landini M, Soderberg-Naucler C (2010) Cytomegalovirus-induced autoimmunity. In: Autoimmune Disorders: Symptoms, Diagnosis and Treatment, Chapter 7. Ed: Maria E. Petrov. Nova Science Publishers Inc, New York.
- Varani S, Landini MP (2011) Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae 2: 6.
- Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, et al. (2011) Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6: e16103.
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, et al. (2005) Human immunosenescence: is it infectious? Immunol Rev 205: 257-268.
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A (2009) Cytomegalovirus and human immunosenescence. Rev Med Virol 19: 47-56.
- Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E (2012) The impact of CMV infection on survival in older humans. Curr Opin Immunol 24: 507-511.
- Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, et al. (2010) Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 171: 1144-1152.
- Derhovanessian E, Larbi A, Pawelec G (2009) Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol 21: 440-445.
- Derhovanessian E, Maier A, Hahnel K, Zelba H, de Craen A, et al. (2013) Lower proportion of naïve peripheral CD8+ T cells and an unopposed pro-inflammatory response to human cytomegalovirus proteins in vitro are associated with longer survival in very elderly people. Age 35: 1387-1399.
- Derhovanessian E, Maier AB, Beck R, Jahn G, Hähnel K, et al. (2010) Hallmark features of immunosenescence are absent in familial longevity. J Immunol 185: 4618-4624.
- Walsh B, Roberts HC, Nicholls PG, Lattimer VA (2008) Trends in hospital inpatient episodes for signs, symptoms and ill-defined conditions: observational study of older people's hospital episodes in England, 1995-2003. Age Ageing 37: 455-458.
- Kendrick S, Conway M (2003) Increasing emergency admissions among older people in Scotland: a whole system audit. isd Scotland.
- Kendrick S, Conway M (2003) Increasing emergency admissions among older people in Scotland: a whole system account – Trends 1981-2001. Whole System Project Working Paper 1,Infomation & Statistics Division, Common Services Agency, NHS Scotland.
- Kendrick S, Conway M (2003) Increasing emergency admissions among older people in Scotland: A whole system account. Information & Statistics Division NHS Scotland.
- Blunt I, Bardsley M, Dixon J (2010) Trends in Emergency admissions in England 2004-2009. The Nuffield Trust, London.
- Jones R (2012) A report investigating bed capacity at the Royal North Shore Hospital, Sydney, NSW. Healthcare Analysis & Forecasting, Camberley, UK.
- Roberts DC, McKay MP, Shaffer A (2008) Increasing rates of emergency department visits for elderly patients in the United States, 1993 to 2003. Ann Emerg Med 51: 769-774.
- Fink W, Lipatov V, Kenitzer M (2009) Diagnoses by General Practitioners: accuracy and reliability. International Journal of Forecasting 25: 784-793.
- Balthesen M, Messerle M, Reddehase MJ (1993) Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol 67: 5360-5366.
- Michaelson RA, Benson GS, Friedman HM (1983) Urinary retention as the presenting symptom of acquired cytomegalovirus infection. Am J Med 74: 526-528.
- Phillips AC, Carroll D, Khan N, Moss P (2008) Cytomegalovirus is associated with depression and anxiety in older adults. Brain Behav Immun 22: 52-55.
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46-56.
- Maes M, Berk M, Goehler L, Song C, Anderson G, et al. (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10: 66.
- Varani S, Lazzarotto T, Margotti M, Masi L, Gramantieri L, et al. (2000) Laboratory signs of acute or recent cytomegalovirus infection are common in cirrhosis of the liver. J Med Virol 62: 25-28.
- Cox B, Richardson A, Graham P, Gislefoss RE, Jellum E, et al. (2010) Breast cancer, cytomegalovirus and Epstein-Barr virus: a nested case-control study. Br J Cancer 102: 1665-1669.
- Harkins LE, Matlaf LA, Soroceanu L, Klemm K, Britt WJ, et al. (2010) Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae 1: 8.
- Nagane Y, Utsugisawa K (2008) Ross syndrome associated with cytomegalovirus infection. Muscle Nerve 38: 924-926.
- Chalabi M, Rezaie F, Moghim S, Mogharehabed A, Rezaei M, et al. (2010) Periodontopathic bacteria and herpesviruses in chronic periodontitis. Mol Oral Microbiol 25: 236-240.
- Xie F, Hu Y, Magee LA, Money DM, Patrick DM, et al. (2010) An association between cytomegalovirus infection and pre-eclampsia: a case-control study and data synthesis. Acta Obstet Gynecol Scand 89: 1162-1167.
- Nakase H, Matsumura K, Yoshino T, Chiba T (2008) Systematic review: cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol 43: 735-740.
- Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, et al. (2008) Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300: 413-422.
- Limaye AP, Boeckh M (2010) CMV in critically ill patients: pathogen or bystander? Rev Med Virol 20: 372-379.
- Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME (2008) Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J 5: 47.
- Miggins M, Hasan A, Hohmann S, Southwick F, Casella G, et al. (2011) The potential influence of common viral infections diagnosed during hospitalization among critically ill patients in the United States. PLoS ONE 6: e18890.
- Lisboa LF, Tong Y, Kumar D, Pang XL, Asberg A, et al. (2012) Analysis and clinical correlation of genetic variation in cytomegalovirus. Transpl Infect Dis 14: 132-140.
- Puchhammer-Stöckl E, Görzer I, Zoufaly A, Jaksch P, Bauer CC, et al. (2006) Emergence of multiple cytomegalovirus strains in blood and lung of lung transplant recipients. Transplantation 81: 187-194.
- Puchammer-Stockl E, Gorzer I (2011) Human cytomegalovirus: an enormous variety of strains and their possible clinical significance. Future Virol 6: 259-271.
- Gerna G, Baldanti F, Revello MG (2004) Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol 65: 381-386.
- Erice A (1999) Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev 12: 286-297.
- Tanaka K, Hori T, Yoto Y, Hatakeyama N, Yamamoto M, et al. (2011) Human cytomegalovirus UL97 D605E polymorphism has a high prevalence in immunocompetent Japanese infants and children. Microbiol Immunol 55: 328-330.
- Gandhoke I, Hussain SA, Pasha ST, Chauhan LS, Khare S (2013) Glycoprotein B Genotyping in Congenital/perinatal Cytomegalovirus Infection in Symptomatic Infants. Indian Pediatr 50: 663-667.
- Murph JR, Souza IE, Dawson JD, Benson P, Petheram SJ, et al. (1998) Epidemiology of congenital cytomegalovirus infection: maternal risk factors and molecular analysis of cytomegalovirus strains. Am J Epidemiol 147: 940-947.
- Murthy S, Hayward GS, Wheelan S, Forman MS, Ahn JH, et al. (2011) Detection of a single identical cytomegalovirus (CMV) strain in recently seroconverted young women. PLoS One 6: e15949.
- Waner JL, Nierenberg JA (1985) Natural killing (NK) of cytomegalovirus (CMV)-infected fibroblasts: a comparison between two strains of CMV, uninfected fibroblasts, and K562 cells. J Med Virol 16: 233-244.
- Bughio F, Elliott DA, Goodrum F (2013) An endothelial cell-specific requirement for the UL133-UL138 locus of human cytomegalovirus for efficient virus maturation. J Virol 87: 3062-3075.
- Esteki-Zadeh A, Karimi M, Strååt K, Ammerpohl O, Zeitelhofer M, et al. (2012) Human cytomegalovirus infection is sensitive to the host cell DNA methylation state and alters global DNA methylation capacity. Epigenetics 7: 585-593.
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, et al. (2010) Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A 107: 9470-9475.
- Pignatelli S, Lazzarotto T, Gatto MR, Dal Monte P, Landini MP, et al. (2010) Cytomegalovirus gN genotypes distribution among congenitally infected newborns and their relationship with symptoms at birth and sequelae. Clin Infect Dis 51: 33-41.
- Kropff B, Burkhardt C, Schott J, Nentwich J, Fisch T, et al. (2012) Glycoprotein N of human cytomegalovirus protects the virus from neutralizing antibodies. PLoS Pathog 8: e1002999.
- McWhorter AR, Smith LM, Masters LL, Chan B, Shellam GR, et al. (2013) Natural killer cell dependent within-host competition arises during multiple MCMV infection: consequences for viral transmission and evolution. PLoS Pathog 9: e1003111.
- Chien SM, Chan CK, Kasupski G, Chamberlain D, Fyles G, et al. (1992) Long-term sequelae after recovery from cytomegalovirus pneumonia in allogeneic bone marrow transplant recipients. Chest 101: 1000-1004.
- Tabll A, Shoman S, Ghanem H, Nabil M, El Din NG, et al. (2011) Assessment of human cytomegalovirus co-infection in Egyptian chronic HCV patients. Virol J 8: 343.
- Yanagisawa N, Toyokura Y, Shiraki H (1975) Double encephalitis with herpes simplex virus and cytomegalovirus in an adult. Acta Neuropathol 33: 153-164.
- Chomel J, Allard J, Floret D, Honneger D, David L, et al. (2001) Role of cytomegalovirus infection in the incidence of viral acute respiratory infections in children attending day-care centers. Eur J Clin Microbiol Infect Dis 20: 167-172.
- Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, et al. (2011) Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care 15: R77.
- Razonable RR, Fanning C, Brown RA, Espy MJ, Rivero A, et al. (2002) Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts. J Infect Dis 185: 110-113.
- Prusty BK, Böhme L, Bergmann B, Siegl C, Krause E, et al. (2012) Imbalanced oxidative stress causes chlamydial persistence during non-productive human herpes virus co-infection. PLoS One 7: e47427.
- Jones R (2012) GP referral to dermatology: which conditions? British Journal of Healthcare Management 18: 594-596.
- Carter C (2008) Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophrenia Bulletin 35: 1163-1182.
- Mortensen L, Maier A, Slagbom, P, Pawalec G, Derhovanessian E, et al. (2012) Early-life environment influencing susceptibility to cytomegalovirus infection: evidence from the4 Leiden longevity study and the longitudinal study of aging Danish twins. Epidemiol Infect 140: 835-841.
- Reeves M, Sinclair J (2013) Epigenetic regulation of human cytomegalovirus gene expression: Impact on human cytomegalovirus latency and regulation. In Cytomegalovirus: From Molecular Pathogen to Intervention, Chapter 19. Ed: Matthias J Reddenhase. Caister Academic Press, Norfolk, England.
- Mohseni M (2007) Epigenetic reprogramming by cytomegalovirus infection. Degree project in biology, Biology Education Center, Uppsala University, Department of Clinical Neurosience, Center for Molecular Medicine, Karolinsks Institute, Stockholm.
- Klein S, Scott A, Glass G (2001) Hormonal changes during physiological development can alter immune response to viruses and infections. Presented at American Physiological Society Conference: Genomes and Hormones – An integrative approach to gender differences in physiology.
- Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF (2012) Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med 10: 82.
- Zhu J, Shearer GM, Norman JE, Pinto LA, Marincola FM, et al. (2000) Host response to cytomegalovirus infection as a determinant of susceptibility to coronary artery disease: sex-based differences in inflammation and type of immune response. Circulation 102: 2491-2496.
- Han XY (2007) Epidemiologic analysis of reactivated cytomegalovirus antigenemia in patients with cancer. J Clin Microbiol 45: 1126-1132.
- Villacres MC, Longmate J, Auge C, Diamond DJ (2004) Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum Immunol 65: 476-485.
- Cannon MJ, Hyde TB, Schmid DS (2011) Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 21: 240-255.
- Jones R (2013) Do recurring outbreaks of a type of infectious immune impairment trigger cyclic changes in the gender ratio at birth? Biomedicine International 4: in press.
- Iwasenko JM, Howard J, Arbuckle S, Graf N, Hall B, et al. (2011) Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis 203: 1526-1533.
- Bristow BN, O'Keefe KA, Shafir SC, Sorvillo FJ (2011) Congenital cytomegalovirus mortality in the United States, 1990-2006. PLoS Negl Trop Dis 5: e1140.
- Picone O, Costa JM, Dejean A, Ville Y (2005) Is fetal gender a risk factor for severe congenital cytomegalovirus infection? Prenat Diagn 25: 34-38.
- Jones R (2012) Gender and financial risk in commissioning. British Journal of Healthcare Management 18: 336-337.
- Roberts ET, Haan MN, Dowd JB, Aiello AE (2010) Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 172: 363-371.
- Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, et al. (2012) Higher immunoglobulin G antibody levels against cytomegalovirus are associated with incident ischemic heart disease in the population-based EPIC-Norfolk cohort. J Infect Dis 206: 1897-1903.
- Kytö V, Vuorinen T, Saukko P, Lautenschlager I, Lignitz E, et al. (2005) Cytomegalovirus infection of the heart is common in patients with fatal myocarditis. Clin Infect Dis 40: 683-688.
- Litjens NH, de Wit EA, Betjes MG (2011) Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing 8: 2.
- Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH (2011) Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int 80: 208-217.
- Griffiths PD (2012) Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect Dis 12: 790-798.
- Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, et al. (2013) Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 208: 564-572.
- Chen S, de Craen AJ, Raz Y, Derhovanessian E, Vossen AC, et al. (2012) Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study. Immun Ageing 9: 18.
- Barami K (2010) Oncomodulatory mechanisms of human cytomegalovirus in gliomas. J Clin Neurosci 17: 819-823.
- Melnick M, Sedghizadeh PP, Allen CM, Jaskoll T (2012) Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp Mol Pathol 92: 118-125.
- Soroceanu L, Cobbs CS (2011) Is HCMV a tumor promoter? Virus Res 157: 193-203.
- Lepiller Q, Aziz Khan K, Di Martino V, Herbein G (2011) Cytomegalovirus and tumors: two players for one goal-immune escape. Open Virol J 5: 60-69.
- Tschische P, Tadagaki K, Kamal M, Jockers R, Waldhoer M (2011) Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem Pharmacol 82: 610-619.
- Johnsen JI, Baryawno N, Söderberg-Nauclér C (2011) Is human cytomegalovirus a target in cancer therapy? Oncotarget 2: 1329-1338.
- Fulop T, Larbi A, Kotb R, de Angelis F, Pawelec G (2011) Aging, immunity, and cancer. Discov Med 11: 537-550.
- Chen T, Hudnall SD (2006) Anatomical mapping of human herpesvirus reservoirs of infection. Mod Pathol 19: 726-737.
- Rahbar A, Stragliotto G, Orrego A, Peredo I, Taher C, et al. (2012) Low levels of Human Cytomegalovirus Infection in Glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae 3: 3.
- Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, et al. (2008) Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res 106: 268-274.
- Novotná M, Hanusova J, Klose J, Preiss M, Havlicek J, et al. (2005) Probable neuroimmunological link between Toxoplasma and cytomegalovirus infections and personality changes in the human host. BMC Infect Dis 5: 54.
- Bosch J, Rector J, Turner J, Riddell N, o’Hartaigh B, Burns V (2013) Psychoneuromicrobiology: Cytomegalovirus infection as a putative link between stress, aging, and immunity. In Immunosenescence, pp 81-100. Bosch J, et al. (Edn). Springer Science & Business Media, New York.
- Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG (2013) Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 70: 176-184.
- Payne RA, Abel GA, Guthrie B, Mercer SW (2013) The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. CMAJ 185: E221-228.
- Lindsay G, Marie M, Hamid K, Jeanne de Clantal R-C, Vincent G, et al. (2011) Impact of the somatotrope growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis upon thymus function: Pharmicological implications in regeneration of immune functions. Immunol Endocrine &metabol Agents- Medicinal Chem 11: 10-20.
- Chentoufi AA, Polychronakos C (2002) Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes 51: 1383-1390.
- Reggiani PC, Morel GR, Cónsole GM, Barbeito CG, Rodriguez SS, et al. (2009) The thymus-neuroendocrine axis: physiology, molecular biology, and therapeutic potential of the thymic peptide thymulin. Ann N Y Acad Sci 1153: 98-106.
- Ye P, Kirschner DE (2002) Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants. J Immunol 168: 4968-4979.
- Goronzy JJ, Fujii H, Weyand CM (2006) Telomeres, immune aging and autoimmunity. Exp Gerontol 41: 246-251.
- Holländer GA, Krenger W, Blazar BR (2010) Emerging strategies to boost thymic function. Curr Opin Pharmacol 10: 443-453.
- McFarland RD, Douek DC, Koup RA, Picker LJ (2000) Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci U S A 97: 4215-4220.
- Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, et al. (2009) Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood 114: 1445-1453.
- Wong CP, Song Y, Elias VD, Magnusson KR, Ho E (2009) Zinc supplementation increases zinc status and thymopoiesis in aged mice. J Nutr 139: 1393-1397.
- Ferrando-MartÃnez S, Franco JM, Hernandez A, Ordoñez A, Gutierrez E, et al. (2009) Thymopoiesis in elderly human is associated with systemic inflammatory status. Age (Dordr) 31: 87-97.
- Ferrando-MartÃnez S, Romero-Sánchez MC, Solana R, Delgado J, de la Rosa R, et al. (2013) Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Dordr) 35: 251-259.
- Brown JA, Stevenson K, Kim HT, Cutler C, Ballen K, et al. (2010) Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood 115: 4111-4119.
- Chen W, Kuolee R, Austin JW, Shen H, Che Y, et al. (2005) Low dose aerosol infection of mice with virulent type A Francisella tularensis induces severe thymus atrophy and CD4+CD8+ thymocyte depletion. Microb Pathog 39: 189-196.
- Jaïdane H, Caloone D, Lobert PE, Sane F, Dardenne O, et al. (2012) Persistent infection of thymic epithelial cells with coxsackievirus B4 results in decreased expression of type 2 insulin-like growth factor. J Virol 86: 11151-11162.
- Brilot F, Chehadeh W, Charlet-Renard C, Martens H, Geenen V, et al. (2002) Persistent infection of human thymic epithelial cells by coxsackievirus B4. J Virol 76: 5260-5265.
- Permar SR, Moss WJ, Ryon JJ, Douek DC, Monze M, et al. (2003) Increased thymic output during acute measles virus infection. J Virol 77: 7872-7879.
- Heieren MH, Kim YK, Balfour HH Jr (1988) Human cytomegalovirus infection of kidney glomerular visceral epithelial and tubular epithelial cells in culture. Transplantation 46: 426-432.
- Numazaki K, Goldman H, Bai XQ, Wong I, Wainberg MA (1989) Effects of infection by HIV-1, cytomegalovirus, and human measles virus on cultured human thymic epithelial cells. Microbiol Immunol 33: 733-745.
- Numazaki K, DeStephano L, Wong I, Goldman H, Spira B, et al. (1989) Replication of cytomegalovirus in human thymic epithelial cells. Med Microbiol Immunol 178: 89-98.
- Just H (2006) Thymic function in inflammatory skin diseases. PhD Thesis, Faculty of Health Sciences, University of Aarhus, Sweden.
- Nowacki T, Bettenworth D, Ross M, Heidemann J, Lehmann P, et al. (2012) Cytomegalovirus (CMV)- specific perforin and granzyme B ELLISPOT assays detect reactivation of CMV infection in Infalammatory Bowel Disease. Cells 1: 35-50.
- Moses AM, Thomas DG, Canfield MC, Collins GH (2003) Central diabetes insipidus due to cytomegalovirus infection of the hypothalamus in a patient with acquired immunodeficiency syndrome: a clinical, pathological, and immunohistochemical case study. J ClinEndocrinol Metabolism 88(1): 51-54.
- Ferreiro J, Vinters HV (1988) Pathology of the pituitary gland in patients with the acquired immune deficiency syndrome (AIDS). Pathology 20: 211-215.
- Smelt MJ, Faas MM, de Haan BJ, Draijer C, Hugenholtz GC, et al. (2013) Susceptibility of human pancreatic ß cells for cytomegalovirus infection and the effects on cellular immunogenicity. Pancreas 41: 39-49.
- Stathis A, La Rosa S, Proserpio I, Micello D, Chini C, et al. (2009) Cytomegalovirus infection of endocrine system in a patient with diffuse large B-cell lymphoma. Report of a case. Tumori 95: 119-122.
- Teraishi F, Shimamura H, Suzuki T, Nakamoto M, Chikuba A, et al. (2008) Cytomegalovirus colitis after systemic chemotherapy in a patient with recurrent colon cancer: a case report. J Med Case Rep 2: 289.
- Kalaitzis J, Basioukas P, Karzi E, Markakis C, Liarmakopoulos E, et al. (2012) Small-bowel necrosis complicating a cytomegalovirus-induced superior mesenteric vein thrombosis in an immunocompetent patient: a case report. J Med Case Rep 6: 118.
- Taglietti F, Drapeau CM, Grilli E, Capone A, Noto P, et al. (2010) Hemolytic anemia due to acute cytomegalovirus infection in an immunocompetent adult: a case report and review of the literature. J Med Case Rep 4: 334.
- Katsoulis K, Minasidis I, Vainas A, Bikas C, Kontakiotis T, et al. (2009) Platypnea and orthodeoxia associated with Pneumocystis jiroveci and Cytomegalovirus pneumonia: a case report. J Med Case Rep 3: 9319.
- Atzmony L, Saar N, Chundadze T, Arbel Y, Justo D, et al. (2008) Cytomegalovirus-associated splenic infarcts in a female patient with Factor V Leiden mutation: a case report. J Med Case Rep 2: 385.
- Ma Y, Feng J, Qi Y, Dou XG (2011) An immunocompetent adult patient with hepatitis and guillain-barré syndrome after cytomegalovirus infection. Virol J 8: 95.
- Kauffman CA, Linnemann CC Jr, Alvira MM (1979) Cytomegalovirus encephalitis associated with thymoma and immunoglobulin deficiency. Am J Med 67: 724-728.
- van Duin D, Miranda C, Husni E (2011) Cytomegalovirus viremia, pneumonitis, and tocilizumab therapy. Emerg Infect Dis 17: 754-756.
- Kalil AC, Florescu DF (2009) Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med 37: 2350-2358.
- Osawa R, Singh N (2009) Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care 13: R68.
- Rennekampff HO, Hamprecht K (2006) Cytomegalovirus infection in burns: a review. J Med Microbiol 55: 483-487.
- von Müller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, et al. (2006) Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis 12: 1517-1522.
- Ho M (1998) Cytomegalovirus infection in patients with bacterial sepsis. Clin Infect Dis 26: 1083-1084.
- Sinclair J (2010) Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection. Biochim Biophys Acta 1799: 286-295.
- Presti RM, Pollock JL, Dal Canto AJ, O'Guin AK, Virgin HW 4th (1998) Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med 188: 577-588.
- Coulson AS, Lucas ZJ, Condy M, Cohn R (1974) Forty-day fever. An epidemic of cytomegalovirus disease in a renal transplant population. West J Med 120: 1-7.
- Gurevich I, Cunha BA (1981) Non-parenteral transmission of cytomegalovirus in a neonatal intensive care unit. Lancet 2: 222-224.
- Shats VJ, Kozacov SM, Miron D (1998) Outbreak of cytomegalovirus infection in the geriatric department. J Am Geriatr Soc 46: 930-931.
- Meunier YA (2005) Infectious mononucleosis-like syndrome and gastrointestinal disorders in acute acquired cytomegalovirus infection. Singapore Med J 46: 421-423.
- Jones R (2012) End of life care and volatility in costs. British Journal of Healthcare Management 18: 374-381.
- Jones R (2013) Trends in unscheduled care. British Journal of Healthcare Management 19: 301-304.
- Jones R (2103) Hidden complexity in A&E trends in Engaland. British Journal of Healthcare Management 19: 354-355.
- Arturo J (2008) Theories of immunoscenescence and infection: Cytomegalovirus, inflammation and homotoxicology. J Biochem Therapy 2: 16-18.
- Clarke NM, May JT (2000) Effect of antimicrobial factors in human milk on rhinoviruses and milk-borne cytomegalovirus in vitro. J Med Microbiol 49: 719-723.
Citation: Jones RP (2013) Widespread Outbreaks of a Subtle Condition Leading to Hospitalisation and Death. Epidemiol 3:137. DOI: 10.4172/2161-1165.1000137
Copyright: © 2013 Jones RP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16954
- [From(publication date): 10-2013 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 11928
- PDF downloads: 5026