Case Report Open Access
18F-FDG PET/CT for Diagnosis of Castleman Disease: A Case Report
Sayumi Suzuki1, Karin Takeda1, Takemichi Okada2, Hitoshi Yamazaki3, Yasutaka Senpuku4 and Hiroaki Yokomori1*
1Department of Internal Medicine and Radiology Kitasato University Medical Center, Kitamoto, Saitama, Japan
2Department of Pathology, Kitasato University Medical Center, Kitamoto, Saitama, Japan
3Department of Radiology, Kitasato University Medical Center, Kitamoto, Saitama, Japan
4Ageo Central General Hospital, Saitama, Japan
- *Corresponding Author:
- Hiroaki Yokomori, M.D.
Kitasato University Medical Center, 6-100 Arai
Kitamoto-shi, Saitama, 364-8501, Japan
Tel: +81-48-593-1212
Fax: +81-48-593-1239
E-mail: yokomori@insti.kitasato-u.ac.jp
Received date: May 18, 2016; Accepted date: May 25, 2016; Published date: May 31, 2016
Citation: Suzuki S, Takeda K, Okada T, Yamazaki H, Senpuku Y, et al. (2016) 18F-FDG PET/CT for Diagnosis of Castleman Disease: A Case Report. J Gastrointest Dig Syst 6:431. doi:10.4172/2161-069X.1000431
Copyright: © 2016 Suzuki S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and reproduction in any medium; provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
A 73-year-old man was admitted to our hospital with fever of unknown origin (FUO). Laboratory findings showed high levels of C-reactive protein (25.99 mg/dL) and interleukin-6 (IL-6) (14.7 pg/mL). Computed tomography revealed right lateral pleural effusion and ascites. 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT revealed intense tracer accumulation in the cervical and para-aortic nodal chains, and lymph node excisional biopsy showed mixed type Castleman disease. Prednisolone and IL-6 receptor antibody, tocilizumab, achieved dramatic results. FDG PET/CT is a valuable modality for assessment of etiology in patients with FUO. This can help identify suitable glands for excisional biopsy in such cases.
Keywords
Castleman disease; 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/Computed tomography (CT); Electron microscopy; Immunohistochemistry
Introduction
The classical definition of fever of unknown origin (FUO), coined by Petersdorf and Beeson in 1961, includes fever that is measured to be above 38.3°C on several occasions during a period longer than three weeks and for which the underlying etiology cannot be diagnosed at the end of at least one week of hospital stay [1]. The last criterion has undergone modification and is now generally interpreted as no diagnosis after appropriate inpatient or outpatient evaluation [2].
18F-Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT), which has become an established imaging tool in oncology, is attracting increasing interest in the field of infectious diseases [3] and can aid in diagnosis of FUO cases.
Castleman disease (CD), first described in 1956, is a rare lymph proliferative disorder [4] that is commonly found in lymphoid tissues of mediastinum and lung hila. CD can present either as unicentric CD (UCD) confined to a single anatomic lymph node or as multicentric CD (MCD) characterized by fever with chills, anemia, generalized lymphadenopathy, and hepatosplenomegaly; the latter follows a more aggressive clinical course. Histopathologically, CD can categorized into four variants: hyaline vascular (HV), plasma cell (PC), mixed, and plasmablastic [5]. HV is characterized by widened mantle zones composed of concentric rings of small lymphocytes in an “onion skin” pattern around small atrophic germinal centers with penetrating hyalinized vessels and dysplastic follicular dendritic cells (FDCs). A recent study of MCD lymph nodes found plasma cells, endothelial cells, macrophages, and FDCs [5].
The pathogenesis of CD is unknown, although a defect in immune regulation, resulting in excessive proliferation of B lymphocytes and plasma cells in lymphoid organs, is proposed to be the underlying mechanism [6]. Most cases of CD are either the HV variant (80%-90%) or the PC variant (10%-20%); a small percentage present with a mixed histologic appearance [6,7]. Patients with the HV form of CD may exhibit no symptoms or present only with lymphadenopathy, whereas those with the PC variant of MCD typically present with fever, weight loss, rash, and anemia [6-8].
PET appears promising as a useful test to evaluate FUO. Many studies have been performed using this modality in patients with FUO, all showing a percentage helpfulness that exceeds that of CT, MRI, or other diagnostic possibilities [5,9]. In this report, we present a patient admitted with FUO who was diagnosed with mixed-type CD using FDG PET/CT and immunohistochemical and electron microscopic evaluations.
Case Report
A 73-year-old man with fever was admitted to our hospital for 1 week, exhibiting leg edema on the day of presentation. The dry mouth symptom was unrecognized. Physical examination confirmed the absence of bilateral cervical and axillary lymphadenopathy and revealed pitting edema in the lower extremities. Moreover, physical examination findings were not confirmed for xerostomia and xerophthalmia.
There were no dermatological, neurological, or ophthalmological abnormalities. Laboratory assessment revealed the following findings: white blood cell (WBC) count, 7.400/μL (normal; 4000-9000); Creactive protein (CRP), 25.99 mg/dL (normal; ≤ 0.30);; total bilirubin (T-bil), 0.8 mg/dL (normal; 0.2-1.0); aspartate transaminase (AST), 33 IU/L (normal; 10-35) ; alanine transaminase (ALT), 18 IU/L (normal; 5-40); alkaline phosphatase (ALP), 2001 IU/L (normal; 115-359); gamma-glutamyl transferase (γ-GTP), 242I U/L (normal; ≤ 80); blood urea nitrogen (BUN), 18 mg/dL (normal; 8.0-22.0); serum keratinize (Cr), 0.86 mg/dL (normal; 0.60-1.10); serum soluble IL-2 receptor (sIL-2R), 1260 U/ml (normal; 124-466); serum IL-6, 14.7 pg/mL (normal; 0.221-4.6).
Additional studies were performed to rule out hepatitis infection; tests for Human Herpes Virus (HHV)-8, Human T-Cell Lymph Trophic Virus (HTLV)-1, and Epstein-Barr Virus (EBV) were negative. The patient had anemia and was positive for antinuclear antibody (ANA), with a titer of 1/640 and a fine speckled immunofluorescence pattern.
Tests for autoantibodies including anti-Ro/SSA, anti-La/SSB, and rheumatoid factor were positive. Serum levels of tumor markers were within normal limits other than the prostate specific marker. Urine analysis revealed proteinuria.
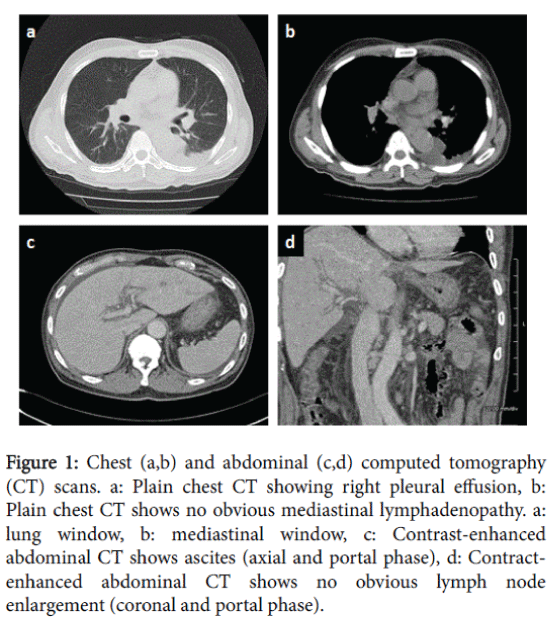
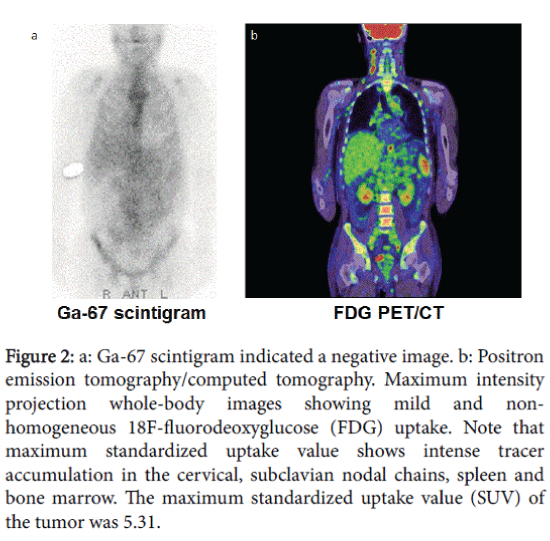
Chest and abdominal computed tomography (CT) imaging revealed pleural effusion and ascites, respectively (Figures 1a, 1b and 1c). Because of a high suspicion of neoplastic etiology, the Ga-67 scintigram indicated a negative image (Figure 2a), it was important to exclude cancer of unknown malignancy as a cause of the fever of unknown origin, and we recommended an examination of PET-CT for the patient and family. Whole-body FDG PET/CT was performed.
Figure 1: Chest (a,b) and abdominal (c,d) computed tomography (CT) scans. a: Plain chest CT showing right pleural effusion, b: Plain chest CT shows no obvious mediastinal lymphadenopathy. a: lung window, b: mediastinal window, c: Contrast-enhanced abdominal CT shows ascites (axial and portal phase), d: Contractenhanced abdominal CT shows no obvious lymph node enlargement (coronal and portal phase).
Figure 2: a: Ga-67 scintigram indicated a negative image. b: Positron emission tomography/computed tomography. Maximum intensity projection whole-body images showing mild and nonhomogeneous 18F-fluorodeoxyglucose (FDG) uptake. Note that maximum standardized uptake value shows intense tracer accumulation in the cervical, subclavian nodal chains, spleen and bone marrow. The maximum standardized uptake value (SUV) of the tumor was 5.31.
Maximum intensity projection (MIP) images revealed intense tracer accumulation in the cervical nodal chains with discrete foci in the para-aortic nodal chain, spleen, and bone marrow. The maximum standardized uptake value (SUV) of the tumor was 5.31 (Figure 2b).
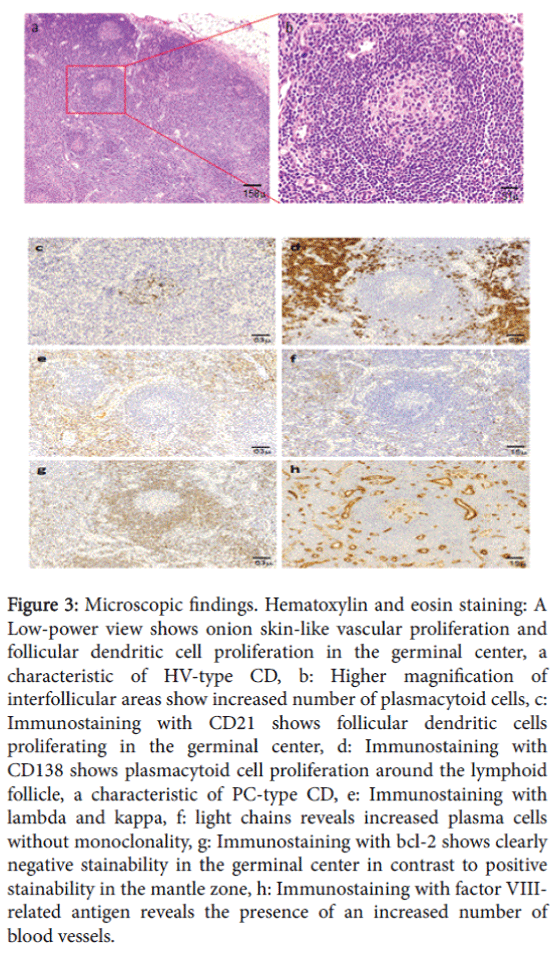
Histological analysis of a right deep cervical lymph node showed the presence of mixed-type lymphoid proliferation. The follicles were regressed or depleted of germinal center cells and had expanded mantle zones with small lymphocytes arranged concentrically in an “onion skin” pattern, with overlapping hyperplastic follicles of varying sizes and mildly increased intermolecular vascularity and focally patent medullary sinuses (Figure 3a).
Higher magnification of the intermolecular areas showed sheets of plasmacytoid cells with eccentric nuclei (Figure 3b). Further studies were conducted for the differential diagnosis among HV, PC, and mixed-type CD. Immunostaining showed CD21-positive proliferative follicular dendritic cells in germinal centers (Figure 3c). Diffuse, proliferative CD138-positive plasmacytoid cells in interfollicular regions, characteristic of PC-type CD, were also observed (Figure 3d). No immunoclonality was observed by immunostaining of kappa/ lambda light chains (Figures 3e and 3f).
Figure 3: Microscopic findings. Hematoxylin and eosin staining: A Low-power view shows onion skin-like vascular proliferation and follicular dendritic cell proliferation in the germinal center, a characteristic of HV-type CD, b: Higher magnification of interfollicular areas show increased number of plasmacytoid cells, c: Immunostaining with CD21 shows follicular dendritic cells proliferating in the germinal center, d: Immunostaining with CD138 shows plasmacytoid cell proliferation around the lymphoid follicle, a characteristic of PC-type CD, e: Immunostaining with lambda and kappa, f: light chains reveals increased plasma cells without monoclonality, g: Immunostaining with bcl-2 shows clearly negative stainability in the germinal center in contrast to positive stainability in the mantle zone, h: Immunostaining with factor VIIIrelated antigen reveals the presence of an increased number of blood vessels.
Moreover, immunohistochemical staining for bcl-2 was negative in atrophic germinal centers and positive in mantle zones, with sharp borders in between (Figure 3g). In intermolecular regions, numerous blood vessels were present, and proliferative endothelial cells expressed vascular markers such as factor VIII-related antigen (Figure 3h).
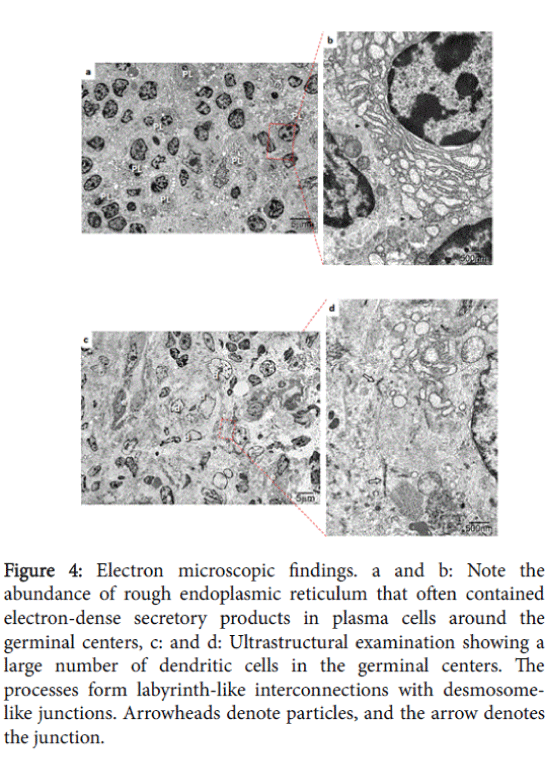
At the ultrastructure level, a large number of dendritic cells were observed in the germinal centers by electron microscopy; these cells possessed labyrinth-like interconnections with desmosome-like junctions (Figures 4a and 4b). Furthermore, rough endoplasmic reticulum was typically abundant and often contained electron-dense secretory products in plasma cells around the germinal center (Figures 4c and 4d).
Figure 4: Electron microscopic findings. a and b: Note the abundance of rough endoplasmic reticulum that often contained electron-dense secretory products in plasma cells around the germinal centers, c: and d: Ultrastructural examination showing a large number of dendritic cells in the germinal centers. The processes form labyrinth-like interconnections with desmosomelike junctions. Arrowheads denote particles, and the arrow denotes the junction.
The patient was diagnosed with mixed-type CD, and there was no evidence of sarcoidosis or any other lymph proliferative disorders. Twelve days after admission, steroid pulse therapy with prednisolone (40 mg/d) for three days achieved control of the fever, ascites, and pleural effusion.
Although serum IL-6 level was only slightly elevated, tocilizumab (400 mg/body), an IL-6 receptor inhibitor, was administered and had dramatic results. He was discharged on the 34th day after admission, and he was followed up every 2 weeks as an outpatient for tocilizumab therapy. However, he suddenly died of a cardiopulmonary arrest on the 66th day after admission.
Discussion
This case report clearly reflects the clinical and morphological picture of mixed-type MCD. The initial manifestations observed in the current case of FUO include infectious diseases such as tuberculosis, neoplastic diseases such as malignant lymphoma and myeloid neoplasms, autoimmune diseases, and drug fever. Initially, superficial lymph nodes were not palpable, and systemic lymphadenopathy was scarce, as observed on CT. An elevated serum sIL2R level suggested a possible diagnosis of atypical malignant lymphoma. Based on increased FDG uptake by the lymph nodes, a lymph node biopsy was performed. FDG PET/CT has recently been used to examine lymph proliferative diseases including MCD and hyper metabolic lymphadenopathy [10], and it offers help in distinguishing between the two; CD closely mimics lymphoma on FDG PET/CT. However, wholebody FDG PET/CT helps in detection of sites of involvement, separating UCD from MCD and CD from lymphoma. Thus, FDG PET/CT can have as significant impact on disease management as localized disease is amenable to surgery [11]. Based on other clinical manifestations, laboratory data, and histopathological findings of the lymph node biopsy, we diagnosed the patient with mixed-type MCD despite its atypical presentation.
MCD is likely a morphologic diagnosis unifying a group of diseases with various etiologies; it was occasionally reported in association with autoimmune manifestations and connective tissue diseases [7,8,12]. Tests for autoantibodies, including anti-Ro/SSA, anti-La/SSB, rheumatoid factor, and ANA, were positive in this case. Connective tissue diseases and MCD share many pathophysiological characteristics that may cause diagnostic difficulties. Moreover, a review of the presence of autoimmune diseases concomitant to DC revealed an association with connective tissue disease [12]. However, in our patient, systemic involvement and plasmacytic histological presentation allowed us to classify our patient as having MCD.
Unfortunately, the average life expectancy after CD diagnosis is ten years, indicating that CD sometimes results in mortality despite treatment. Including the present case, there have been only five reported case in which tocilizumab with prednisolone therapy was used; of these, three patients were successfully treated, and one died due to sepsis [13]. A nationwide survey was required to further clarify the epidemiology, clinical features, pathological aspect, clonal analysis, and optimal treatment strategies of MCD in Japan.
Before establishing a diagnosis of CD, B cell lymphomas (BCLs) including follicular lymphoma (FL) should be ruled out. In our patient, HV-type CD was diagnosed based on bcl-2-negative atrophic germinal centers separated with sharp borders from bcl-2-positive mantle zones by immunostaining [14].
At the ultrastructural level, we observed endothelial cell spouting concomitant with abundant plasma cells around germinal centers and dendritic cells with labyrinth-like interconnections with desmosomelike junctions in the germinal centers [15]. Ultrastructural findings suggested that activated dendritic cells might be exerting proangiogenic activity mediated by the prototypic angiogenic growth factor VEGF-A. In turn, pro- and anti-angiogenic mediators might affect dendritic cell biology, modulating their differentiation and maturation, and might stimulate the proliferation of endothelial cells in follicles of HV cells of CD [16]. This mechanism of disease warrants further investigation. Recent findings of monoclonality in MCD cases suggest that stromal cells, such as myoid cells and follicular dendritic cells, might constitute the pathologic cellular component of MCD [7,17]. IL-6 expression was increased in the germinal centers of hyperplastic lymph nodes in CD. In addition, a correlation between serum IL-6 concentration and clinical features was shown, which suggested that dysregulated IL-6 production from affected lymph nodes might be responsible for the systemic manifestations of this disease [7,8].
FDG PET/CT was proposed to assess FUO or for diagnosis and evaluation of lymphoproliferative disorders such as CD. FDG PET/CT may also aid in choosing the specific lymph node for biopsy. 18F-FDG uptake is well correlated with disease multicentricity and clinical manifestation, suggesting that it can serve as an important imaging marker in patients with CD.
References
- Petersdorf RG, Beeson PB (1961) Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 40: 1-30.
- Petersdorf RG (1992) Fever of unknown origin. An old friend revisited. Arch Intern Med 152: 21-22.
- Meller J, Sahlmann CO, Scheel AK (2007) 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med 48: 35-45.
- Castleman B, Iverson L, Menendez VP (1956) Localized mediastinallymphnode hyperplasia resembling thymoma. Cancer 9: 822-830.
- Cronin DM, Warnke RA (2009) Castleman disease: an update on classification and the spectrum of associated lesions. AdvAnatPathol 16: 236-246.
- Shahidi H, Myers JL, Kvale PA (1995) Castleman's disease. Mayo ClinProc 70: 969-977.
- van Rhee F, Stone K, Szmania S, Barlogie B, Singh Z (2010) Castleman disease in the 21st century: an update on diagnosis, assessment, and therapy. ClinAdvHematolOncol 8: 486-498.
- Fajgenbaum DC, van Rhee F, Nabel CS (2014) HHV-8-negative, idiopathic multicentricCastleman disease: novel insights into biology, pathogenesis, and therapy. Blood 123: 2924-2933.
- Kubota K, Nakamoto Y, Tamaki N, Kanegae K, Fukuda H, et al. (2011) FDG-PET for the diagnosis of fever of unknown origin: a Japanese multi-center study. Ann Nucl Med 25: 355-364.
- Bonekamp D, Horton KM, Hruban RH, Fishman EK (2011) Castleman disease: the great mimic. Radiographics 31: 1793-1807.
- Enomoto K, Nakamichi I, Hamada K, Inoue A, Higuchi I, et al. (2007) Unicentric and multicentricCastleman's disease. Br J Radiol 80: e24-26.
- Muskardin TW, Peterson BA, Molitor JA (2012) Castleman disease and associated autoimmune disease. CurrOpinRheumatol 24: 76-83.
- Masaki Y, Nakajima A, Iwao H (2013) Japanese variant of multicentricCastleman’s disease associated with serositis and thrombocytopenia: a report of two cases: is TAFRO syndrome (Castleman-Kojima Disease) a distinct clinicopathological entity? J ClinExpHematop 53: 79-85.
- Siddiqi IN, Brynes RK, Wang E (2011) B-cell lymphoma with hyaline vascular Castleman disease-like features: a clinicopathologic study. Am J ClinPathol 135: 901-914.
- Muretto P, Cinti S, Staccioli MP (1985) Multicentric giant lymph-node hyperplasia: immunohistochemical and ultrastructural study of two cases. Haematologica 70: 120-131.
- Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M (2007) Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol 28: 385-392.
- Chang KC, Wang YC, Hung LY, Huang WT, Tsou JH, et al. (2014) Monoclonality and cytogenetic abnormalities in hyaline vascular Castleman disease. Mod Pathol 27: 823-831.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 12693
- [From(publication date):
June-2016 - Aug 28, 2025] - Breakdown by view type
- HTML page views : 11721
- PDF downloads : 972