A Concept Map of Neonatal Hypoglycemia
Received: 14-Oct-2017 / Accepted Date: 16-Nov-2017 / Published Date: 27-Nov-2017 DOI: 10.4172/2572-4983.1000140
Abstract
Objectives: To analyze current management of neonatal hypoglycemia and establish a concept map based on testimonials from physicians who use capillary blood glucose tests to guide management.
Methods: This was an observational, descriptive, mixed qualitative and quantitative study. A questionnaire was administered to physicians, seeking to characterize their responses to neonatal hypoglycemia in pregnancy, labor and delivery, and the immediate neonatal period. Data collection was performed with two groups of Brazilian physicians: neonatologists (group GN) and pediatricians (group GP). A Likert-type scale was used to collect responses. The Mann-Whitney test and Fisher’s exact test were used for statistical analysis of continuous and categorical variables respectively. Statistical significance was accepted at 5%. Principal components analysis with varimax rotation and Kaiser normalization was used to verify the structure of question/answer factors in the two groups of professionals. A concept map was constructed using the Cmap Tools Knowledge Kit, version 5.05.01.
Results: Of the 98 questionnaires analyzed, 34.7% were completed by neonatologists (n=34/98) and 65.3% by pediatricians (n=64/98). Mean age in the two groups (GN and GP) was 42.4 years (SD: 11.78; 95% CI: 40.10, 44.88; p=0.597), with a significant difference in age in men (mean=50 years; SD: 10.35; 95% CI: 45.20, 54.90; p<0.001); 79.2% of respondents (n=78/98) were women. Overall, 32.7% of respondents (n=32/98) claimed they did not currently work with neonates. Respondents in GN had completed more postgraduate courses (p=0.38). The two groups responded similarly to all questions. The responses highlighted the importance of values lower than 40 mg/dL in the diagnosis of neonatal hypoglycemia, as well as the indication of intravenous infusion when glucose was below 40 mg/dL in symptomatic neonates. Immediate institution of periodic capillary blood glucose measurement was recommended for the following groups of neonates: those born to diabetic mothers, those with intrauterine growth restriction, small for gestational age, large for gestational age, preterm neonates, septic neonates, and those with birth asphyxia.
Conclusions: Capillary blood glucose is part of routine neonatal management, especially preventive, in light of the possibility of neonatal hypoglycemia. Our findings highlight that, in high-risk gestational groups, the entire neonatal team should be focused on the risk of hypoglycemia. The development of management algorithms based on the use of peripheral blood glucose test strips has contributed to streamlining the management of neonatal hypoglycemia.
Keywords: Hypoglycemia; Newborn; Maps; Practice guideline; Principal components analysis
Introduction
Hypoglycemia is a matter of heated debate in neonatology, particularly regarding its clinical definition and the optimal timing for intervention [1]. It is noteworthy that high levels of blood glucose can also cause damage during infant development, depending on the preference for glycolysis, oxidative and/or ketone metabolism, time, energy demand, deposition, etc. as discussed by Adamkin [2,3]. The current evidence base for glucose screening and treatment of asymptomatic infants includes reducing the risk of achieving higherthan- desirable glucose levels, which may be associated with glucose instability and, perhaps, adverse neurological outcomes with nonketotic hyperglycinemia.
It is known that some neonates are more likely to develop hypoglycemia than others [4], and that low blood glucose levels can lead to permanent central nervous system damage [5]. The cut off level which should prompt treatment remains unclear, especially in asymptomatic neonates [6].
Cut off points for diagnostic and management considerations have been discussed since the 1930s [7], including in preterm neonates [8], the most important factor to bear in mind is that glucose meters overestimate the actual plasma level of glucose [9]. Glucose levels in arterial blood are 10-15% higher than in venous blood, and levels in plasma are 10-15% higher than in whole blood [10].
In low-complexity neonatal units, screening for hypoglycemia is done using test strips, which allow rapid bedside quantification of glucose levels. The specimens collected for test-strip reading in standard glucose meters are usually obtained from capillary blood. Any capillary glucose level <40 mg/dL should prompt confirmation of actual plasma glucose levels as soon as possible. A recent study measured capillary blood glucose levels using three different glucose meters and compared these measurements to levels quantitated by the hexokinase method. The authors concluded that none of the devices yielded satisfactory results. However, they noted that the overestimation of glucose levels by these devices could enable early detection of hypoglycaemia [9].
Obtaining immediate accurate measurements of actual blood glucose has long been difficult or even impossible in low-complexity settings, considering that symptomatic hypoglycemia – especially in high-risk groups – must be addressed quickly, and that the decision to infuse glucose intravenously (or not) must be made immediately.
Starting from the premise that all newborns are vulnerable to declines in blood glucose levels, we presumed that physicians acting in the perinatal setting would be able to contribute with descriptions of their practical attitudes toward suspected cases of neonatal hypoglycemia, and that these descriptions could be used to develop a flowchart for management of this condition based on samples obtained from rapid capillary blood glucose testing.
Within this context, the present study sought to construct a concept map of neonatal hypoglycemia based on the testimonials of specialist physicians whose management of this condition is based on point-ofcare capillary blood glucose testing.
Subjects and Methods
This was a mixed qualitative/quantitative, descriptive, observational study. A semi-structured questionnaire was administered to specialized physicians in order to collect information on their attitudes toward management of neonatal hypoglycemia.
The sample included two groups
Generalist pediatricians and specialist neonatologists, all agreed to take part in the study, in accordance with Brazilian National Health Council Resolution No. 466/12.
The questionnaire was divided into two parts. The first part was designed to collect general data, including age, sex, month and year of medical school graduation, and whether the respondent had pursued postgraduate studies in Neonatology after completing training in Pediatrics.
The second part of the questionnaire was subdivided into two stages
Eight multiple-choice questions with three to five possible answers each, followed by two open-ended questions. One open-ended question inquired as to management practices for asymptomatic neonatal hypoglycemia, regardless of cause; the second such question asked the respondent to identify any variable they regarded as important when deciding how to manage neonatal hypoglycemia which had not been addressed in the questionnaire.
The questionnaire then collected information on several factors related to neonatal hypoglycemia, including maternal problems related to hypoglycemia (Diabetes, Hypertensive disease, Systemic disease, Breastfeeding), delivery (Route of delivery: Vaginal, Surgical), neonatal factors (Gestational age/Birth weight: SGA, AGA, LGA; Neonatal conditions: sepsis, asphyxia, IUGR, congenital infections), and analytic factors (Capillary glucose level deemed diagnostic of neonatal hypoglycemia; Level deemed to prompt oral supplementation of breastfeeding; Level deemed to prompt intravenous glucose infusion). Responses were scored on a Likert-type scale {Completely disagree (1); Disagree (2); Neither agree nor disagree (3); Agree (4); Completely agree (5); or Never (1); Occasionally (2); Sometimes (3); Often (4); Always (5)}.
During the study (2013), questionnaires were delivered personally by one of the investigators to Pediatrics and Neonatology departments in the state of Rio de Janeiro, Brazil. Prospective respondents were asked to reply by e-mail. They received an example questionnaire demonstrating how the research instrument should be completed. Throughout the data collection period, the investigator was available to clarify any doubts regarding proper completion of the instrument.
Initially, each respondent was identified only by level of proficiency in neonatal care, divided into two groups: non-neonatologist pediatricians (GP) and neonatologists (GN). The latter group comprised pediatricians who had completed a residency program in Neonatology, and/or had at least 5 years’ experience working in a specialized neonatal unit, and/or were board-certified as neonatologists by the Brazilian Society of Pediatrics.
The demographic variables collected were gender (male or female), time since graduation (<10 years or >10 years), postgraduate medical studies (residency/specialist training), setting of postgraduate studies (university hospital or otherwise), and whether the respondent currently works at a service that provides Neonatology training (yes or no).
The eight statements concerning neonatal hypoglycemia were scored from 1 to 5 on the aforementioned Likert-type scale of agreement. The wording of questionnaire items was guided by keywords selected on the basis of the recent literature on the topic. Multiple-choice questions were also based on the literature [10-26].
To construct the concept map, a graphic-based tool was used to organize and represent content. Concepts were represented by circles or boxes, and relationships between them were denoted by lines. Respondents’ answers to the research instrument were the basic substrate for plotting the concept flow.
Our analysis strategy consisted of first calculating Cronbach’s alpha coefficients, which define the reliability of different respondents’ answers on the same measurement scale by displaying the mean correlation between questionnaire items. Cronbach’s alpha values range from 0 to 1, and are deemed to represent consistency that is very good (>0.9), good (0.8-0.9), fair (0.7-0.8), poor (0.6-0.7), or unacceptable (<0.6).
The Keiser-Meyer-Olkin (KMO) test measure of sampling adequacy shows the strength of connection between variables. High values (0.5-1.0) are indicative of appropriateness of analysis. Values below 0.5 suggest the factor analysis may be inadequate.
Bartlett’s test of sphericity tests the null hypothesis that the correlation matrix is an identity matrix (variables are not correlated; correlation coefficients would be zero). In this case, principal component analysis cannot be performed. We want this test to be significant and have a value less than 0.05. Principal components analysis was used to evaluate the association between the constructed model and the degree of agreement between respondents. The core purpose of this analysis was to establish, in a homogeneous group with a strong correlation between variables, a coefficient without the initially existing correlation. The first component corresponds to the axis with the greatest variability, and the second component, to the axis with the second greatest variability. These exploratory models are based on the total variance of responses, and the two first components explain the largest percentage of response variability. Eigenvectors are a set of axes (components) extracted from a similarity matrix. Eigenvalues correspond to the length of the eigenvectors, and, thus, to their importance to explaining total variance in the data. Orthogonal Varimax Rotation maximizes the loading of each variable onto the new components and minimizes the number of variables that have high loadings on each component.
Principal component analysis was used to evaluate the association between the constructed model and the degree of agreement between respondents. The concept maps themselves were built in Cmap Tools Knowledge Kit – version 5.05.01 – Institute for Human and Machine Cognition. A University Affiliated Research Institute.
The return rate of completed questionnaires during the study period fell short of expectations. However, there were enough instruments to establish two groups for comparative purposes and to serve as inputs for construction of the concept map.
Data were processed in SPSS Statistics for Windows, Version 22.0 (Armonk, NY: IBM Corp.). Continuous variables were evaluated descriptively to yield means, medians, standard deviations, variance, and ranges (minimum and maximum). The quantitative continuous variables of interest were age (in years), time since completion of medical training (in years), and ordinal responses to the eight questionnaire items on hypoglycemia (scored on a scale of 1 to 5).
The Kolmogorov-Smirnov or Shapiro-Wilk methods were used as appropriate to test for normality of data distribution in both groups (GP and GN), and ranked means of these variables were compared by the Mann-Whitney U test. To test for association between categorical variables, the chi-square test and Fisher’s exact test were used. The significance level was set at 5% for all hypothesis tests.
Divergence in answers to the eight questionnaire items was observed and the two groups were separated to test for associations and examine percent agreement and disagreement, mode, mean, and standard deviation in each group.
Using the principal components analysis method, a model was constructed for probabilistic weighting of variables related to the full set of questions about neonatal hypoglycemia and cluster analysis of responses regarding blood glucose cut off levels, routes of glucose administration, variables related to intrauterine growth, and maternal and delivery variables. The software was set to describe coefficients using the Pearson correlation matrix and conduct Kaiser-Meyer-Olkin (KMO) and Bartlett tests. For extraction, a table was created showing the variance explained by each set of answers, for eigenvalues >1. Varimax rotation was selected and results were ranked by weight. Values below 0.66 were suppressed in the interest of clarity and better visualization. To facilitate analysis, the software was set to generate factor loadings (including plot generation) only on the first two components.
Results
Of the 223 questionnaires sent out, 98 were completed and returned; 34.7% were completed by neonatologists (GN=34/98) and 65.3% by generalist pediatricians (GP=64/98).
The mean age of respondents across the two groups was 42.4 years (SD: 11.66; 95% CI: 40.10, 44.78), with no significant difference in ranked means between the two groups (GN mean=42.91; GP mean=42.19; p=0.597).
Regarding gender, 79.6% of respondents (n=78/98) were female. There was a significant age difference between men and women (male mean=50.05, SD=10.35; 95% CI: 45.20, 54.90; female mean=40.49, SD=11.22; 95% CI: 37.96, 43.02; p<0.001). There was no association between gender and group allocation (p=0.431). There was also no significant between-group difference (p=0.455) in time since graduation (GN mean=17.88; GP mean=16.47).
Overall, 84.7% (n=83/98) of respondents claimed to have completed a residency program and 75.5% (n=74/98) claimed to be board-certified in Pediatrics and/or Neonatology by the Brazilian Society of Pediatrics. There was a significant association between belonging to either group (GP=25/34; GN=58/64) and having completed residency (p=0.038), as well as a significant association between being board-certified by the Brazilian Society of Pediatrics and belonging to group GP (n=58/64).
Table 1 reports the main results for the two groups. The reliability of responses was acceptable (GN=0.814; GP=0.793). As shown by the median and mode, both groups provided bery similar responses. Regarding maternal problems, nearly 100% of respondents agreed with blood glucose controls in case of diabetic mothers (GN=fabs 34/34, median 5.0, mode 5; GP=fabs 60/64, median 5.0, mode 5); conversely, few believed that route of delivery should mandate screening (GN=median 1.0, mode 1; GP=median 2.0, mode 1). All major neonatal problems, except for congenital infections, were also highly regarded (GN=median 3.0, mode 3; GP=median 3.0, mode 3). Both groups chose 20 mg/dL as the cutoff for administration of IV dextrose (GN=median 5.0, mode 5; GP=median 5.0, mode 5), and believed that higher blood glucose values should prompt oral intervention for prevention and/or treatment of neonatal hypoglycemia (GN=median 4.0, mode 4; GP=median 4.0, mode 4).
| Groups | GN (n=34) | GP (n=64) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cronbach’s alfa | 0.814 | 0.793 | ||||||
| KMO/B | 0.586/p=0.000 | 0.628/p=0.000 | ||||||
| Variable | N(fabs) | Median/Mode | 1 C | 2 C | N(fabs) | Median/Mode | 1 C | 2 C |
| Maternal problems | ACG | ACG | ||||||
| Diabetes | 34/34 | 5.0/5 | - | - | 60/64 | 5.0/5 | 0.968 | |
| Hypertensive disease | 6/34 | 3.0/3 | 0.971 | 9/64 | 3.0/3 | |||
| Systemic disease | 8/34 | 3.0/3 | 0.927 | 8/64 | 3.0/3 | 0.878 | ||
| Breastfeeding | 2/34 | 3.0/3 | 0.782 | 3/64 | 2.0/2 | 0.880 | ||
| Delivery route | NCG | NCG | ||||||
| Vaginal | 21/34 | 1.0/1 | 0.913 | 34/64 | 1.0/1 | 0.905 | ||
| Surgical | 17/34 | 1.5/1 | 0.913 | 27/64 | 2.0/1 | 0.905 | ||
| Weight-for-GA | ACG | ACG | ||||||
| SGA | 30/34 | 5.0/5 | 0.845 | 50/64 | 5.0/5 | 0.844 | ||
| AGA | 2/34 | 2.0/2 | 0.964 | 2/64 | 2.0/1 | 0.992 | ||
| LGA | 30/34 | 5.0/5 | 0.842 | 56/64 | 5.0/5 | 0.875 | ||
| Gestational age | ACG | ACG | ||||||
| <34 weeks | 31/34 | 5.0/5 | 0.917 | 53/64 | 5.0/5 | 0.677 | ||
| 34-36 weeks | 23/34 | 5.0/5 | 0.91 | 25/64 | 4.0/4 | 0.879 | ||
| 37-40 weeks | 2/34 | 2.0/1 | 0.845 | 2/64 | 2.0/2 | 0.839 | ||
| >40 weeks | 1/34 | 1.5/1 | 0.847 | 3/64 | 2.0/2 | 0.716 | ||
| Neonatal problems | ACG | ACG | ||||||
| Likely sepsis | 21/34 | 5.0/5 | 0.696 | 46/64 | 5.0/5 | 0.875 | ||
| Asphyxia | 19/34 | 5.0/5 | 0.756 | 33/64 | 5.0/5 | 0.724 | ||
| IUGR | 27/34 | 5.0/5 | 0.881 | 33/64 | 5.0/5 | 0.925 | ||
| Congenital infection | 5/34 | 3.0/3 | 0.875 | 13/64 | 3.0/3 | |||
| Hypoglycemia | DV | DV | ||||||
| <20 mg/dL | 28/34 | 5.0/5 | 0.94 | 48/64 | 5.0/5 | 0.896 | ||
| 20-39 mg/dL | 27/34 | 5.0/5 | 0.953 | 43/64 | 5.0/5 | 0.888 | ||
| 40-50 mg/dL | 3/34 | 4.0/4 | 14/64 | 4.0/4 | 0.866 | |||
| >50 mg/dL | 2/34 | 1.0/1 | 0.77 | 3/64 | 2.0/1 | 0.860 | ||
| ABM or PBM | PO | PO | ||||||
| <20 mg/dL | 9/34 | 1.0/1 | 0.783 | 20/64 | 2.0/1 | 0.872 | ||
| 20-39 mg/dL | 22/34 | 5.0/5 | 0.791 | 26/64 | 4.0/5 | 0.878 | ||
| 40-50 mg/dL | 8/34 | 4.0/4 | 19/64 | 4.0/4 | 0.853 | |||
| >50 mg/dL | 2/34 | 2.0/1 | 0.912 | 8/64 | 2.0/1 | 0.850 | ||
| IV glucose | TA | TA | ||||||
| <20 mg/dL | 31/34 | 5.0/5 | 0.695 | 57/64 | 5.0/5 | 0.868 | ||
| 20-39 mg/dL | 8/34 | 4.0/4 | 0.819 | 26/64 | 4.0/4 | 0.717 | ||
| 40-50 mg/dL | 2/34 | 2.0/2 | 0.729 | 8/64 | 2.0/2 | 0.893 | ||
| >50 mg/dL | 2/34 | 1.0/1 | 0.885 | 1/64 | 1.0/1 | 0.863 | ||
Principal components analysis. Varimax rotation with Kaiser normalization. Factors extracted on the basis of eigenvalue >1. Cronbach: Cronbach’s alpha; KMO/B: Kaiser-Meyer-Olkin/Bartlett tests; N(fabs): frequency of agreement; 1 C: Factor Loading of First Component; 2 C: Factor Loading of Second Component; ACG: Always Control Glucose; NCG: Never Control Glucose; GA: Gestational Age; IUGR: Intrauterine Growth Restriction; SGA: Small for Gestational Age; AGA: Adequate for Gestational Age; LGA: Large for Gestational Age; DV: Diagnostic Value for Hypoglycemia; ABM or PBM: Artificial Baby Milk or Pumped Breast Milk; PO: per os; IV: Intravenous; TA: Treat Always at this Cutoff Level.
Table 1: Agreement, Cronbach’s alpha coefficients, and two principal components solution with KMO/Bartlett statistics in the neonatologist (GN) and pediatrician (GP) groups.
The Kaiser-Meyer-Olkin measure of sampling adequacy and Bartlett test (GN=0.586/p=0.000; GP=0.586/p=0.000) supported the use of principal components analysis on this database. All measurements clearly showed that the two first components explained 65.52% to 100.00% of absolute variance in GN and 65.52% to 100.00% in GP, respectively.
In both groups, the contribution of each component showed consistency between the loaded values and the absolute frequencies of each item answered, both for the first (1C) and for the second (2C) component. Unfilled fields corresponded to factor loadings <0.66. There was also an evident lack of correspondence of loadings in GN, both regarding maternal diabetes – which respondents unanimously (34/34) agreed should prompt blood glucose controls – and regarding cutoff blood glucose values that should prompt oral feeds and IV dextrose. High factor loadings (>0.66) and a slightly higher variability of responses in GN might explain these higher loads of responses regarding blood glucose values >50 mg/dL.
In the subjective portion of the instrument, with open-ended questions, when respondents were asked to describe their immediate management of asymptomatic neonatal hypoglycemia, as determined by a low capillary blood glucose measurement during rooming-in, regardless of cause, GP responses agreed with GN responses regarding encouragement of breastfeeding and/or complementary oral formula/ pasteurized pumped breast milk, in addition to measuring blood glucose again after management.
When asked whether any variables other than those covered in the research instrument were important when deciding how to manage neonatal hypoglycemia, and, if so, to describe these variables in their own words, just over 26% of GN respondents and more than 53% of GP respondents replied that no additional variables were needed other than those already covered in the questionnaire. Among those who replied that other variables were relevant, most noted that consideration of the clinical picture/symptoms and of the possible causes of neonatal hypoglycemia were important variables when deciding how to manage such episodes.
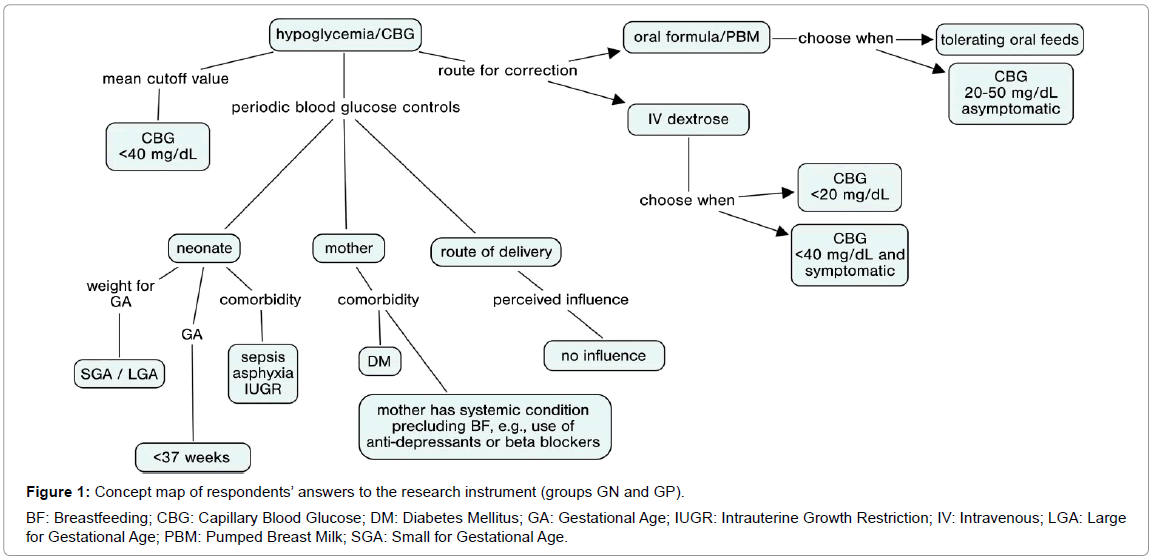
The concept map constructed from the responses of physicians involved in neonatal care is shown in Figure 1.
This map was constructed using pooled data from both groups, as their responses were highly similar.
Proper assistance at the time of delivery is essential, as low vitality (perinatal asphyxia) is associated with hypoglycemia. The pathological changes caused by neonatal asphyxia can often cause derangements in glucose metabolism. Anaerobic glycolysis supervenes, with a decline in energy production and increased glucose consumption. These neonates often feed less and consequently absorb less nutrients, becoming more susceptible to hypoglycemia after rapid depletion of glycogen stores [27,28].
Magnetic resonance imaging (MRI) suggests that the occipital lobe may be particularly vulnerable to insults caused by hypoglycemia. Other cerebral complications involve global visual perception (areas within the dorsal visual cortex) and problems of executive function (skills essential for learning, interaction with the environment, working memory, reasoning, task switching, and problem-solving). At age 2 years, these neonates exhibit higher rates of sensorineural involvement, processing difficulties, and multiple secondary problems related to growth and development. Therefore, providers involved in neonatal care must be ever vigilant to any potential inability of newborns to consistently maintain preprandial glucose concentrations >50 mg/dL up to 48 h of age and >60 mg/dL after 48 h of age [1].
Discussion
Assessment of the clinical practices of physicians who provide perinatal care revealed that both those trained as generalist pediatricians and those trained as specialist neonatologists respond similarly to neonatal hypoglycemia.
Among maternal problems occurring in the prenatal period that should prompt periodic blood glucose controls in the neonate, diabetes was cited most often by respondents in both groups. Some studies have reported improvements in the management of diabetes with more appropriate drug combinations8, new proposed therapeutic approaches [12] and even development of screening methods for gestational diabetes [13]. These advances are necessary, as glucose imbalances during pregnancy can cause irreversible damage to the fetus and newborn, neonatal hypoglycemia remains a leading cause of morbidity [14]. Although neither group demonstrated a direct concern with hypertensive disease of pregnancy, studies have raised concerns regarding increasing body mass index in pregnant women [15], and it is becoming increasingly clear that obesity and diabetes go hand in hand [16]. It is also important to address the relationship between pregnancy-induced hypertension and disorders of fetal development, from intrauterine growth restriction (IUGR) [17,18] to neonates large for gestational age (LGA) [19] and even preterm birth [20,21] all of which facilitate the development of hypoglycemia. In their answers to the open-ended questionnaire items, the respondents also noted maternal medication use as an important factor, as many drugs (beta blockers, chlorpropamide, tocolytics, benzthiazide) can suppress the catecholamine response and prevent glycogenolysis, or stimulate the pancreatic beta cells. An even greater concern is the growing use of antidepressants during pregnancy, which may be facilitating the development of neonatal hypoglycaemia [22]. After birth, breastfeeding-related problems must be prevented, as they can be a cause of metabolic disorders, including derangements in blood glucose. This could be prevented by strict hypoglycemia prevention protocols [23] or by existing, well-established programs (kangaroo mother care) [24].
The mode of delivery had no influence on the decision to screen for hypoglycemia. Conversely, most respondents in both groups would never request blood glucose controls on the basis of this factor alone.
There was no surprise regarding management of SGA [16] and LGA [17] neonates: respondents’ practices confirmed that inadequacy for gestational age raises concerns with regard to hypoglycemia. Likewise, preterm birth (<37 weeks GA) was concerning to all respondents as a driving factor of hypoglycemia. Responses showed that, in clinical practice, there is a trend to prescribe blood glucose controls when neonates are born premature. GP respondents were more concerned with the need to control blood glucose in preterm infants born at <34 weeks, whereas in GN respondents, the preferred cut off point was 37 weeks.
In both groups, when inquired as to the role of continuous blood glucose control in the presence of neonatal morbidity, sepsis was the situation most likely to prompt glucose measurement orders, despite wide variance in responses. This was to be expected, as sepsis and asphyxia are the leading causes of interfacility transfer of neonates [25]. Secondary but still great importance was assigned to asphyxia and intrauterine growth restriction, while in utero infections were mentioned but less likely to prompt concern. It bears stressing that perinatal asphyxia, especially in neonates with intrauterine growth restriction, leads to poor metabolic adaptation in the first hour after birth [26].
Most physicians in both groups defined hypoglycemia as a blood glucose level <40 mg/dL, although they highlighted the importance of assessing the clinical picture/symptoms as well as blood glucose measurements alone to ensure more effective measurement. There is an increasing concern with optimal cutoff values for diagnosis of hypoglycemia and initiation of therapy, as the plasma glucose concentration associated with neurological damage is still unknown [25], although said damage is known to be permanent [5]. Thus, the current trend would be to consider a diagnosis os hypoglycemia at relatively higher glucose levels (<2.6 mmol/L or 47 mg/dL) [27]. So as to select a wider population for intervention and decrease the likelihood of failing to treat neonates who may develop future morbidity as a result of undiagnosed hypoglycemia. Within this context, it bears stressing that point-of-care test strips are known to overestimate serum glucose levels [9], which should be taken into account when addressing potential cases of hypoglycemia. Respondents revealed that, in clinical practice, they tend to prescribe complementary oral feeding when blood glucose is 20-50 mg/dL in asymptomatic infants, while prescribing IV dextrose in cases of blood glucose <20 mg/dL, regardless of the presence of symptoms, or <40 mg/dL in the presence of symptoms. It should be noted that the proportions of responses in the GN group were in agreement as to this concept of prescribing oral feeds vs. IV dextrose.
Several problems were encountered when conducting this study. These included resistance to diagnosing hypoglycemia on the basis of capillary blood screening alone, considering that most clinical algorithms mandate peripheral rather than capillary blood collection. Traditionally, glucose measurements in peripheral blood are considered the standard to define bedside management. However, it is known measurements in plasma from freshly centrifuged blood obtained from a central venous catheter would be the gold standard for accurate confirmation of low glucose levels. Other challenges included the low response rate, which led to a sample size insufficient for generalization of our findings. We believe that, in view of the small sample size, some statistical tests may have behaved unexpectedly. The most rewarding part of this study was the construction of a concept map based on the opinions of experienced providers, which demonstrated the simple response required in an often harrowing clinical event (neonatal hypoglycemia).
In conclusion, our findings highlight the fact that, in high gestational risk groups, the entire neonatal care team should be focused on the risk of hypoglycemia. The use of certain specific drugs during pregnancy should raise red flags of this risk. The development of an algorithm for neonatal hypoglycemia management based on peripheral blood samples and bedside screening using test strips and glucose meters could contribute to streamlining management of neonatal blood glucose levels. Based on the experience of our respondents, we were able to conclude that intravenous glucose infusion is always indicated in cases of capillary blood glucose <20 mg/dL, regardless of the presence of symptoms, or <40 mg/dL if symptomatic.
What is already known on this topic:
• Hypoglycemia in newborns causes permanent damage to the central nervous system.
• Problems related to pregnancy, birth conditions, and neonatal and postnatal morbidity are all implicated in the development of neonatal hypoglycemia.
• Although there is no consensus about the optimal serum glucose cutoff levels which define hypoglycemia, procedures to address low blood glucose in newborns (glucose gel) have been implemented at several maternity facilities.
What this study adds:
• The presence of glucose disorders, feeding/dietary disorders, and/ or the use of specific drugs during pregnancy should alert the perinatal care team to the risk of neonatal hypoglycemia.
• The development of neonatal hypoglycemia management algorithms based on the use of peripheral blood glucose test strips can contribute to streamlining the management of neonatal blood glucose levels.
• In our respondents’ experience, intravenous glucose infusion is indicated whenever blood glucose is <20 mg/dL, regardless of the presence of symptoms, or <40 mg/dL when symptoms are present.
References
- Hawdon JM, Beer J, Sharp D, Upton M (2016) Neonatal hypoglycaemia: learning from claims. Archives of disease in childhood Fetal and neonatal edition. Epub.
- Adamkin DH (2017) Neonatal hypoglycemia. Semin Fetal Neonatal Med 22: 36-41.
- Adamkin DH (2015) Metabolic screening and postnatal glucose homeostasis in the newborn. Pediatr Clin North Am 62: 385-409.
- Bulut C, Gursoy T, Ovali F (2016) Short-Term Outcomes and Mortality of Late Preterm Infants. Balkan medical journal. Epub 33: 198-203.
- Yang G, Zou LP, Wang J, Shi X, Tian S, et al. (2016) Neonatal hypoglycemic brain injury is a cause of infantile spasms. Experimental and therapeutic medicine. Epub 11: 2066-2070.
- Rozance PJ (2013) Update on neonatal hypoglycemia. Curr Opin Endocrinol Diabetes Obes. Epub 21: 45-50.
- Hartmann A, Jaudon J (1937) Hypoglycemia. Jornal de Pediatria 11: 1-36.
- Castrodale V, Rinehart S (2014) The golden hour: improving the stabilization of the very low birth-weight infant. Advances in neonatal care: official journal of the National Association of Neonatal Nurses 14: 9-14.
- Ben Ameur K, Chioukh FZ, Bouanene I, Ghedira ES, Ben Hamida H, et al. (2016) [Evaluation of the measurement of capillary glucose concentration versus plasma glucose in the newborn]. Archives de pediatrie: Organe officiel de la Societe francaise de pediatrie 23: 908-912.
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, et al. (2003) Tests of glycemia in diabetes. Diabetes Care 26: S106-S108.
- Nachum Z, Zafran N, Salim R, Hissin N, Hasanein J, et al. (2017) Glyburide Versus Metformin and Their Combination for the Treatment of Gestational Diabetes Mellitus: A Randomized Controlled Study. Diabetes Care 40: 332-337.
- Gao F, Wang G, Wang L, Guo N (2017) Phytosterol nutritional supplement improves pregnancy and neonatal complications of gestational diabetes mellitus in a double-blind and placebo-controlled clinical study. Food & Function 8: 424-428.
- Koivunen S, Torkki A, Bloigu A, Gissler M, Pouta A, et al. (2017) Towards national comprehensive gestational diabetes screening - consequences for neonatal outcome and care. Acta Obstetricia et Gynecologica Scandinavica 96: 106-113.
- De Leon DD, Stanley CA (2017) Congenital Hypoglycemia Disorders: New Aspects of Etiology, Diagnosis, Treatment and Outcomes: Highlights of the Proceedings of the Congenital Hypoglycemia Disorders Symposium, Philadelphia April 2016. Pediatric Diabetes 18: 3-9.
- Suk D, Kwak T, Khawar N, Vanhorn S, Salafia CM, et al. (2016) Increasing maternal body mass index during pregnancy increases neonatal intensive care unit admission in near and full-term infants. The journal of maternal-fetal & neonatal medicine 29: 3249-3253.
- Langer O (2016) Obesity or diabetes: which is more hazardous to the health of the offspring? The journal of maternal-fetal & neonatal medicine 29: 186-190.
- Sharma D, Shastri S, Sharma P (2016) Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clinical Medicine Insights Pediatrics 10: 67-83.
- Sharma D, Farahbakhsh N, Shastri S, Sharma P (2016) Intrauterine growth restriction – part 2. The journal of maternal-fetal & neonatal medicine 29: 4037-4048.
- Yamamoto JM, Kallas-Koeman MM, Butalia S, Lodha AK, Donovan LE (2017) Large-for-gestational-age (LGA) neonate predicts a 2.5-fold increased odds of neonatal hypoglycaemia in women with type 1 diabetes. Diabetes/metabolism research and reviews 33: e2824.
- Dickson JL, Chase JG, Lynn A, Shaw GM (2016) Model-based glycaemic control: methodology and initial results from neonatal intensive care. Biomedizinische Technik Biomedical engineering.
- Sharma D (2016) Golden 60 minutes of newborn's life: Part 1: Preterm neonate. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine 30: 2716-2727.
- Smit M, Dolman KM, Honig A (2016) Mirtazapine in pregnancy and lactation - A systematic review. European neuro-psycho pharmacology: The Journal of the European College of Europsychopharmacology 26: 126-135.
- Stewart CE, Sage EL, Reynolds P (2016) Supporting 'Baby Friendly': a quality improvement initiative for the management of transitional neonatal hypoglycaemia. Archives of Disease in Childhood Fetal and Neonatal Edition 101: F344-F347.
- Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, et al. (2016) Kangaroo Mother Care and Neonatal Outcomes: A Meta-analysis. Pediatrics 137.
- Rathod D, Adhisivam B, Bhat BV (2015) Transport of sick neonates to a tertiary care hospital, South India: condition at arrival and outcome. Tropical Doctor 45: 96-99.
- Lazic Mitrovic T, Mikovic Z, Mandic Markovic V, Mihailovic S (2016) Impact of Transient Period of Metabolic Adaptation on Perinatal Asphyxia in Neonates with Intrauterine Growth Retardation. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine 30: 2665-267.
- Tin W (2014) Defining neonatal hypoglycaemia: a continuing debate. Seminars in Fetal & Neonatal Medicine 19: 27-32.
- Zhou W, Yu J, Wu Y, Zhang H (2015) Hypoglycemia incidence and risk factors assessment in hospitalized neonates. J Matern Fetal Neonatal Med 28: 422-425.
Citation: Macedo Lima G, Lima Cabral Martins ACPM, Moraes Barbosa AD, Figueiredo Jr I (2017) A Concept Map of Neonatal Hypoglycemia. Neonat Pediatr Med 3: 140. DOI: 10.4172/2572-4983.1000140
Copyright: 2017 Macedo Lima G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9982
- [From(publication date): 0-2017 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 8982
- PDF downloads: 1000