Research Article Open Access
Absorption process of salazosulfapyridine in human intestinal epithelial cells and rat intestine
Kazumasa Naruhashi*, Akiko Kamino, Ena Ochi, Erina Kusabiraki, Megumi Ueda, Shinichi Sugiura, Hirokazu Nakanishi and Nobuhito Shibata
Faculty of Pharmaceutical Sciences, Doshisha Women’s College of Liberal Arts, Kyoto, Japan
- *Corresponding Author:
- Kazumasa Naruhashi
Faculty of Pharmaceutical Sciences
Doshisha Women’s College of Liberal Arts
Kodo Kyotanabe-shi, Kyoto 610-0395, Japan
Tel: 81774658495
Fax: 81774-658479
E-mail: knaruhas@dwc.doshisha.ac.jp
Received date: September 14, 2016; Accepted date: September 23, 2016; Published date: September 28, 2016
Citation: Naruhashi K, Kamino A, Ochi E, Kusabiraki E, Ueda M, et al. (2016) Absorption Process of Salazosulfapyridine in Human Intestinal Epithelial Cells and Rat Intestine. Clin Pharmacol Biopharm 5:165. doi:10.4172/2167-065X.1000165
Copyright: © 2016 Naruhashi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Introduction: Salazosulfapyridine (SASP) is an oral medication used to treat rheumatoid arthritis and inflammatory bowel disease, particularly ulcerative colitis. It has been reported that various transporters such as P-glycoprotein (Pgp) and multidrug resistance-associated protein 2 (MRP2), are involved in the transport of SASP. P-gp and MRP2 are expressed in the brain, intestine, and various tissues in both humans and rats. In the intestine, P-gp limits the absorption of certain drugs, however, its mode of absorptive action with regard to SASP has not, as of yet been studied. The aim of this study was to investigate the intestinal transport of SASP and examine whether transporters such as P-gp and MRP2 are involved in this process.
Method: Bidirectional permeability and inhibition transport studies were performed using Caco-2 and T84 cell lines. Transcellular transport studies were conducted using isolated rat intestinal tissue mounted in an Ussing-type chamber. The intestinal absorption was examined using an in-situ closed-loop experiment in rats.
Results: SASP showed secretory-directed transport across Caco-2 and T84 cells, as well as rat intestinal tissue. Cyclosporine A (CsA), a P-gp inhibitor, was found to decrease this secretory-directed transport. In the intestinal closedloop experiment, addition of CsA resulted in increased accumulation of SASP; however, this was too miniscule to be considered. The inhibition study showed that both secretory and absorptive transporters sensitive to probenecid are involved in the process of SASP absorption in the small intestine.
Conclusion: In the process of SASP absorption in the intestine, CsA-sensitive secretory transporters including P-gp as well as probenecid-sensitive absorptive and secretory-transporters are involved and the total of the absorption of SASP is regulated by these transporters. It is likely that these transporters are coordinated in a complex manner to effectively regulate absorption of SASP and other biochemically similar drugs.
Keywords
Salazosulfapyridine; P-glycoprotein; Multidrug resistance-associated protein 2; Absorption; Rat; Caco-2; T84; Intestine
Abbreviations
ABC: ATP-binding cassette; BCRP: Breast cancer resistance protein; CsA: cyclosporine A; HBSS: Hanks’ balanced salt solution; HPLC: High-performance liquid chromatography; MES: 2-(N-morpholino) ethanesulfonic acid; MRP2: Multidrug resistance-associated protein 2; PBS: Phosphate buffered saline; P-gp: P-glycoprotein, SASP: Salazosulfapyridine
Introduction
Salazosulfapyridine (SASP) was originally proposed as a treatment for rheumatoid arthritis. It was subsequently discovered that SASP was also efficacious in treating inflammatory bowel disease, particularly ulcerative colitis [1,2].
SASP is a prodrug composed of 5-aminosalicylic acid (5-ASA) linked to sulfapyridine through an azo bond. SASP is partially absorbed in the small intestine after oral administration and the remainder passes into the colon, where it is reduced by coliform bacterial enzyme, azoreductase, to sulfapyridine and 5-ASA [3,4]. SASP and 5-ASA are effective for rheumatoid disease, while 5-ASA provides an antiinflammatory effect for inflammatory bowel diseases symptoms [5-7]. The bioavailability of SASP is relatively low, less than 15% [8]. This is thought to be due to its low solubility and poor permeability [9].
In addition to passive permeation through the intestinal epithelial cell membrane, both absorptive (influx) and secretory (efflux) transporters are involved in the drug absorption from the intestine to blood and vice versa. A number of absorptive transporters for nutrients such as glucose, amino acids, and oligopeptides have been identified to be expressed at the apical membranes of intestinal epithelial cells [10]. One of the most well studied absorptive transporters is peptide transporter 1 (PepT1), which recognizes oligopeptides as its substrate. β-Lactams and other molecules with chemical structures similar to those of oligopeptides are capable of crossing cell membranes through PepT1 carriers [11]. Several efflux transporters with broad substrate specificity were reported to be expressed in the intestine. These include P-glycoprotein (P-gp) [12] and multidrug resistance-associated protein 2 (MRP2) [13], MRP3, breast cancer resistance protein (BCRP) and so on. P-gp was one of the first transporters to be vigorously studied [12].
Intestinal absorption studies of SASP have shown to involve a combination of both several absorptive and efflux transporters. For intestinal absorption of SASP, MRP2 [14,15] and BCRP [14] have previously been identified as secretory transporters, and OATP2B1 [15] as an absorptive transporter.
Various in-vitro and in-vivo methods can be applied to the study of for drug administration. Caco-2 cells are connected by tight junctions possess microvilli. They develop the morphological characteristics of simple columnar enterocytes when grown on plastic dishes or nitrocellulose filters. In addition, Caco-2 cells express numerous transporters that contribute to both absorptive and secretory functions, such as PepT1 and P-gp, respectively [16]. Because they undergo similar differentiation when grown on Transwell polycarbonate membranes, Caco-2 monolayers are suitable as intestinal epithelial transport model systems [17]. They are being widely used as a standard permeabilityscreening assay for predicting drug intestinal permeability and for assessing the fraction of oral drug doses absorbed in humans. This is because permeability through Caco-2 cell monolayers correlates well with in-vivo absorption of orally administered compounds in humans [18-19]. T84 cell line, also a human carcinoma cell line, was derived from a lung metastasis of a colon carcinoma in a 72-year-old male. Madara et al. [20] demonstrated that the cells grew to confluence as a monolayer with the basolateral membrane attached to the surface of the culture dish and showed the existence of a microvillus-studded apical membrane facing the media. T84 cells also grow as polarized monolayers and display a morphology similar to that of undifferentiated crypt cells of the small intestine. They have been used extensively as a model system for studying epithelial electrolyte transport and its regulation by various hormones and neurotransmitters [20-21]. It has been suggested that this cell line can also serve as a model system for studying electrolyte transport processes [20].
In addition to human epithelial cell lines, model animals are often used for studies concerning intestinal absorption. Rats are favorable owing to their small size and the fact that they can be used for in-situ and in-vitro study. For this work, both in-situ and in-vitro studies were employed in the form of a closed-loop experiment and Ussing chamber method, respectively.
Using the aforementioned strategies, a set of experiments were set up to determine whether the intestinal transport of quinolone antimicrobials is regulated by P-gp and MRP2 [22-23] in rat intestinal tissue and human intestinal cell line Caco-2, and transport mechanism of intestinal absorption of μ opioid receptor agonists and contribution of P-gp in rat intestine and in Caco-2 cells [24].
As described above, several transporters are shown to be involve in the absorption of SASP from intestine [14,15], however, absorption process in various methods have not been compared in one study comprehensively. In answering these questions, this work was carried out by considering and experimenting with a range of methods, representing a comprehensive study with could relevant to future research in this area.
Materials and Methods
Chemicals
SASP was purchased from Sigma Co (St. Louis, MO). All other reagents were commercial products of reagent grade and were used without further purification.
Culture of Caco-2 and T84 cells
Caco-2 cells were grown in Dulbecco’s modified Eagle’s medium containing 10% of bovine serum and 1% non-essential amino acids, 2 mM L-glutamine, 100 units/mL penicillin G and 100μg/mL streptomycin, as described previously. Caco-2 cells were seeded onto Transwell insert at 1.26×105 cells/insert, and cultured for 14 and 21 days respectively [22-25].
T84 cells were grown in a 1: 1 mixture of Dulbecco’s modified Eagle’s medium with 4.5 g/L of D-glucose and Ham’s F12 Nutrient mixture containing 5% of bovine serum. T84 cells were seeded onto Transwell insert at 0.565×106 cells/insert, and cultured for 7-9 or 12-14 days, respectively [25].
The transepithelial electrical resistance of the monolayer of Caco-2 and T84 cells was 250~350 and 550~700 Ω cm2, respectively.
Transepithelial transport experiment
The transepithelial transport study with Caco-2 or T84 cells grown on polycarbonate filter of Transwell was performed as described previously [22-25]. The buffer used was HBSS (0.952 mM CaCl2, 5.36 mM KCl, 0.441 mM KH2PO4, 0.812 mM MgSO4, 136.7 mM NaCl, 0.385 mM Na2HPO4, 25 mM D-glucose and 10 mM Hepes, pH 7.4; the osmolarity was 315 mOsm/kg).
The volume of apical and basolateral compartments was 0.5 and 1.5 mL, respectively. To measure apical-to-basolateral (absorptive) or basolateral-to-apical (secretory) flux, a test compound was included in the apical or basolateral side, respectively. At the designated time, 0.5 mL of the basolateral or 0.2 mL of the apical side solution was withdrawn and replaced with an equal volume of HBSS, respectively [22-25].
Animals
Wistar/ST male rats were used at the age of 6 to 8 weeks. The animal studies were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Doshisha Women’s College of Liberal Arts.
Transport experiments by the Ussing-type chamber method
Rat intestinal tissue sheets were prepared as described previously [22-24]. The tissue sheets, consisting of the mucosa and most of the muscularis mucosa, were mounted vertically in an Ussing-type chamber that provided an exposed area of 0.5 cm2. The volume of bathing solution on each side was 5mL, and the temperature was maintained at 37°C. The test solution was composed of 128 mM NaCl, 5.1 mM KCl, 1.4 mM CaCl2, 1.3 mM MgSO4, 21 mM NaHCO3, 1.3 mM KH2PO4, 10 mM NaH2PO4, and 5 mM glucose at pH 7.4 (basolateral side) or 6.0 (mucosal side) and was gassed with O2 before and during the transport experiments. In the inhibition studies, modulators were added to the same side as the substrate.
Intestinal absorption study by the closed loop method
Intestinal absorption study by the closed loop method was performed as previously reported [22-24]. After the rats were anesthetized by intraperitoneal administration of pentobarbital (5 mg/kg), their intestines were exposed by midline abdominal incisions. Closed intestinal loops of 10 cm were prepared by ligating both ends after clearing the gut with slowly passed warmed isotonic 2-(N-morpholino)ethanesulfonic acid (MES) buffer (5 mM KCl, 100 mM NaCl, 10 mM MES, 85 mM mannitol, 0.01% polyethylene glycol; pH 6.4; 290 mOsm/kg osmolarity) until the effluent became clear and expelling the remaining solution by means of air pumped through a syringe. Isotonic MES buffer (50 μL/cm), including SASP with or without inhibitor, was administered into the loops as a bolus. The animals were kept warm at 37°C. After 15 min, the solution in the loops was collected and the loops were rinsed with isotonic MES buffer to give a total volume of 10 mL. After collecting the solution in the loops, the mucosa was also collected.
Analytical methods
Samples of experimental solution from transepithelial transport experiment by Caco-2 and T84 cells were properly diluted in HBSS when needed and was then subjected to high-performance liquid chromatography (HPLC) determination.
Samples of experimental solution from the Ussing-type chamber and closed loop experiments were properly diluted in 0.1 M phosphate buffered saline (PBS) and was then subjected to HPLC. To samples of intestinal mucosa from the closed loop experiments, 1 mL of mobile phase solution (described below) was added and homogenized. The homogenate was centrifuged to yield the supernatant. The supernatant was then subjected to HPLC determination.
The HPLC system consisted of a constant-flow pump (LC-20AD; Shimadzu Co., Kyoto, Japan), a UV detector (SPD-20A; Shimadzu Co.), a system controller (CBM-20A; Shimadzu Co.), and an automatic sample injector (SIL-20A; Shimadzu Co.); a Cosmocil 5C18 MS-II column (150 mm height×4.6 mm I.D; Nacalai Tesque, Kyoto, Japan) was used as the analytical column. The mobile phase comprised 20mM potassium phosphate buffer (pH 7.0): methanol (60: 40, [v/v]) with a column temperature of 40°C, flow rate of 1.2 mL/min, and UV detection at 254 nm.
Data analysis
Transport was estimated in terms of permeation (μL/cm2) by dividing the amount transported (μmol/cm2) with the initial concentration of the test compound on the donor side (μmol/μL). The permeability coefficient (μL/cm2/min) was obtained from the slope of the linear portion of the plots of permeation against time (min). The secretory ratio was calculated from the permeability coefficient of absorptive transport divided by that of secretory transport. All data are expressed as the means ± SEM and statistical analysis was performed with Student’s t-test. A difference of P<0.05 between means was considered significant.
Results
Transport of SASP across intestinal epithelial monolayers
We studied SASP permeation in Caco-2 and T84 cell monolayers cultured on polycarbonate filters.
The secretory transport of SASP across Caco-2 and T84 monolayer was significantly greater than the absorptive transport (Figure 1). The absorptive transport rates in Caco-2 and T84 were very similar, while the secretory transport rate of Caco-2 was much faster than that of T84. The calculated secretory ratios (secretory transport divided by absorptive transport) were 15.4 and 1.86 in Caco-2 and T84, respectively, indicating secretory-directed transport and the degree is higher in Caco-2 cells than in T84 cells.
Inhibitory study in transport of SASP across intestinal epithelial monolayers
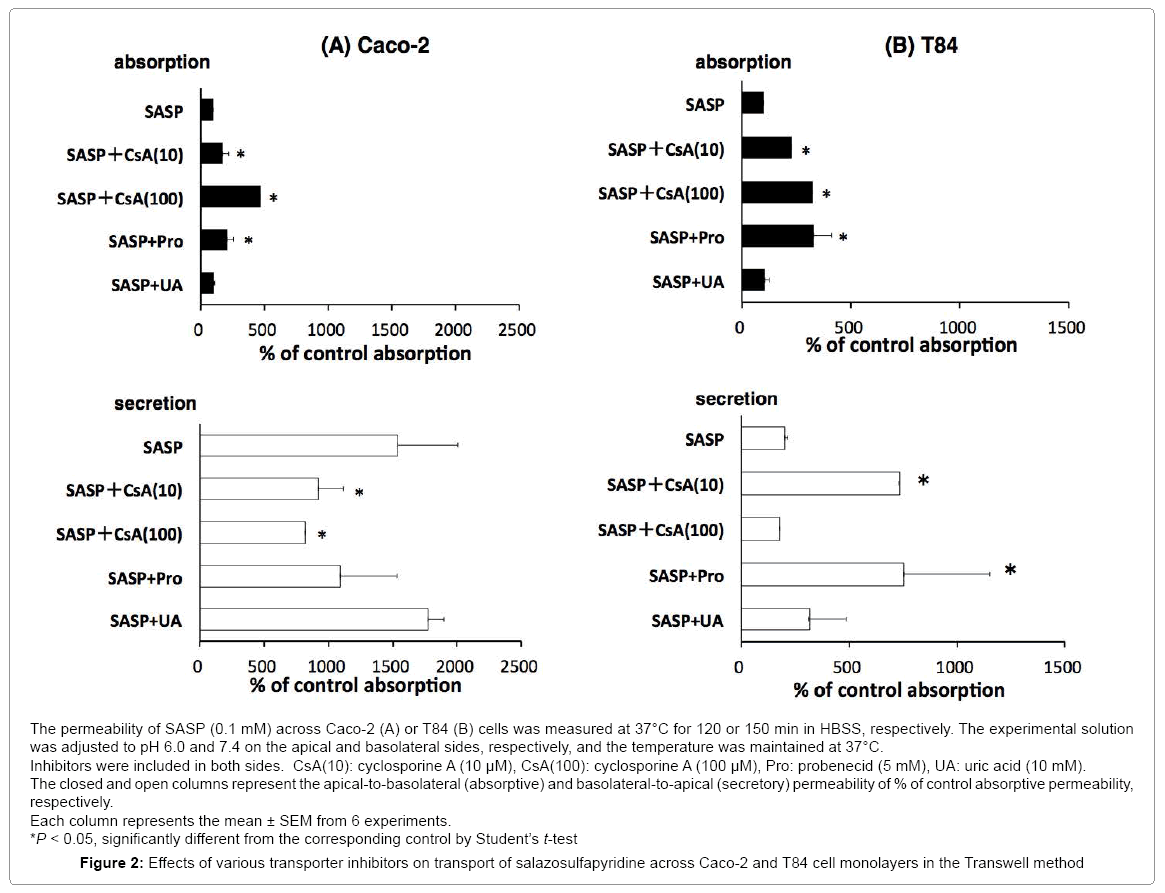
We examined the effect of various transporter inhibitors on the secretory-directed transport in these cells (Figure 2).
In Caco-2, addition of cyclosporine A (CsA) (10, 100 μM) significantly increased absorptive transport of SASP and decreased secretory transport in a concentration depending manner. In T84, addition of CsA (100 μM) significantly increased absorptive transport of SASP but did not alter secretory transport.
Probenecid (5 mM) in Caco-2 showed the same tendency as CsA although the changes were not significantly different. In T84, probenecid (5 mM) increased both absorptive and secretory transport; significantly uric acid did not change both directions of SASP transport in Caco-2 and T84.
Transport of SASP across rat intestinal tissue mounted in an Ussing chamber and inhibition experiments
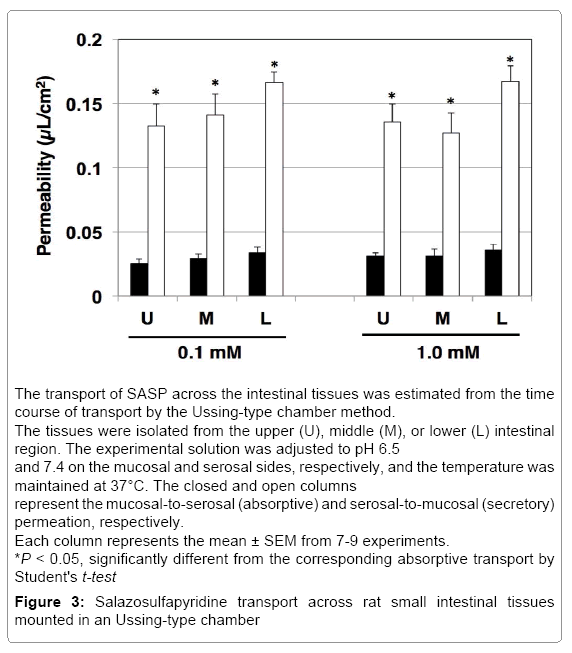
We studied SASP permeation in rat intestinal tissue mounted in Ussing chambers to determine the characteristics of absorption and secretion.
In the isolated rat intestinal tissue, the transport of SASP (0.1 and 1.0 mM) in the secretory direction was faster than that in absorptive direction, showing secretory-directed transport in all segments. The most prominent secretory ratio was 4.92 SASP 0.1 mM in the intestinal tissue from lower segment (Figure 3). Therefore, we performed inhibitory study using only in lower segment at SASP 0.1 mM (Table 1). CsA (10 μM) increased absorptive transport but did not change secretory transport, resulting in decrease in secretory ratio. Probenecid (1.0 mM) increased absorptive transport as well as decreased secretory transport, significantly and secretory ratios were decreased since the increase of absorptive permeability was more drastic than that of secretory permeability. Uric acid (5.0 mM) did not alter either absorptive or secretory transport.
| Absorptive (µL/min) | Secretory (µL/min) | Secretory ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SASP 0.1mM | 0.0338 | ± | 0.0042 | 0.1664 | ± | 0.0082 | 4.92 | ||
| + CsA (10 µM) | 0.0793 | ± | 0.0033 | * | 0.1613 | ± | 0.0061 | 2.03 | |
| + Pro (1.0 mM) | 0.0953 | ± | 0.0133 | * | 0.2615 | ± | 0.0041 | * | 2.74 |
| + UA (5.0 mM) | 0.0333 | ± | 0.0022 | * | 0.1615 | ± | 0.0033 | 4.85 | |
CsA: cyclosporine A, Pro: probenecid, UA: uric acid
Each column represents the mean ± SEM from 7-9 experiments.
*P < 0.05, significantly different from the corresponding control by Student's t-test
Table 1. Effects of various transporter inhibitors on the transport of salazosulfapyridine across isolated rat tissues mounted in an Ussing-type chamber.
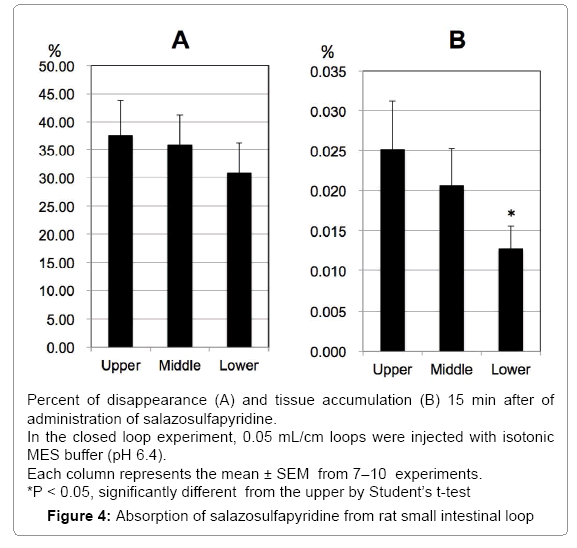
Absorption of SASP from rat small intestinal loops
We examined the intestinal absorption of SASP by the in-situ closed loop method in rats. After 15 min of experimental time, 37.5, 35.8 and 30.9% of the administered amount of SASP was disappeared from upper, middle and lower loops of the rat intestine, respectively, and no regional difference was observed, although the disappearance of lower segment tended to be higher than the others (Figure 4A). Accumulation in the mucosa was 0.025, 0.021, and 0.013% in upper, middle and lower loops (Figure 4B). The accumulation of mucosa in lower segment was significantly lower than that in upper segment, although the percentage of accumulation was very small.
CsA (10 μM) did not affect disappearance and accumulation except the tissue accumulation in lower segment. Probenecid (0.5 mM) has no effect but probenecid (5.0 mM) decreased disappearance from upper and lower loops significantly. Significant decrease was also observed in the disappearance from middle loop by uric acid (5 mM) (Table 2).
| Dissaperance | Tissue Accumulation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper | SASP (0.1 mM) | 37.50 | ± | 6.25 | 0.02514 | ± | 0.00610 | ||
| + CsA (10 µM) | 34.29 | ± | 3.39 | 0.02514 | ± | 0.00480 | |||
| + Pro (0.5 mM) | 40.00 | ± | 5.93 | 0.02179 | ± | 0.00320 | |||
| + Pro (5.0 mM) | 28.39 | ± | 3.93 | * | 0.02400 | ± | 0.00419 | ||
| + UA (5.0 mM) | 30.36 | ± | 2.14 | 0.02149 | ± | 0.00137 | |||
| Middle | SASP (0.1 mM) | 35.83 | ± | 5.35 | 0.02055 | ± | 0.00472 | ||
| + CsA (10 µM) | 37.43 | ± | 3.92 | 0.02268 | ± | 0.00370 | |||
| + Pro (0.5 mM) | 34.22 | ± | 5.53 | 0.02780 | ± | 0.00551 | |||
| + Pro (5.0 mM) | 27.63 | ± | 2.88 | * | 0.01874 | ± | 0.00087 | ||
| + UA (5.0 mM) | 25.85 | ± | 3.21 | 0.01669 | ± | 0.00315 | |||
| Lower | SASP (0.1 mM) | 30.86 | ± | 5.39 | 0.01270 | ± | 0.00288 | ||
| + CsA (10 µM) | 28.81 | ± | 3.72 | 0.01929 | ± | 0.00298 | |||
| + Pro (0.5 mM) | 26.39 | ± | 4.65 | 0.01606 | ± | 0.00273 | |||
| + Pro (5.0 mM) | 25.09 | ± | 5.39 | 0.00830 | ± | 0.00186 | * | ||
| + UA (5.0 mM) | 23.23 | ± | 3.72 | * | 0.01343 | ± | 0.00107 | ||
CsA: Cyclosporine A, Pro: Probenecid, UA: Uric acid
Each column represents the mean ± SEM from 7-10 experiments.
*P< 0.05, significantly different from the corresponding control by Student’s t-test
Table 2. Effect of various transporter inhibitors on salazosulfapyridine absorption from rat intestinal loop
Discussion
SASP has been clinically used for rheumatoid and inflammatory bowel disease as an oral medication. After SASP is administered orally, it is absorbed from intestine; however, the absorptive characteristic of SASP from intestine is not well understood. Therefore, we studied SASP absorption by using two intestinal epithelial cells and rat intestine invitro and in-situ.
First, we examined the transport characteristics of SASP in human intestinal cell lines, Caco-2 and T84 cells (Figure 1). In both Caco-2 and T84 cells, significant secretory-directed transport was observed, suggesting that secretory transporter(s) may also be involved in human intestine when SASP is absorbed from small intestine. The degree of secretory-directed transport was much higher in Caco-2 cells (secretory ratio: 15.4) than in T84 cells (1.86). Caco-2 cells express numerous transporters that contribute to both absorptive and secretory functions [10]. Previously, we reported the comparison of the expression and function of ATP-binding cassette (ABC) transporters in Caco-2 and T84 cells cultured on plastic dishes or polycarbonate filters of transwell [25]. It was found that the contribution of the transporter function of ABC transporters, including P-gp, to the total transepithelial transport is likely to be stronger in Caco-2 cells than in T84 cells [25]. Therefore, the greater secretory ratio observed in the Caco-2 cells than in T84 cells may be due to differences in the expression levels of transporters contributing to the SASP secretion.
We also used rat intestine to confirm that secretory transporters may be involved in the process of SPSA intestinal absorption. In rat intestinal tissue mounted in the Ussing-type chamber, we observed significant secretory-directed transport of SASP (Figure 3) as observed in Caco-2 cells and T84 cells. This result suggests the involvement of secretory transporters in SASP absorption process in rats. In the closedloop study, only about 30% of administered SASP was absorbed (Figure 4). The absorption of SASP from the rat intestinal loop was relatively slow compared to that of loperamide absorption (approximately 80% in 15 min by the same method) [24].
Taken together, these results suggest that secretory transporters are indeed involved in the absorption process of SASP in rat intestine as well as in human intestinal epithelial cells, and the involvement of secretory transporters may be extrapolated that bioavailability of SASP is very poor in human.
Inhibitory study was carried out to specify the transporters involved in the process of SASP absorption. CsA is a substrate of P-gp that inhibits various ABC transporters. We demonstrated previously that P-gp is involved in the intestinal absorption process of grepafloxacin, loperamide and morphine by the same methods using CsA in Caco-2 and T84 cells, and rat intestine [12,24]. Many studies, including ours, have shown that CsA at lower concentrations (5~10 μM) inhibits P-gp exclusively. At higher concentrations (>50 μM), however, it was found to inhibit not only P-gp, but also various ABC transporters and others non-specifically [22,24,25]. In this study, significant increases of absorptive transport coupled with significant decreases of secretory transport were observed when CsA was added within SASP solution in Caco-2 cells (Figure 2A). As for T84 cells, CsA increased not only SASP absorptive transport but also secretory transport at a higher concentration (100 μM) (Figure 2B). In the Ussing-type chamber experiment, addition of CsA resulted in a significant increase in absorptive transport with no alteration in secretory transport (Table 1). These results strongly indicate that CsA-sensitive transporters, such as P-gp, are involved. As mentioned above, expression of P-gp is higher in Caco-2 cells with higher function than in T84 cells. The results observed in T84 cells suggest that apart from P-gp acting as a secretory transporter, there are other absorptive transporters involved in the transport of SASP, with the contribution of the latter to the net transport being greater than that of the former. In the rat loop experiment, only the accumulation of SASP in the lower segment of mucosa increased significantly on addition of CsA (Table 2). The other segments remained unaffected. Taken together, these results suggest that CsA-sensitive secretory transporters such as P-gp are involved in the SASP absorption process; however, their contribution to the net absorption is likely to be very minimal.
Probenecid is an anionic compound and a substrate/inhibitor of multidrug resistance protein 2 (MRP2) [26]. We have previously reported that probenecid inhibits MRP2-mediated transport of p-aminohippuric acid [27]. There are some reports that probenecid inhibits various absorptive and secretory transporters such as anionic transports, MRP3, BCRP and so on [26]. In this study, probenecid significantly increased absorptive transport without altering secretory transport in Caco-2 cells. In T84 cells and in rat tissue mounted in Ussing-type chamber, both absorptive and secretory transports were significantly increased, suggesting that both absorptive and secretory transporters might be involved. In the intestinal loop method, significant decrease in absorption from upper and middle intestinal loops was observed. This result may be a result of inhibition of absorptive transporters sensitive to probenecid in the rat intestine. These observations suggest that various absorptive and secretory transporters sensitive to probenecid are involved in the absorption process of SASP.
It was suggested that uric acid might be transported by a transporter which is known as BCRP [28] and others reported that an orphan transporter expressed in Caco-2 cells was urate transporter with high affinity to nicotine [29]. Despite this, the expression and function of these transporters within the intestine have not been studied in detail. Nonetheless, these reports indicate a possibility that a transporter would be expressed in the intestine and transport certain medication including SASP. In this study, the effect of uric acid was very exclusive with the only significant effect observed being an increase in absorptive transport in rat tissue mounted in the Ussing-type chamber (Table 1). Despite this result, it should be acknowledged that a very high concentration (5 mM) was required to bring about this effect. This result suggests that probenecid- and uric-acid-sensitive transporters with low affinity may also be involved in the SASP absorption process; however, their collective contribution is minimal.
Tomaru et al [15] suggested the possible involvement of some influx (absorptive) transporters in the intestinal absorption of SASP as well as efflux (secretory) transporters, which would explain why the absorptive clearance did not appear to change at various SASP concentrations in mice. We also found that the inhibitors of various transporters affect SASP transport differently depending on the experimental method employed. The effect of CsA, for example, was not well observed in the rat intestinal loop method when compared with the other methods used in our study. However, the effect of probenecid was observed to be more than that of CsA. This may be ascribed to the ratio of contribution of each transporter to the total SAPSA transport. The contribution of probenecid-sensitive transporters may have masked CsA-sensitive transporters in the loop method.
In conclusion, in the absorption process in the intestine involves CsA-sensitive secretory transporters such as P-gp as well as probenecidsensitive absorptive and secretory transporters. It is likely that these transporters are coordinated in a complex manner to effectively regulate absorption of SASP and other biochemically similar drugs.
References
- Peppercorn MA (1984) Sulfasalazine. Pharmacology, clinical use, toxicity, and related new drug development. Ann Intern Med 101: 377–386.
- Rains CP, Noble S, Faulds D (1995) Sulfasalazine. A review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs 50: 137–156.
- Houston JB, Day J, Walker J (1982) Azo reduction of sulphasalazine in healthy volunteers. Br J Clin Pharmacol 14: 395–398.
- Peppercorn MA, Goldman P (1972) The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther 181: 555–562.
- Bird HA (1995) Sulphasalazine, sulphapyridine or 5-aminosalicylic acid-which is the active moiety in rheumatoid arthritis? Br J Rheumatol 34 (Suppl. 2): 16–19.
- Pullar T, Hunter JA, Capell HA (1985) Which component of sulphasalazine is active in rheumatoid arthritis? Br Med J (Clin. Res. Ed.) 290: 1535–1538.
- Das KM, Dubin R (1976) Clinical pharmacokinetics of sulphasalazine. Clin Pharmacokinet 1: 406–425.
- Klotz U (1985) Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid. Clin Pharmacokinet10: 285-302.
- Yazdanian M1, Glynn SL, Wright JL, Hawi A (1988) Correlating partitioning and caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm Res 15: 1490-1494.
- Nakanishi T, Tamai I (2015) Interaction of Drug or Food with Drug Transporters in Intestine and Liver. Curr Drug Metab 16: 753-764.
- Tamai I, Nakanishi T, Hayashi K, Terao T, Sai Y, et al. (1997) The predominant contribution of oligopeptide transporter PepT1 to intestinal absorption of beta-lactam antibiotics in the rat small intestine. J Pharm Pharmacol 49: 796-801.
- Terao T, Hisanaga E, Sai Y, Tamai I, Tsuji A (1996) Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J Pharm Pharmacol 48: 1083-1089.
- Gotoh Y, Suzuki H, Kinoshita S, Hirohashi T, Kato Y, Sugiyama Y (2000) Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J Pharmacol Exp Ther 292: 433-439.
- Dahan A, Amidon GL (2009) Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. Am J Physiol Gastrointest Liver Physiol 297: G371-G377.
- Tomaru A, Morimoto N, Morishita M, Takayama K, Fujita T, et al. (2012) Studies on the Intestinal Absorption Characteristics of Sulfasalazine, a Breast Cancer Resistance Protein (BCRP) Substrate Studies on the intestinal absorption characteristics of sulfasalazine, a breast cancer resistance protein (BCRP) substrate. Drug Metab Pharmacokinet 28: 71-74.
- Li Q, Sai Y, Kato Y, Tamai I and Tsuji A (2003) Influence of drugs and nutrients on transporter gene expression levels in Caco-2 and LS180 intestinal epithelial cell lines. Pharm Res20: 1119-1124.
- Hidalgo IJ, Raub TJ and Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology96: 736-749.
- Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun175: 880-885.
- Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, et al. (2000) Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci10: 195-204.
- Madara JL, Stafford J, Dharmsathaphorn K, Carlson S (1987) Structural analysis of a human intestinal epithelial cell line. Gastroenterology92: 1133-1145.
- Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H (1984) A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol 246: G204-G208.
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, et al. (2001) Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein. J Pharm Pharmacol 53:699-709.
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, et al. (2002) Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob Agents Chemother 46: 344-349.
- Naruhashi K, Kamino A, Ochi E, Kusabiraki E, Ueda M, et al. (2016) Transport Mechanism of Intestinal Absorption of μ Opioid Recept or Agonists and Contribution of P-Glycoprotein in Rats and Human Intestinal Epithelial Caco-2. Clin Pharmacol Biopharm 5: 154.
- Naruhashi K, Kurahashi Y, Fujita Y, Kawakita E, Yamasaki Y, et al. (2011) Comparison of the expression and function of ATP binding cassette transporters in Caco-2 and T84 cells on stimulation by selected endogenous compounds and xenobiotics. Drug Metab Pharmacokinet 26: 145-153.
- Horikawa M, Kato Y, Tyson CA, Sugiyama Y (2002) The potential for an interaction between MRP2 (ABCC2) and various therapeutic agents: probenecid as a candidate inhibitor of the biliary excretion of irinotecan metabolites. Drug Metab Pharmacokinet 17 :23-33.
- Naruhashi K, Tamai I, Sai Y, Suzuki N, Tsuji A (2001) Secretory transport of p-aminohippuric acid across intestinal epithelial cells in Caco-2 cells and isolated intestinal tissue. J Pharm Pharmaco 53: 73-81.
- Takada T, Ichida K, Matsuo H, Nakayama A, Murakami K, et al. (2014) ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides Nucleotides Nucleic Acids 33: 275-281.
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, et al. (2008) Identification of a New Urate and High Affinity Nicotinate Transporter, hOAT10 (SLC22A13) J Biol Chem 283: 16332-16341.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 12172
- [From(publication date):
November-2016 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 11230
- PDF downloads : 942