Case Report Open Access
Acute Thoracic Aortic Dissection (Stanford B): Challenges in Early Detection and Management
| Noraini Sarina Abdullah1* and Fathinul Fikri Ahmad Saad2 | |
| 1Medical Unit, Pusat Perubatan Universiti Kebangsaan Malaysia, Jln Yaakob Latif 56100 Kuala Lumpur, Malaysia | |
| 2Pusat Pengimejan Diagnostik Nuklear, Fakulti Perubatan Sains Kesihatan, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia | |
| Corresponding Author : | Noraini Sarina Abdullah Medical Unit, Pusat Perubatan Universiti Kebangsaan Malaysia Jln Yaakob Latif 56100 Kuala Lumpur, Malaysia Tel: +60391455555 E-mail: surihatiku.sn@gmail.com |
| Received: June 29, 2015 Accepted: August 03, 2015 Published: August 07, 2015 | |
| Citation: Abdullah NS, Saad FFA (2015) Acute Thoracic Aortic Dissection (Stanford B): Challenges in Early Detection and Management. OMICS J Radiol 4:200. doi:10.4172/2167-7964.1000200 | |
| Copyright: © 2015 Abdullah NS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Background: Diagnosis of acute thoracic dissection is crucial given its potential fatal complications in delayed treatment response. Early and appropriate intervention renders obviation of futile surgery.
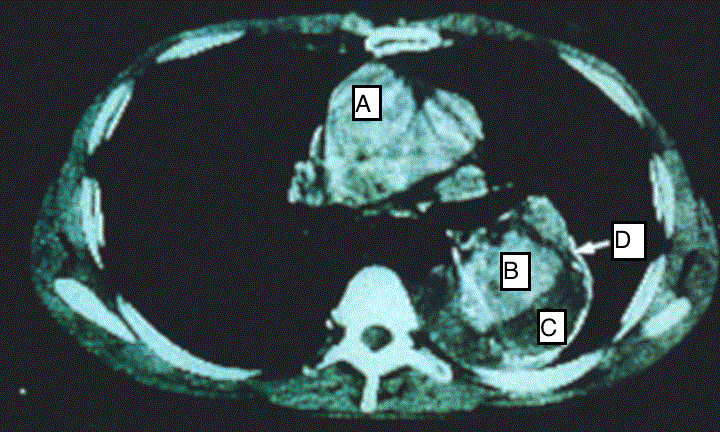
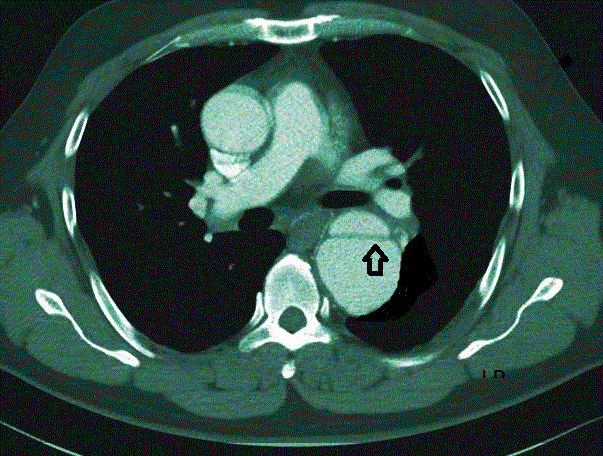
Case report: A 63 years old man with uncontrolled hypertension who presented to emergency department with sharp excruciating chest pain which radiated to the back. A Computed Tomography Angiography (CTA) was done which confirmed thoracic aortic dissection (Stanford type B) (Figures 1 and 2). An uneventful endovascular stent graft with Captivia system was done to contain the entry tear. The patient was doing well post procedure with wellcontrolled blood pressure.
Conclusion: This case reports documents challenges in making immediate diagnosis of acute aortic dissection and in providing appropriate intervention to avert fatal outcomes.
|
Abstract
Background: Diagnosis of acute thoracic dissection is crucial given its potential fatal complications in delayed treatment response. Early and appropriate intervention renders obviation of futile surgery.
Case report: A 63 years old man with uncontrolled hypertension who presented to emergency department with sharp excruciating chest pain which radiated to the back. A Computed Tomography Angiography (CTA) was done which confirmed thoracic aortic dissection (Stanford type B) (Figures 1 and 2). An uneventful endovascular stent graft with Captivia system was done to contain the entry tear. The patient was doing well post procedure with well-controlled blood pressure. Conclusion: This case reports documents challenges in making immediate diagnosis of acute aortic dissection and in providing appropriate intervention to avert fatal outcomes. Keywords
Aortic dissection; TEVAR; Computed tomography; Angiography
Introduction
Aortic dissection is rare but it is fatal. Undiagnosed aortic dissection can have mortality rate as high as 33 percent within the first 24 hours, 50 percent within 48 hours and 75 percent within two weeks [1]. The Stanford classification divides dissections into two types: Type A and Type B. Type A involves the ascending aorta and the type B affects the descending aortic segment. Aortic dissection arises from a tear in the intima which results in a separation of the aortic intima layers with infiltration of bleeding and the danger of impending aortic rupture or organ malperfusion or thrombosis. Clinically, the patient presents with sudden severe chest pain radiating to the back [1]. The most common risk factors are elderly, chronic smoker, atherosclerosis, uncontrolled hypertension and blunt trauma to the chest. According to a study, of all aortic dissection, 10% are chronic [2]. Typically they arise distal to the left subclavian artery and have reentry points into the true lumen. Pain maybe minimal or absent and patients often present with cardiac failure. Chronic dissection is more likely to appear radiographically as atherosclerotic aneurysm on chest film than an acute dissection [3]. In acute stage(<2 weeks), according to the classifications on the region of aortic dissection, the condition of false channel and the onset, appropriate medical, surgical or endovascular treatments including endovascular aneurysm repair followed by rapid and accurate diagnosis should be performed without delay [4]. Any delay of detection and management may cause fatal or severe complication like organ damage. This case report documents the challenges in making an immediate diagnosis and the choice of an appropriate intervention to avert fatal outcomes.
Case Report
A 63 years old man with chronic uncontrolled hypertension presented to Emergency Department (ED) with sudden onset of central pressing chest pain started since 4 hours prior to presentation while he was squatting in the toilet. The pain was sharp pressing in nature with pain score 7/10 which radiated to back and along the spine. The pain intensity reduces overtime which become continuously dull aching. It was associated with sweating, nausea and shortness of breath. It was partially relieved by rest. There was no fever, no cough, no palpitation, dizziness, headache or abdominal pain or weakness of lower limb and no pedal edema. He had history of gouty attack, chronic hypertension and, hyperlipidemia with poor compliance. He was given sublingual glyceryl trinitrates without much relief. The pain was dissipated with intravenous Morphine (2 mg).
On examination, his pulse was weak with bradycardia (57 bpm), hypertensive with blood pressure of (180/92 mmHg), oxygen saturation (96%) and normal glucose level (5.8 mmol/L). There was no sign of thrombosis or acute vascular occlusion. His renal profiles and the serum creatinine were normal. Bedside echocardiogram done showed good heart contractility with no regional wall motion abnormalities (RMWA), from the 5 chamber views, aortic root was not dilated. There was aortic septation and flaps were noted at the subxyphoid level about 2.5 cm for which findings were suspicious for a dissecting aneurysm. Chest X-ray revealed cardiomegaly with electrocardiography showed signs of infero lateral myocardial infarction as evidence by elevation of ST segment in ECG leads (AVF) and the lateral chest leads (V4, V5 and V6). The patient was referred to surgical department and admitted to High Dependency Unit (HDU) for further workout and monitoring. Urgent Computed Tomography (CT) angiograph scan was done which showed a continuous double-lumen sign indicating acute luminal dissection with flap formation. The dissecting segment emanating from the commencement of the descending aorta down to the level of xiphisternum for which changes confirmed the diagnosis of aortic dissection Type B (Figures 1 and 2). There is no evidence of aortic rupture or developing hematoma. He was kept monitored in HDU and antihypertensive drug was given to ensure a targeted BP of <140/90 mmHg could be achieved. After 10 days in HDU, the blood pressure (BP) was still high despite intravenous labetolol. We tried to stop Labetalol to see any control of BP but it was on the raising trend A Stanford B Thoracic Endovascular Aortic Repair (TEVAR) was performed as a definitive treatment for the thoracic aortic dissection. Discussion
This patient presented to ED with acute sharp chest pain which radiated to the back. At that juncture, differentiation from aortic dissection and acute myocardiac infarction were difficult. The symptom of back pain and the associated non-specific ECG changes of the anterolateral infarction gave a good prediction to the evolution of the dissecting aneurysm. The echocardiogram revealed an important lead for which CTA confirmed the aortic dissection. This is substantiated by the Michael Leitman et al study [5] on early recognition of acute thoracic aortic dissection and aneurysm concluded that increasing heart rate, chest pain, diabetes, head and neck pain, dizziness and history of myocardial infarction (MI) can be used to differentiate acute coronary syndrome (ACS) from thoracic aortic dissection/aneurysm. Nevertheless, both diseases could manifest concomitantly whereby the dissecting flap may extend as far as the commencement of the main coronary artery at the root of the ascending aorta which is more commonly in type A aortic dissection [6]. Prompt recognition of aortic dissection or aneurysm and differentiation from ACS is difficult yet crucial.
In addition, the gender and age factor of this patient should remain the main index of suspicion for aortic dissection who presented with uncontrolled hypertension and hypercholesterolemia. Data showed that average age for aortic dissection to occur is in the 60s and that two thirds of dissections occur in men [5]. The damage of the intimal explains the weakened tunica layers of the aorta as a result of artherosclerotic vessel in hypercholestrolaimic individual [4]. Furthermore, the hemodynamic of hypertension could potentially cause for hydrodynamic or shearing forces which lead to aorta cause tear of intima [5]. The most frequently performed tests to diagnose aortic dissection and its complications include CT scan, Transesophageal echocardiogram and magnetic resonance imaging (MRI). There was a study done by M.Oudkerk suggested that CT is at least as accurate as aortography in the diagnosis of acute aortic dissection [7]. The role of transesophageal echocardiogram (TEE) in early detection of aneurysm is essential as the technique is easy to perform and literally quick. The sensitivity of TEE has higher sensitivity in type A (sensitivity 93% and specificity 97%) than type B (sensitivity 84% and specificity 94%.) [8]. Nevertheless, since the patient was in pain complicated with anterolateral myocardial infacrtion, the procedure was deferred to CT. Diagnosis and management were tailored to the information of its anatomical extent and chronicity. According to study done by Sri G Thrumuthy et al, management of the uncomplicated distal (Type B) is best by intensive drug treatment and the complicated type B dissection requires surgical intervention such as in this case [9]. All patients need a long life antihypertensive therapy and surveillance imaging. In this particular patient, the immediate diagnosis and intervention had preserved him from further fatal deterioration of the aortic dissection with salvage TEVAR for Stanford type B thoracic aorta dissection confirmed on the CTA. Thoracic Endovascular Aortic Repair (TEVAR) was performed via a femoral artery cutdown. The patient was heparinized for a target activated clotting time ≥ 300 sec. A 12F sheath is used for femoral access to accommodate a large diameter occlusion balloon for potential risk of acute rupture occurs. An angled catheter and guidewire are used to access the abdominal aorta, and then advanced under fluoroscopic guidance into ascending aorta. The soft tip of the guidewire should reflect off the aortic valve, back into ascending aorta. The guidewire is then exchanged through the Intraveous ultrasound (IVUS) catheter for a stiff (soft tip) guide wire and the thoracic aorta interrogated with IVUS. A percutaneous 6F sheath is placed in the contralateral femoral artery and a second guidewire positioned in ascending aorta. An endoluminal stent was deployed in the distal descending aorta. The patient was discharged after a week hospitalization and was on regular monthly follow-up. Conclusion
Aortic dissection even though not so common but it still dangerous and fatal if not manage early. Early detection and proper management can prevent further complications.
|
References
- Collins JS, Evangelista A, Nienaber CA, Bossone E, Fang J, et al. (2004) Differences in clinical presentation, management, and outcomes of acute type a aortic dissection in patients with and without previous cardiac surgery. Circulation 110: II237-242.
- Blount KJ, Hagspiel KD (2009) Aortic diameter, true lumen, and false lumen growth rates in chronic type B aortic dissection. AJR Am J Roentgenol 192: W222-229.
- Ambos MA, Rothberg M, Lefleur RS, Weiner S, McCauley DI (1979) Unsuspected aortic dissection: the chronic "healed" dissection. AJR Am J Roentgenol 132: 221-225.
- Baumann F, Makaloski V, Diehm N (2013) [Aortic aneurysms and aortic dissection: epidemiology, pathophysiology and diagnostics]. Internist (Berl) 54: 535-542.
- Leitman IM, Suzuki K, Wengrofsky AJ, Menashe E, Poplawski M, et al. (2013) Early recognition of acute thoracic aortic dissection and aneurysm. World J Emerg Surg 8: 47.
- Neri E, Toscano T, Papalia U, Frati G, Massetti M, et al. (2001) Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg 121: 552-560.
- LePage MA, Quint LE, Sonnad SS, Deeb GM, Williams DM (2001) Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol 177: 207-211.
- Evangelista A, Avegliano G, Aguilar R, Cuellar H, Igual A, et al. (2010) Impact of contrast-enhanced echocardiography on the diagnostic algorithm of acute aortic dissection. Eur Heart J 31: 472-479.
- Thrumurthy SG, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM (2012) The diagnosis and management of aortic dissection. BMJ 344.
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14544
- [From(publication date):
August-2015 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 9933
- PDF downloads : 4611
