Analysis of Mutagenic Potential of Therapeutic Vaccine Based on BPV-1 E6 Recombinant Protein Combined with Different Adjuvants
Received: 18-Dec-2017 / Accepted Date: 25-Dec-2017 / Published Date: 30-Dec-2017
Abstract
Bovine papillomavirus is a worldwide distributed virus that affects at least 60% of Brazilian cattle herd, causing significant economic loses. Considering the great number of BPV-infected bovines, therapeutic vaccines, such those based on E6 protein, are mandatory to control the BPV-related diseases. However, the choice of the adjuvant remains challenging the vaccinology field, since adjuvants are associated with local and systemic reactions. Thus, this study analyzed the cytotoxic and mutagenic potential of BPV-1 E6 protein-based vaccine formulations using different adjuvants: aluminum hydroxide, complete and incomplete Freund’s adjuvants and two anti-oxidant saponin-rich extracts obtained from Agave sisalana-alcoholic and acid hydrolysis extract. Results of these analyzes suggest that acid hydrolysis extract from A. sisalana is an alternative and useful candidate for therapeutic vaccines, being able to reduce the mutagenic potential of antigen.
Keywords: Papillomavirus; Vaccine; E6 protein; Adjuvants; Saponin
Introduction
Vaccines are considered one of the most important medical advances of the last 200 years, reducing the infectious agent-associated deaths [1,2]. Currently, veterinary vaccines are responsible for 23% of all vaccine market [3]. In this sense, with the biotechnological advances, the interest in veterinary vaccines has grown, especially because animal vaccines have a less stringent regulatory and preclinical trials requirement [3]. However, this fact does not dispense the need to evaluate the biosafety of these products though in vitro and in vivo methods.
Bovine papillomavirus (BPV) is the etiological agent of bovine papillomatosis (BP), infectious and neoplastic disease, characterized by the presence of multiple papillomas that can regress spontaneously or persists, leading to malignancies, including urinary bladder and upper digestive carcinomas [4]. BPV is a worldwide distributed virus that causes significant economic loses, especially for Brazil that has the second largest cattle herd in the world, with 215 million of bovines [5]. According to epidemiological data, at least 60% of Brazilian cattle are infected by the BPV [6]. However, this number can be higher, since the viral infection can be asymptomatic [7,8].
Currently, there are few treatment methods against BP available. Among them are: the papilloma surgical excision [9], self-haemotherapy [10] and control of ectoparasites [11]. However, these methods are few effective, especially for large bovine herds, as verified in Brazil. In this sense, both prophylactic and therapeutic vaccines against BPV are mandatory to control the BPV-related diseases.
The idea to develop a vaccine against BPV had begun in 1940 decade [12]. Since then, different vaccine models were proposed in literature [13-17], however, none of them became a commercial product [5]. Currently, our group demonstrated the efficacy and biosafety of BPV-1 L1 capsomers and virus-like particles (VLPs) produced in Escherichia coli as a candidate for prophylactic vaccine [18]. In contrast to prophylactic vaccines, which stimulates the neutralizing antibodies production, therapeutic vaccines are used to stimulate the cellular immune response by the activation of antigen presenting cells (APCs), CD4+ helper and CD8+ cytotoxic T-lymphocytes (CTLs) [19-21]. For this reason, therapeutic vaccines can eliminate the infection and prevent the cancer progression [22]. In this sense, studies based on HPV have been demonstrated that E6 protein is a useful candidate as antigen for therapeutic vaccines (Table 1).
| Vaccines models | Adjuvant | Reference |
|---|---|---|
| - recombinant vaccinia virus expressing E6 and E7 proteins of HPV-16 and 18 (TA-HPV) | - | Borysiewwicz et al. [68] |
| - recombinant vaccinia virus expressing either HPV-16 E6 and E7 proteins | Meneguzzi et al. [69] | |
| He et al. [70] | ||
| - long peptide of HPV-16 E6 and E7 proteins | Montanide ISA-51 | Welters et al. [71] |
| - long peptide of HPV-16 E6 alone and combined with E7 | Kenter et al. [72] | |
| - vaccination with 9-amino acid of HPV-16 E7 protein | IFA | Muderspach et al. [73] |
| - E6 recombinant protein | - | Lin et al. [74] |
| - HPV L2, E6 and E7 single fusion protein (TA-CIN) | - | Jons et al. [75] |
| - HPV-16 E6, E7 and L2 fusion protein (TA-CIN) | Saponin GPI-0100 | Peng et al. [32] |
| - DNA vaccine encoding calreticulin linked to E6 protein (CRT/E6) | Peng et al. [76] | |
| - DNA vaccine targeting HPV-16 and 18 E6 and E7 proteins (VGX-3100) | Trimble et al. [77] | |
| Morrow et al. [78] | ||
| - DNA vaccine encoding HPV-16 E6/E7 fusion antigen | Yan et al. [79] | |
| - DNA vaccine encoding a single chair trimer of MHC-I linked to HPV-16 E6 | Huang et al. [80] | |
| - HPV-16 uploaded in engineered exomes | ISCOMATRIX | Manfredi et al. [59] |
Table 1: Vaccines based on HPV E6 protein. Previous studies employing the HPV E6 protein as antigen for therapeutic vaccine.
Based on this data, our group cloned, expressed and purified the BPV-1 E6 protein as candidate to therapeutic vaccine [14]. However, we verified that the BPV-1 E6 protein alone is able to induce DNA damages, neosis [23] and oxidative stress in BPV-free epithelial cells [24]. These data put in check the biosafety of the BPV-1 E6 protein as antigen. Despite that, the capability to eradicate the viral infection, as well as the antigenicity of the protein makes it an important therapeutic vaccine candidate. For this reason, to explore vaccine formulations, evaluating the use of different adjuvants, can be an alternative to improve the biosafety of final products.
Different adjuvants were developed, including emulsions, mineral salt, surfactants, bacterial-derived adjuvants, liposomes, polymeric microspheres and cytokines [25]. Despite this, few adjuvants are used in vaccine formulations due to their toxicity, that can lead to unwanted reactions [26-28]. These reactions can be occur at local level, resulting in pain, inflammation, necrosis, swelling, granuloma, ulcer and sterile abscess formation or, at systemic levels, leading to nausea, fever, uveitis, eosinophilia, allergy, anaphylaxis, adjuvant arthritis and organ-specific toxicity [25].
Ideally, adjuvants must be safety, stable, biodegradable, immunologically inert and able to produce an appropriated immune response [26,28]. On the one hand the choice of adjuvant can be a challenge for the vaccine success [29], on the other hand, it reduces the antigen quantity and can improve the biosafety of final formulation. For this reason, the discovery of novel adjuvants has aroused a great research interest in vaccinology.
In this context, the saponins (Quil A and QS-21) and semi-synthetic triterpene glycosides from naturally occurring saponins (GPI-0100) are promising adjuvant candidates, once they can elicit a Type 1 helper T cell response for those diseases in which a cytotoxic T lymphocyte (CTL) response is desired [30-34]. Beside this, the antioxidant property [35-37] make the saponins useful candidate as adjuvant, especially for BPV-1 E6 protein-based vaccines, which antigen (E6 protein) has a oxidant action [24,38,39].
Saponins can be obtained from many different plant species, including Agave sisalana Perrine, popularly known as sisal [40,41]. Currently, Brazil is the world’s largest producer of A. sisalana for the supply of the sisal fiber [41]. However, only four percent of sisal leaves are used to fiber production [42]. The excessive waste of material (mucilage and sisal juice), which comprises 95% of plant byproduct, are generally discarded in the soil [42-44], making the sisal juice an unexplored source of biomolecules with pharmacological interest, including saponins.
However, natural products are not necessary safe [44-47]. Therefore, the safety and efficacy should be evaluated to ensure the best conditions of use [44]. Among the tests recommended to determine the biosafety of drugs candidate to be licensed are the micronucleus test and comet assay [45]. Although commonly used due its high statistical potential, the micronucleus test is less sensitivity than comet assay [45,48,49]. For this reason, this study analyzed the mutagenic potential of BPV- 1 E6 protein-based therapeutic vaccines using different adjuvants (aluminum hydroxide, complete and incomplete Freund’s adjuvant, ethanolic and acid hydrolysis extracts obtained from A. sisalana) by the micronucleus test, comet and histone γ-H2AX.
Material and Methods
BPV-1 E6 recombinant oncoprotein expression and purification
BPV-1 E6 recombinant oncoprotein was expressed in Escherichia coli BL21, according to Mazzuchelli-de-Souza et al. [14]. The oncoprotein was subjected to dialysis to remove urea and imidazole, used during the BPV-1 E6 recombinant oncoprotein production. Dialysis was performed using Slide-A-Lyzer Dialysis Cassette (3K-12 ml) (Thermo Scientific, Carlsbad, USA). For this step, 10 mL of purified protein were dialyzed against two liters of dialysis buffer (20 mM Tris- HCl and 500 mM NaCl, pH 8.0) for eight hours at 4°C under constant agitation. The BPV-1 E6 recombinant oncoprotein identity was verified by Western blot and mass spectrometry. Results of these analyses confirmed the protein identity, as reported by Araldi et al. [23].
In silico prediction of BPV-1 E6 protein antigenicity based on bovine MHC-1
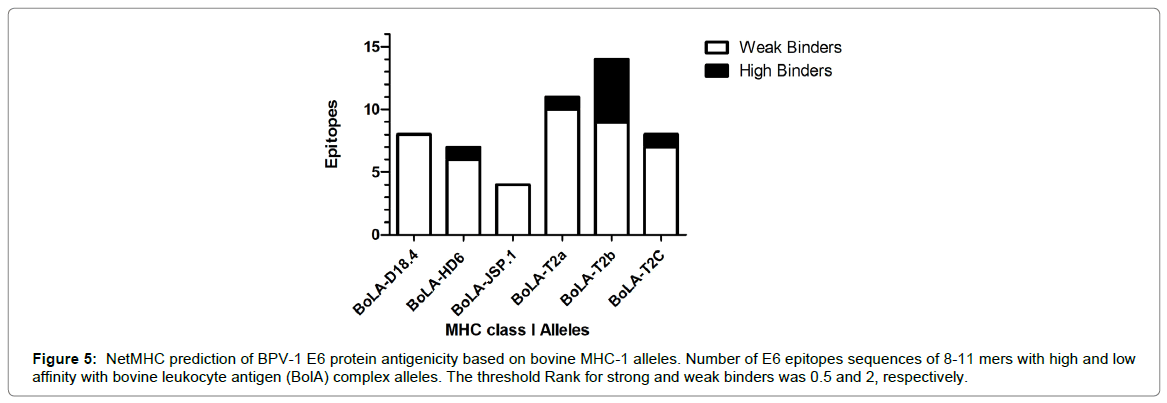
The BPV-1 E6 recombinant oncoprotein antigenicity was pre-dicted by in silico analysis. For this, the epitopes for bovine major histocompatibility class I (MHC-I) alleles of BPV-1 E6 protein sequence (MDLKPFARTNPFSGLDCLWCREPLSEVDAFRCMVKDFHVVIREGCRYGACTTCLENCLATERRLWQGVPVTGEEAELLHGKTLDRLCIRCCYCGGKLTKNEKHRHVLFNEPFCKTRANIIRGRCY DCCRHGSRSKYP), available in Mazzuchelli-de-Souza et al. [14], were identified using the software NetMHC 4.0 Server (http://www.cbs.dtu.dk/services/NetMHC/). In this analysis, it was identified peptides sequences with 8-11 mers for bovine leukocyte antigen (BolA) complex (BoLA-D18.4, BoLA-HD6, BoLA-JSP.1, BoLA-T2a, BoLAT2b and BoLA-T2c). The threshold Rank for strong and weak binders was 0.5 and 2, respectively. The GraphPad Prism 5 (GraphPad Software Inc., USA) software was used for graphical visualization of the results.
Obtaining the sisal extracts used as adjuvant
The two saponin-rich extracts used as adjuvant were obtained from Agave sisalana Perrine. Fresh leaves were collected on a sisal farm located in Valente, in the state of Bahia (Brazil). Leaves were dried at 50ºC using electric drier and crushed with the aid of a mechanical grinder to the powder form. For the ethanolic extract (EEAS) obtaining, 10 g of A. sisalana powder form were subjected to static maceration with 100 mL of ethanol 70ºC for seven days in dark room. The product of this maceration was filtered and liofilized (EEAS). The acid hydrolysis extract of A. sisalana (AHEAS) was prepared according to Duender et al. [40]. In details: the sisal juice was heated at 100ºC for ten times to increase its concentration. The obtained product was hydrolyzed with 2N HCl for four hours, under agitation. The precipitated was separated from acid solution by filtration at room temperature. Both EEAS and AHEAS were kept at 4ºC until use.
Determination of A. sisalana dried extract saponin concentration
The saponin concentration was determined by spectrophotometric analysis, according to the literature [50,51]. The A. sisalana in powder form was dissolved in distilled water at three concentrations: 0.25, 0.35 and 0.50 mg/mL. An aliquot of 1.0 mL of each solution was incubated for 20 min with 1.0 mL of 0.2% cobalt chloride chromogenic reagent and 1.0 mL concentrated sulphuric acid. These solutions were analysed in UV-Vis spectrophotometer at 284 nm. A solution of commercial saponin (Merck, Germany) at 0.2 mg/mL was used as control. The saponin concentration was expressed in: 1) mg/mL for saponins in extract solution and 2) mg/g for saponins in dried extract. These concentrations were obtained from the linear regression curve with the control (saponin, Merck).
Adjuvants and vaccine formulations
The aluminum hydroxide (Alum), complete and incomplete Freund’s adjuvants (CFA and IFA, respectively) were obtained from Sigma (Germany). The A. sisalana extracts EEAS and AHEAS were diluted in PBS with 2% Tween 80 (Sigma, Germany) at a final concentration of 50 μg/mL. The cytotoxic and mutagenic potential of these adjuvants were analyzed individually, as well as in association with the antigen (dialyzed BPV-1 E6 recombinant oncoprotein) at a final concentration of 1.0 μg/mL, as proposed by Araldi et al. [23]. The different formulations tested are showed in Table 2.
| Drugs tested | Concentration | Abbreviation |
|---|---|---|
| Negative control | - | Control |
| A. Sisalan extracts diluent (PBS + 2% Tween 80) | 5 µL/mL | T80 |
| Positive control (cyclophosphamide)1 | 50 µg/mL | C+ |
| Positive control (H2O2)2 | 100 µM | H2O2 |
| BPV-1 E6 recombinant protein | 1 µg/mL | E6 |
| Aluminium hydroxyde | 5 µL/mL | Alum |
| Complete Freund’s adjuvant | 5 µL/mL | CFA |
| Incomplete Freund’s adjuvant | 50 µg/mL | IFA |
| Ethanolic extract of A. sisalana* | 50 µg/mL | EEAS |
| Acid hydrolysis extract of A. sisalana* | 50 µg/mL | AHEAS |
| BPV-1 E6 recombinant protein + aluminium hydroxyde | 1 µg/mL + 50 µg/mL | E6 + Alum |
| BPV-1 E6 recombinant protein + complete Freund’s adjuvant | 1 µg/mL + 50 µg/mL | E6 + CFA |
| BPV-1 E6 recombinant protein + incomplete Freund’s adjuvant | 1 µg/mL + 50 µg/mL | E6 + IFA |
| BPV-1 E6 recombinant protein + ethanolic extract of A. sisalana* | 1 µg/mL + 50 µg/mL | E6 + EEAS |
| BPV-1 E6 recombinant protein + acid hydrolysis extract of A. sisalana* | 1 µg/mL + 50 µg/mL | E6 + EHEAS |
1Positive control employed in mutagenic analyses (micronucleus test and histone γH2AX)
2Positive control employed in ROS production analysis using the DCF-DA assay
*diluted in PBS + 2% Tween 80.
Table 2: Vaccine formulations analyzed. Description of controls and experimental groups, treated with antigen, adjuvants and vaccine formulations.
Cell culture
Both cytotoxic and mutagenic tests were performed in vitro, using the CRIB cell line, which was already used to determinate the mutagenic potential of BPV-1 E6 recombinant oncoprotein [23]. CRIB cells were extended in cultures flasks of 25 cm2, containing 5.0 mL of complete medium (Eagle’s minimal essential medium-MEM, supplemented with 10% of fetal bovine serum) (Cultilab, Brazil) as proposed by Flores and Donis [52]. All analysis was performed without antibiotic supplementation, since the antibiotics can lead to DNA damages, resulting in false-positive results [53].
Annexin V-PI assay
The annexin V-PI assay was used to analyze the cytotoxic potential of antigen, adjuvants and vaccine formulations. Cells were expanded in culture flasks of 25 cm2 with 5.0 mL of complete medium until a confluence of 70%. Cells were subjected to different treatments (Table 2) for 48 h at 37ºC, time necessary for two duplication rounds. After, the medium was transferred for Falcon tubes of 15.0 mL and the cells and incubated with 2.0 mL of trypsin/EDTA solution (Cultilab, Brazil) for five minutes at 37ºC to promote the monolayer disaggregation. Cell suspension was transferred to Falcon tube containing the medium removed and centrifuged at 200 x g for five minutes, discarding the supernatant. The pellet was homogenized with 1.0 mL of cold sterile PBS and transferred for polypropylene tube of 1.5 mL. Cells were centrifuged at 200 x g for five minutes, discarding the supernatant. Cells were homogenized with 100 μL of binding buffer and incubated with 5.0 μL of Annexin V-FITC and 5.0 μL of propidium iodide (PI) (Quatro G, Brazil) for 15 min at room temperature. Cells were homogenized with 100 μL of binding buffer and centrifuged at 200 x g for five minutes, discarding the supernatant. Cells were homogenized with 100 μL of cold binding buffer and analyzed in BD Accuri C6 flow cytometry (BD Biosciences, USA), using the channels FL1 (Annexin V-FITC) and FL3 (PI). Analyses were performed in triplicate, being analyzed 10,000 events/analyses. Data were analyzed through dot plot using the BD Accuri C6 software (BD Biosciences, USA).
ROS detection by DCF-DA assay
Considering that previous study showed that BPV-1 E6 promotes the oxidative stress [24], the reactive oxygen species (ROS) generation was determined using the dichlorofluorescein diacetate (DCF-DA) (Sigma, Germany), according to the de-Sá-Júnior et al. [46]. CRIB cells were expanded in culture flasks of 25 cm2 with 5.0 mL of complete medium until a confluence of 80%. The medium was discarded by inversion and the cells were washed with 2.0 mL of PBS sterile at 37ºC to remove death cells. A volume of 5.0 mL of complete medium was transferred to the culture flasks with 100 mM of DCF-DA diluted in dimethylsulfoxide (DMSO) (Merck, Germany). Cells were incubated at 37ºC at 5% CO2 for 30 min. Cells were washed with 2.0 mL of PBS sterile at 37ºC. A volume of 5.0 mL of complete medium was transferred to the culture flasks and subjected to different treatments (Table 2) for 24 min at 37ºC at 5% CO2. The monolayer was disaggregated using 2.0 mL of trypsin/EDTA solution (Cultilab, Brazil). The cell pellet was homogenized with 1.0 mL of cold sterile PBS and transferred for polypropylene tube of 1.5 mL. Cells were centrifuged at 200 x g for five minutes and homogenized in 400 μL of PBS. As positive control, it was employed 100 mM of hydrogen peroxide. Cells were analyzed in in BD Accuri C6 flow cytometry (BD Biosciences, USA), using the FL1 channel in a total of 10,000 events. This analysis was performed in triplicate.
Micronucleus test
To verify the genotoxic potential of antigen, adjuvants and vaccine formulations (Table 2), it was performed the micronucleus test. A total of 1 x 105 CRIB cell was transferred to six-well plate, containing a sterile coverslip of 24 x 24 mm with 2 mL of MEM medium, supplemented with 10% of fetal bovine serum. After one hour, cultures were subjected to different treatments (Table 2), remaining incubated for 48 h, time necessary to two replication cycles. After this time, medium was removed, and the cells were washed three times with PBS for five minutes and fixed with methanol for 30 min. Cells were stained with solution 1:4 Giemsa-PBS for 3 min and, after, washed twice with PBS. Coverslips containing the biological material were mounted on slides using Entellan (Merck, Germany). Slides were analyzed in Axiophot binocular microscope (Carl Zeiss, Germany) to observe the frequency of micronucleated cells in a total of 1,000 cells, according to Araldi et al. [53]. As negative control, it was employed cell without any drug and, as positive control, cells were treated with 50 μg/mL of cyclophosphamide (Sigma, Germany).
Histone γ-H2AX assay
The histone H2AX variant is unique in eukaryotes due to its carboxyl tail, that include a high conserved sequence, comprised by one serine residue at position 139, which is phosphorylated in the presence of DNA double strand-breaks (DSBs) [54]. The histone H2AX (p Ser139) is also known as γ-H2AX [54]. Thus, the immunodetection of this histone is recognized as a hallmark of DSBs and, therefore, clastogenesis [55-57]. Currently studies show that the histone γ-H2AX assay is 100 times more sensitivity than comet assay [54,58]. For this reason, this method was additionally performed to evaluate the clastogenic potential of therapeutic vaccines.
CRIB cells were expanded in culture flasks of 25 cm2 with 5 mL of complete medium until a confluence of 60-70%. Cells were incubated with different tested drugs (Table 2) for 24 h at 37ºC. Cells were subjected to monolayer disaggregation with 2.0 mL of trypsin/ EDTA solution (Cultilab, Brazil). Cells were transferred to 1.5 mL polypropylene tubes and fixed in 1.0 mL of 1.0% formalin solution at 4ºC for 2 h. Cell suspension was centrifuged and washed twice with 1.0 mL of PBS at 4ºC to remove the formalin residues. Cell were incubated with 1.0% BSA at 4ºC for 20 min, washed with 200 μL of PBS, and incubated overnight at 4ºC with the polyclonal anti histone γ-H2AX (p Ser139) antibody produced in rabbit (Novusbio, USA) at a dilution of 1:200 in PBS with 0.01% Triton X-100 (Sigma, Germany). Cells were centrifuged under described conditions, washed twice with PBS and incubated at 4ºC for two hours with anti-rabbit conjugated with Alexa Fluor 488 secondary antibody (Invitrogen, USA) at 1:2000 dilution in 1% BSA. Next, cells were washed with PBS, centrifuged and homogenized in 100 μL of PBS. Cells were analyzed in BD Accuri C6 cytometer (BD Bioscience, USA), employing the FL1 channel. A total of 10,000 events were analyzed. This analyzis was performed in triplicated. Results were analyzed in FlowJo software (TreeStar, USA), using the mean of percentage of immune-labeled cells. CRIB cells incubated exclusively with secondary antibody and non-incubated with any primary, neither secondary antibodies were used as controls.
Statistical analyses
Statistical analyses of annexin V-PI, DCF-DA and histone γ-H2AX assays were performed based on ANOVA followed by Tukey’s multiple comparison tests. Statistical analyses of micronucleus test were performed by non-parametric methods, using the Friedman test followed by the Dunn post-hoc test. These analyzes were performed using the GraphPad Prism version 5 software (GraphPad Software Inc., USA), with 5% of significance level.
Results
Saponin concentration of Agave sisalana dry extract
Based on the linear regression curve (R2=0.9963) of absorbance values of spectrophotometric analysis of commercial saponin (Merck), it was obtained the following equation: A=1.984 [s]+0.0995 (A-absorbance and [s]-saponin concentration in mg/mL), which was used to determine the saponin concentration in dry extract of A. sisalana. Results of this analysis are showed a concentration of 908.4 mg saponins/g of dry extract, indicating that saponins represent 90.84% of weight of the dry extract (Table 3).
| (Concentration) mg/mL | Absorbance | (Saponin) mg/mL* | (Saponin) mg/g** |
|---|---|---|---|
| 0.25 | 0.537 | 0.22 | 882.68 |
| 0.35 | 0.737 | 0.32 | 918.01 |
| 0.5 | 1.017 | 0.46 | 924.52 |
*Results expressed in “mg” of saponins per “mL” of dry extract solutions of A. sisalana;
**results expressed in “mg” of saponins per “g” of dry extract of A. sisalana.
Table 3: Saponin concentration. Analysis of saponin content in A. sisalana.
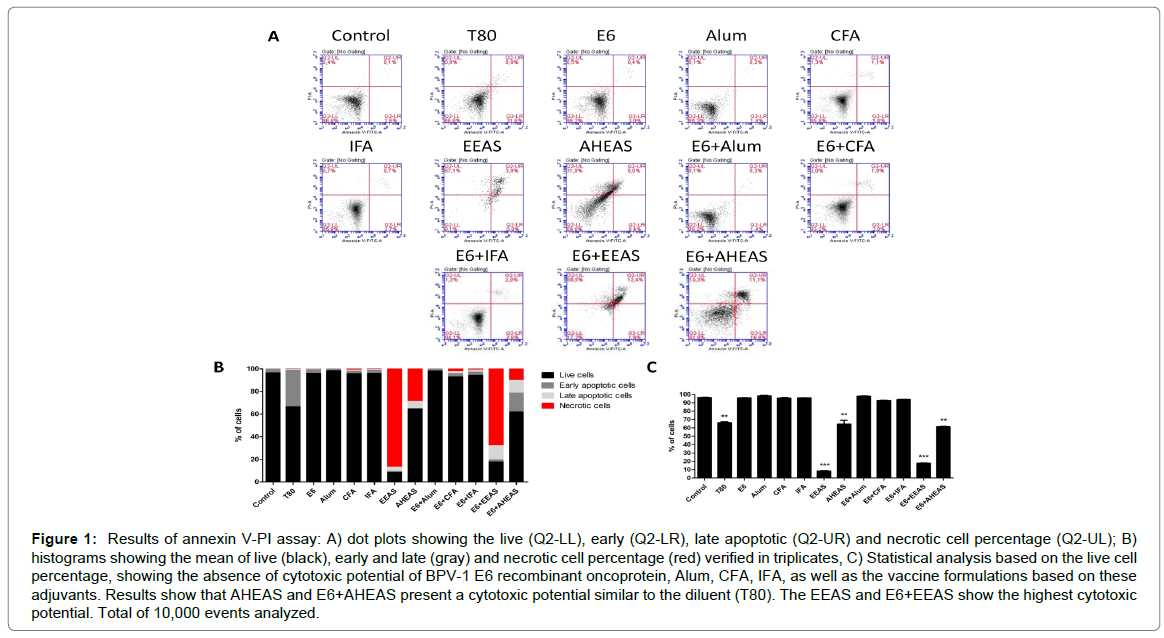
Cytotoxic potential analysis of adjuvants and vaccine formulations
Results of annexin V-PI assay (Figures 1A-C and Table 4), employed to analyze the cytotoxicity, showed significant statistical differences among the different vaccine formulations (F=1446, R2=0.9986 and p<0.0001). Based on this result, it was performed the Tukey’s multiple comparison test, which showed that both the A. sisalana extracts (EEAS and AHEAS), as well as the vaccine formulations using these extracts as adjuvants (E6+EEAS and E6+AHEAS) are cytotoxic in relation to control (Figure 1 and Table 4). A similar cytotoxicity was verified in cell treated with the A. sisalana extracts diluent (T80) (Figure 1B and Table 4). These data were reinforced by the statistical analysis based on the live cell percentage, that pointed out that the A. sisalana extracts (EEAS and AHEAS), the vaccines formulations based on these saponin-rich extracts (E6+EEAS and E6+AHEAS) and the diluent (T80) showed a cytotoxic potential (Figure 1C and Table 4). By the contrast, the Alum, CFA, IFA and the vaccine formulations based on these adjuvants did not show significant statistical differences when compared with the control (Figure 1C and Table 4), indicating that these adjuvants are safe.
| Cytotoxicity – Annexin V-PI assay | ROS production – DCF-DA assay | |||||
|---|---|---|---|---|---|---|
| Live | Early apoptotic | Late apoptotic | Necrotic | % of cells | MFI | |
| Group | ͞x ± SD | ͞x ± SD | ͞x ± SD | ͞x ± SD | ͞x ± SD | ͞x ± SD |
| Control | 96.3 ± 0.43 | 2.93 ± 0.30 | 0.20 ± 0.10 | 0.56 ± 0.15 | 31.0 ± 0.40 | 3373 ± 176.1 |
| T80 | 66.1 ± 1.01** | 32.4 ± 1.05 | 0.83 ± 0.05 | 0.60 ± 0.17 | 48.0 ± 0.40*** | 8055 ± 1563.0 |
| H2O2 | - | - | - | - | 84.1 ± 0.25*** | 26510 ± 479.9 |
| E6 | 95.7 ± 0.37 | 3.30 ± 0.26 | 0.40 ± 0.00 | 0.56 ± 0.05 | 79.3 ± 0.40*** | 9081 ± 964.3 |
| Alum | 98.1 ± 0.10 | 1.53 ± 0.11 | 0.20 ± 0.00 | 0.13 ± 0.05 | 5.63 ± 0.05*** | 3479 ± 72.5 |
| CFA | 95.5 ± 0.20 | 1.96 ± 0.20 | 1.30 ± 0.17 | 1.16 ± 0.15 | 38.2 ± 0.41*** | 5950 ± 163.6 |
| IFA | 95.7 ± 0.15 | 2.70 ± 0.10 | 0.80 ± 0.10 | 0.76 ± 0.05 | 64.7 ± 0.70*** | 4932 ± 942.2 |
| EEAS | 8.16 ± 0.11*** | 0.86 ± 0.15 | 3.90 ± 0.30 | 87.0 ± 0.55 | 1.80 ± 0.34*** | 8309 ± 424.1 |
| AHEAS | 64.3 ± 4.75** | 0.30 ± 0.10 | 6.36 ± 1.42 | 28.9 ± 3.63 | 6.23 ± 1.30*** | 8370 ± 264.3 |
| E6+Alum | 97.8 ± 0.35 | 1.80 ± 0.36 | 0.26 ± 0.05 | 0.10 ± 0.00 | 1.30 ± 0.30*** | 11830 ± 747.2 |
| E6+CFA | 92.8 ± 0.35 | 2.73 ± 0.30 | 1.76 ± 0.32 | 2.60 ± 0.52 | 64.7 ± 0.70*** | 5950 ± 163.6 |
| E6+IFA | 94.0 ± 0.10 | 2.80 ± 0.17 | 1.93 ± 0.11 | 1.26 ± 0.15 | 56.9 ± 0.20*** | 3546 ± 54.1 |
| E6+EEAS | 17.5 ± 0.30*** | 1.90 ± 0.10 | 12.5 ± 0.26 | 68.1 ± 0.55 | 8.50 ± 0.10*** | 6766 ± 118.2 |
| E6+AHEAS | 61.6 ± 0.30** | 16.8 ± 1.15 | 11.2 ± 0.28 | 10.3 ± 0.00 | 7.26 ± 0.57*** | 4145 ± 123.1 |
͞x : mean of cell percentage, SD – standard deviation, MFI – mean of fluorescence intensity.
***p
Table 4: Annexin V-PI and DCF-DA assay results. Results of annexin V-PI and DCF-DA assay.
Figure 1: Results of annexin V-PI assay: A) dot plots showing the live (Q2-LL), early (Q2-LR), late apoptotic (Q2-UR) and necrotic cell percentage (Q2-UL); B) histograms showing the mean of live (black), early and late (gray) and necrotic cell percentage (red) verified in triplicates, C) Statistical analysis based on the live cell percentage, showing the absence of cytotoxic potential of BPV-1 E6 recombinant oncoprotein, Alum, CFA, IFA, as well as the vaccine formulations based on these adjuvants. Results show that AHEAS and E6+AHEAS present a cytotoxic potential similar to the diluent (T80). The EEAS and E6+EEAS show the highest cytotoxic potential. Total of 10,000 events analyzed.
ROS production
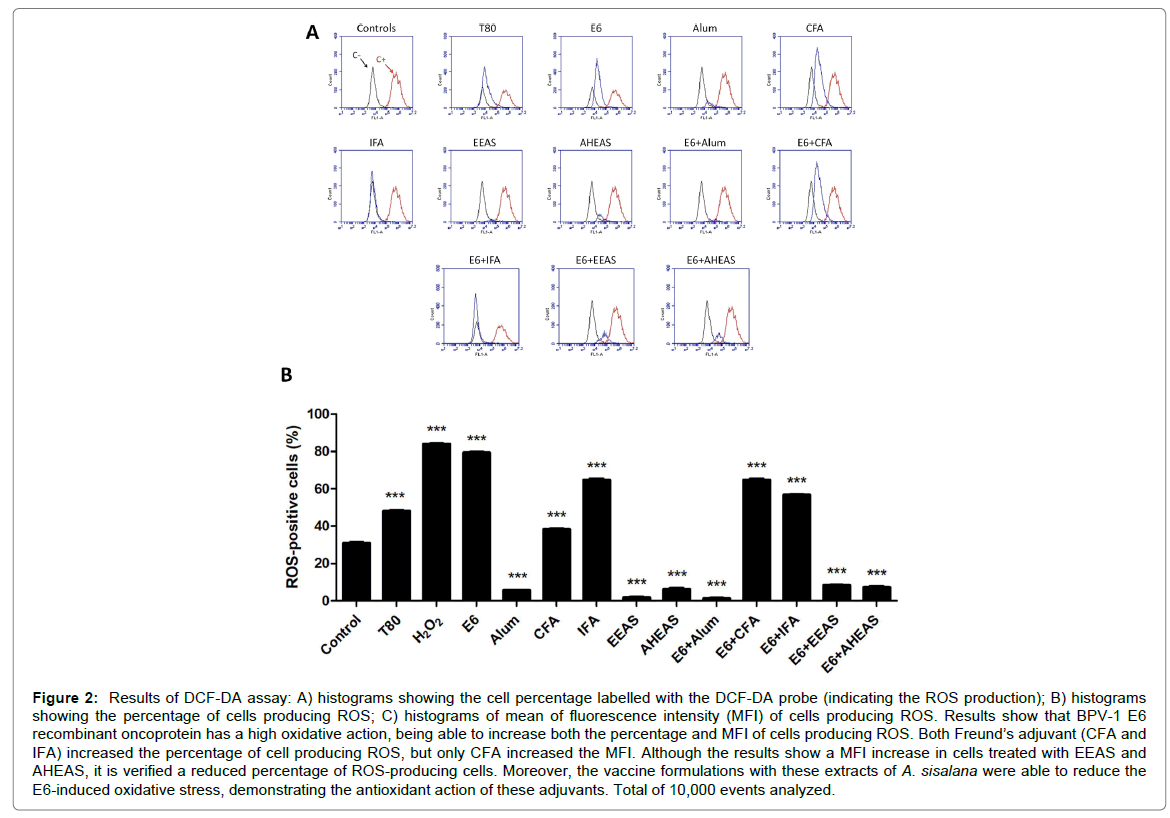
Based on the ROS-positive cell percentage (Figure 2 and Table 4) it was performed an ANOVA analysis, that pointed out significant statistical differences among the formulations tests (F=13930, R2=0.9999 and p<0.0001). For this reason, it was performed the Tukey’s multiple comparison test. Result of this test showed that the BPV-1 E6 recombinant protein, the adjuvants CFA and IFA, as well as the diluent of A. sisalana extracts T80 increased the percentage of ROS-producing cells, suggesting an oxidative potential (Figure 2A and Table 4). Similar results were also verified in vaccine formulations E6+CFA and E6+IFA (Figure 2B and Table 4). By the contrast, the Alum, EEAS and AHEAS reduced significantly the percentage of ROS-producing cells, suggesting an antioxidant potential (Figure 2B and Table 4). Similar results were also verified in vaccine formulations E6+Alum, E6+EEAS and E6+AHEAS (Figure 2B and Table 4).
Figure 2: Results of DCF-DA assay: A) histograms showing the cell percentage labelled with the DCF-DA probe (indicating the ROS production); B) histograms showing the percentage of cells producing ROS; C) histograms of mean of fluorescence intensity (MFI) of cells producing ROS. Results show that BPV-1 E6 recombinant oncoprotein has a high oxidative action, being able to increase both the percentage and MFI of cells producing ROS. Both Freund’s adjuvant (CFA and IFA) increased the percentage of cell producing ROS, but only CFA increased the MFI. Although the results show a MFI increase in cells treated with EEAS and AHEAS, it is verified a reduced percentage of ROS-producing cells. Moreover, the vaccine formulations with these extracts of A. sisalana were able to reduce the E6-induced oxidative stress, demonstrating the antioxidant action of these adjuvants. Total of 10,000 events analyzed.
Micronucleus test
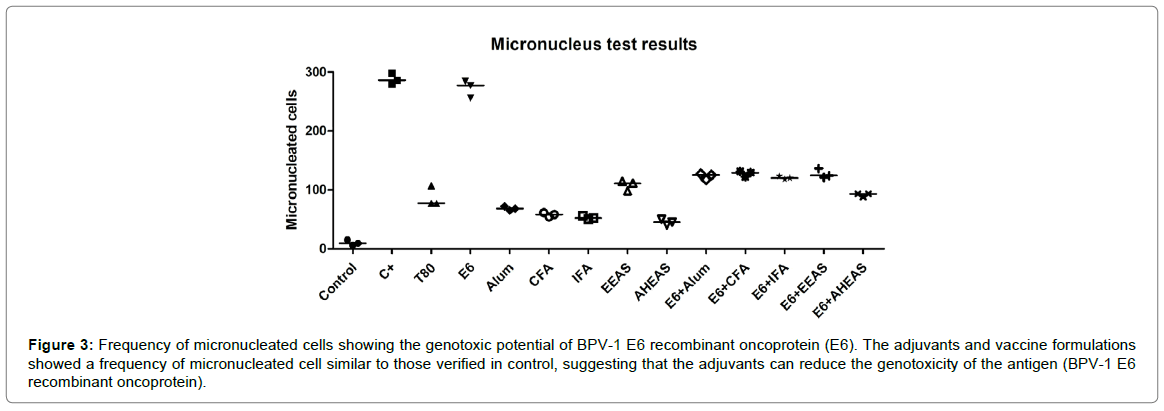
Based on the number of micro-nucleated cells (Figure 3 and Table 5), it was performed the Friedman test, which pointed out significant statistical differences among the formulations tested (Friedman statistic=38.28, p=0.0001). Considering this result, it was performed the Dunn post hoc test, that showed that BPV-1 E6 recombinant protein has a similar genotoxic potential to cyclophosphamide, employed as positive control (Figure 3 and Table 5). However, the results did not show statistical differences among the adjuvants, neither among the vaccine formulations using the BPV-1 E6 recombinant protein in relation to negative control (Figure 3 and Table 5). This data suggest that the tested adjuvants can reduce the antigen genotoxicity.
| Genotoxicity-Micronucleus test | Clastogenicity–histone γH2AX assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nomal cells | Micronucleated cells | % of cells | |||||||
| ͞x Group | 1 | 2 | 3 | Median | 1 | 2 | 3 | Median | ͞x ± SD |
| Control | 985 | 991 | 994 | 991 | 15 | 9 | 6 | 9 | 0.20 ± 0.02 |
| C+ | 702 | 714 | 721 | 714 | 298 | 286 | 279 | 286*** | 94.2 ± 0.17*** |
| T80 | 893 | 923 | 923 | 923 | 107 | 77 | 77 | 77 | 0.32 ± 0.08 |
| E6 | 728 | 744 | 716 | 728 | 277 | 256 | 284 | 256*** | 95.2 ± 0.30*** |
| Alum | 932 | 935 | 928 | 932 | 68 | 65 | 72 | 68 | 6.79 ± 0.96 |
| CFA | 942 | 946 | 939 | 942 | 58 | 54 | 61 | 58 | 93.1 ± 0.17** |
| IFA | 950 | 944 | 948 | 948 | 50 | 56 | 52 | 52 | 87.8 ± 3.14*** |
| EEAS | 902 | 889 | 886 | 889 | 98 | 111 | 114 | 111 | 1.94 ± 0.16*** |
| AHEAS | 960 | 955 | 950 | 955 | 40 | 45 | 50 | 45 | 84.0 ± 1.59*** |
| E6+Alum | 883 | 875 | 873 | 875 | 117 | 125 | 127 | 125 | 5.07 ± 0.27 |
| E6+CFA | 878 | 869 | 871 | 871 | 122 | 131 | 129 | 129 | 93.8 ± 0.20* |
| E6+IFA | 882 | 877 | 880 | 880 | 118 | 123 | 120 | 120 | 90.7 ± 0.92*** |
| E6+EEAS | 876 | 864 | 913 | 876 | 124 | 136 | 121 | 124 | 1.82 ± 0.10*** |
| E6+AHEAS | 912 | 907 | 907 | 907 | 88 | 93 | 93 | 93 | 84.3 ± 8.38*** |
***p<0.0001, **p
Table 5: Micronucleus test and histone H2AX assay. Results of mutagenic analyses (micronucleus test and histone γH2AX assay).
Figure 3: Frequency of micronucleated cells showing the genotoxic potential of BPV-1 E6 recombinant oncoprotein (E6). The adjuvants and vaccine formulations showed a frequency of micronucleated cell similar to those verified in control, suggesting that the adjuvants can reduce the genotoxicity of the antigen (BPV-1 E6 recombinant oncoprotein).
Expression levels of histone γH2AX
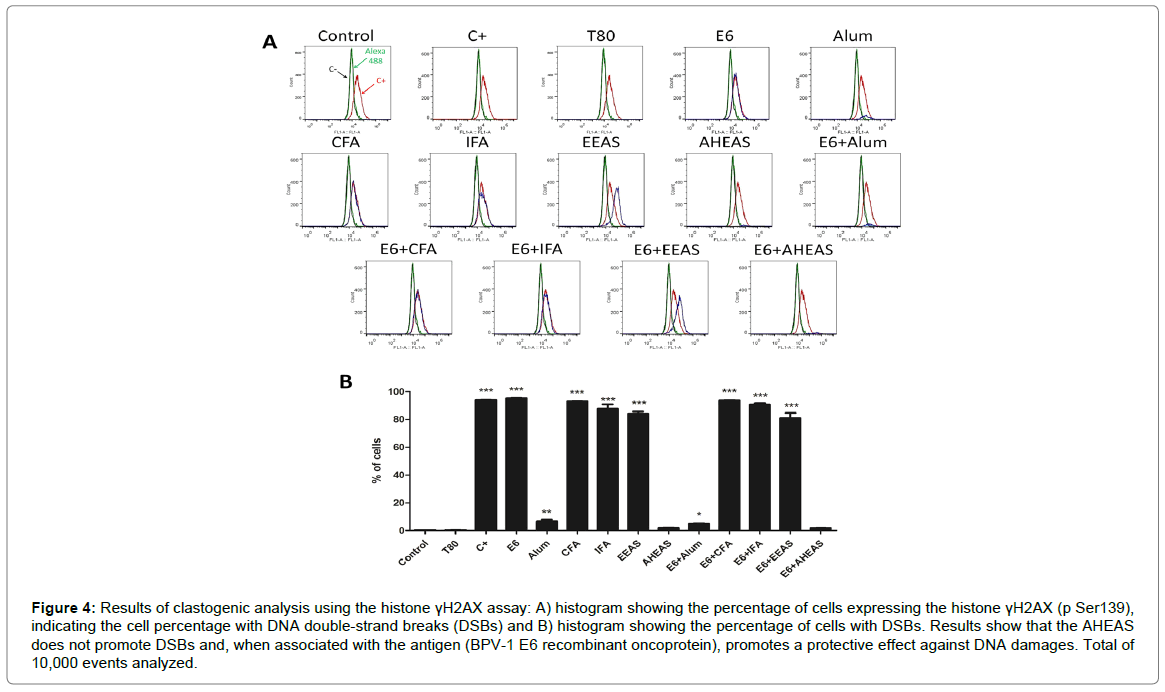
Based on the mean of histone γH2AX-expressing cell percentage (Figures 4A and 4B and Table 5), it was performed the ANOVA analysis, which results pointed out significant statistical differences among the different formulations tested (F=2876, R2=0.9993 and p<0.0001). Considering this result, it was performed the Turkey’s multiple comparison test, which results showed that BPV-1 E6 recombinant protein, CFA, IFA and EEAS, as well as the vaccine formulations based on these adjuvants (E6+CFA, E6+IFA and E6+EEAS) presented similar expression levels of histone γH2AX to those verified in positive control (cyclophosphamide), indicating the clastogenic action of these adjuvants and formulations (Figure 4 and Table 5). Cells treated with Alum and E6+Alum showed an intermediate clastogenic potential (Figure 4 and Table 5). By the opposite, the AHEAS showed expression levels of histone γH2AX similar to the negative control, indicating the absence of clastogenicity (Figure 4 and Table 5). Moreover, the AHEAS reduced the clastogenic potential of BPV-1 E6 recombinant protein) (Figure 4 and Table 5), increasing the biosafety of vaccine.
Figure 4: Results of clastogenic analysis using the histone γH2AX assay: A) histogram showing the percentage of cells expressing the histone γH2AX (p Ser139), indicating the cell percentage with DNA double-strand breaks (DSBs) and B) histogram showing the percentage of cells with DSBs. Results show that the AHEAS does not promote DSBs and, when associated with the antigen (BPV-1 E6 recombinant oncoprotein), promotes a protective effect against DNA damages. Total of 10,000 events analyzed.
Discussion
There are several techniques for the therapeutic vaccines production, but the vaccines based on E6 and E7 protein are the most promising [20]. In this sense, the E6 protein stands out for its stability and relative obtaining facility [14,59,60]. Moreover, the protein contains diverse epitopes for human leukocyte antigens (HLA - HPV E6) [61], as well as for bovine leukocyte antigens (BoLA-BPV E6) identified by in silico analysis [14]. For these reasons, since 2013 we are studying the BPV-1 recombinant protein as antigen candidate to therapeutic vaccine [14,23]. In order to confirm the antigenicity of BPV-1 E6 protein, we analyzed the protein binding-sites with bovine MHC-I alleles using in silico tools. Results of this analysis showed the antigenic potential of BPV-1 E6 recombinant oncoprotein (Figure 5), reinforcing its use as antigen for therapeutic vaccines.
However, previous analysis showed that BPV-1 E6 recombinant oncoprotein alone is able to induce DNA damages in CRIB cells and bovine lymphocytes [23,62]. We also verified that BPV-1 E6 recombinant oncoprotein promotes metabolic deregulations, resulting in ROS production [24] through a supposedly mechanism homologous to HPV-16 E6* oncoprotein [63].
Nevertheless, vaccines based on recombinant protein require the use of adjuvants to increase the immune response, reducing the antigen quantity in final vaccine formulation [26-28]. The addition of adjuvants can promote a better presentation for MHC class I, increasing the endogenous processing of the vaccine, and consequently the uptake by MHC class I, driving more efficiently to dendritic cells, which increase the presentation of MHC class I and the activation of CD8+ cells. In this context, previous study showed that saponin-based adjuvants can stimulate de CD8+ cells activation [64,65]. For this reason, the saponins emerge as additional candidate to adjuvants for E6-based protein vaccines.
Based on these data, we analyzed the cytotoxic and mutagenic potential of the most employed adjuvants (Alum, CFA and IFA) alone and combined with the BPV-1 E6 recombinant protein. Moreover, considering that the mutagenic potential BPV-1 E6 protein is related to their oxidant activity [24], we included two antioxidant saponin-rich extracts obtained from A. sisalana: EEAS and AHEAS.
The cytotoxic potential of antigen, adjuvants and vaccines was evaluated through the annexin V-PI assay. Results of this analysis pointed out that EEAS shows the highest cytotoxic potential, which was verified by the elevate number of necrotic cells (Figure 1B and Table 4). The cytotoxicity of EEAS can be attributed to the surfactant presence, that can bind to cholesterol present in cell membrane, leading to pore formation and hemolysis [27]. The hemolytic action of EEAS also explains the high percentage of necrotic cells verified in the vaccine formulation using this extract as adjuvant (E6+EEAS) (Figure 1B and Table 4). On the one side, the necrotic action of EEAS represents an unwanted effect, by the other side it suggests an unexplored antineoplastic action of ethanolic extract of A. sisalana.
Although the live cell percentage of AHEAS had showed a significant statistical difference in relation to control, it was not verified significant statistical differences between AHEAS/E6+AHEAS and the diluent (T80) (Figure 1C and Table 4). Considering that the acid hydrolysis reduces the hemolytic action of A. sisalana extract [40], the results suggest that the cytotoxicity verified in cells treated with AHEAS alone or in association with BPV-1 E6 recombinant protein can be attributed to the Tween 80, used as diluent. By the opposite, the other adjuvants analyzed (Alum, CFA and IFA) did not show cytotoxic potential in relation to control (Figure 1C and Table 4).
Although not cytotoxic, the Freund’s adjuvants (CFA and IFA) alone or combined with BPV-1 E6 recombinant oncoprotein increased the percentage of ROS-producing cells (Figure 2 and Table 4). This result is alarming, once the BPV-1 E6 recombinant protein has a prominent oxidant capability [24], which was also verified in this study (Figure 2 and Table 4). However, the Alum, EEAS and AHEAS reduced significantly the percentage of ROS-producing cells (Figure 2 and Table 4). Moreover, when combined with the antigen, these adjuvants reduced the percentage of cells labelled by the DCF-DA probe (Figure 2 and Table 4), indicating the antioxidant property of A. sisalana extracts. Interesting, the diluent of A. sisalana extracts (T80) showed to increase the ROS production in relation to control, but when added to EEAS and AHEAS, it was verified a reduction of cell percentage labelled by the probe, reinforcing the antioxidant capability of these extracts (Figure 2 and Table 4). The antioxidant activity of A. sisalana extracts can be justified by the flavonoids present in these extracts [41].
The genotoxic potential analysis showed a high frequency of micronuclei in CRIB cells treated with BPV-1 E6 recombinant protein (Figure 3), reinforcing the mutagenic potential of this protein, previous described in Araldi et al. [23]. The genotoxic activity can be attributed to the fork replication stress and the oxidant action of the BPV-1 E6 recombinant protein. The adjuvants analyzed did not show genotoxic activity when tested alone or combined with the antigen (Figure 3 and Table 5). These data suggest that the antigen genotoxic potential is directly related with the oxidant activity of BPV-1 E6 recombinant oncoprotein.
Results of flow cytometry showed that the treatment with BPV-1 E6 recombinant protein, CFA, IFA, EEAS, E6+CFA, E6+IFA and E6+EEAS promoted an expressive increase in γ-H2AX expression levels (Figure 4). Results of statistical analyses showed these treatments present a clastogenic potential similar to cyclophosphamide, an alkylating drug used as positive control (Figure 4 and Table 5). These data reinforce the antigen mutagenic potential, also verified by micronucleus test (Figure 4 and Table 5), as well as indicates the high sensitivity of the histone γ-H2AX assay to detect DSBs, allowing to identify the clastogenic action of CFA, IFA, EEAS, E6+CFA, E6+IFA and E6+EEAS, that was not verified using the micronucleus assay (Figure 4 and Table 5). Although the expression levels of γ-H2AX in cells treated with Alum and AHEAS were increased in relation to control, they are expressively reduced in relation to the other adjuvants tested (Figure 4 and Table 5). Moreover, these adjuvants were also able to reduce the mutagenic potential of the BPV-1 E6 protein (Figure 4 and Table 5), reinforcing the results of micronucleus test (Figure 3). In this sense, it was not verified significant statistical differences between AHEAS and E6+AHEAS and control (Figure 4 and Table 5). The antioxidant mechanisms involve direct inhibition of the ROS generation or the scavenging of the free radical. According to Dini et al. [66], the antioxidant mechanisms of saponins involves both antiradical and reducing property, which is related to the metal ion chelating activity [67].
In summary, the results suggest that acid hydrolysis extract from A. sisalana (AHEAS) is a useful candidate as adjuvant for BPV E6 recombinant protein-based therapeutic vaccine, being able to increase the vaccine biosafety. Considering the functional homologies among the BPV and HPV E6 protein, and the antigenicity of these proteins, our results indicate that AHEAS could be a useful adjuvant candidate for human vaccines against HPV. In addition, the antinociceptive and anti-inflammatory action of AHEAS [40], could also reduce the eventual local reactions related to the cytotoxicity. Moreover, considering that sisal juice is rich is saponin, but it is discarded as a residue of sisal fibre industry, to use this sisal juice as source of adjuvants emerges as an ecological and cheaper alternative.
Ethical Statement
This study was approved by Ethical Committee of Butantan Institute (Permission number 1319/14).
Acknowledgments
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant numbers 2014/20617-5 and 2016/09450-7).
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Wilson-Welder J, Torres M, Kipper M, Mallapragada S, Wannemuehler M, et al. (2011) Hydrotropy: A promising tool for solubility enhancement: A review. Int J Drug Dev Res 3: 26-33.
- Pulendran B, Li S, Nakaya H (2010) Systems vaccinology. Immunity 33: 516-29.
- Meeusen E, Walker J, Peters A, Pastoret P, Jungersen G (2007) Current status of veterinary vaccines. Clin Microbiol Rev 20: 489–510.
- Munday J (2014) Bovine and human papillomaviruses: A comparative review. Vet Pathol 1–13.
- Araldi RP, Assaf SMR, Carvalho RF, Carvalho MAC, Mazzuchelli-de-Souza J, et al. (2017) Papillomaviruses: a systematic review. Genet Mol Biol 41: 1-21.
- Stocco dos SRC, Lindsey CJ, Ferraz OP, Pinto JR, Mirandola RS, et al. (1998) Bovine papillomavirus transmission and chromosomal aberrations: an experimental model. J Gen Virol 79: 2127–35.
- Araldi RP, Melo T, Diniz NDP, Carvalho RF, Beçak W, et al. (2013) Bovine papillomavirus clastogenic effect analyzed in comet assay. Biomed Res Int 1–7.
- Silva MAR, Albuquerque BMF, Pontes NE, Coutinho LCA, Leitão NCG, et al. (2013) Detection and expression of bovine papillomavirus in blood of healthy and papillomatosis-affected cattle. Genet Mol Res 12: 3150–56.
- Muro L, Botteira C, Piccinin A (2008) Papilomatose bovina. Rev. CientÃfica Eletrônica Med. Veterinária.
- Leto M, Santos-Júnior G, Porro A, Tomimori J (2011) Human papillomavirus infection : etiopathogenesis, molecular biology and clinical manifestations. An Bras Dermatologia 86: 306–317.
- William J, Kirubaharan J, Uthumann K (1992) Survey on incidence and complications of bovine cutaneous papillomatosis Indian J Vet 69: 842–4.
- Shope R (1937) Immunization of rabbits to infectious papillomatosis. J Exp Med 65: 219–231.
- Love A, Chapman S, Matic S, Noris E, Lomonossoff G, et al. (2012) In planta production of a candidate vaccine against bovine papillomavirus type 1. Planta 236: 1305–13.
- Mazzuchelli-de-Souza J, Carvalho RF, Ruiz R, Melo TC, Araldi RP, et al. (2013) Expression and in silico analysis of the recombinant bovine papillomavirus E6 protein as a model for viral oncoproteins studies. Biomed Res Int 2013: 421398.
- Góes L, Freitas AC, Ferraz O, Rieger T, Santosa J, et al. (2008) Bovine papillomavirus type 4 L1 gene transfection in a Drosophila S2 cell expression system: absence of L1 protein expression. Brazilian J Microbiol 39: 1–4.
- Gaukroger JM, Chandrachud LM, O’Neil BW, Grindlay GJ, Knowles G, et al. (1996) Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J Gen Virol 77: 1577–1583.
- Jarret W, Smith K, O’Neil B, Gaukroger J, Chandrachud L, et al. (1991) Studies on vaccination against Papillomavirus: prophylactic and therapeutic vaccination with recombinant structural proteins Virology 184: 2–42.
- Módolo DG, Araldi RP, Mazzuchelli-de-Souza J, Pereira A, Pimenta DC, et al. (2017) Integrated analysis of recombinant BPV-1 L1 protein for the production of a bovine papillomavirus VLP vaccine. Vaccine 35: 1590–1593.
- Yang A, Jeang J, Cheng K, Cheng T, Yang B, et al. (2016) Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 15: 989–1007.
- Yang A, Farmer E, Wu TC, Hung CF (2016) Perspectives for therapeutic HPV vaccine development. J Biomed Sci 23: 75.
- Van Der BSH (2008) Therapeutic Vaccines In Cancer: Moving From Immunomonitoring To Immunoguiding. Expert Rev Vaccines 7: 1–5.
- Hoppe-Seyler X, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F (2017) The HPV E6/E7 oncogenes: Key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol 1–11.
- Araldi RP, Mazzuchelli-de-Souza J, Modolo DG, Souza EB, Melo TC, et al. (2015) Mutagenic potential of Bos taurus papillomavirus type 1 E6 recombinant protein : First description. Biomed Res Int 2015.
- Araldi RP, De-Sá-Júnior P, Magnelli RF, Modolo DG, Mazzuchelli-de-Souza J, et al. (2016) Primary cultures derived from bovine papillomavirus-infected lesions as model to study metabolic deregulation. J Cancer Res Ther Oncol 4: 1–18.
- Petrovsky N, Aguilar J (2004) Vaccine adjuvants: Current state and future trends. Immunol Cell Biol 82: 488–496.
- Reed S, Bertholet S, Coler R, Friede M (2009) New horizons in adjuvants for vaccine development. Trends Immunol 30: 23–32.
- Singh M, O’Hagan D (1995) Advances in vaccine adjuvants. Nat Biotechnol 17: 1075–81.
- Aguilar J, RodrÃguez E (2007) Vaccine adjuvants revisited. Vaccine 25: 3752–3762.
- Fernández-Tejada A, Tan D, Gin D (2016) Development of improved vaccine adjuvants based on the saponin natural product QS-21 through chemical synthesis. Acc Chem Res 49: 1741–1756.
- Liu H, Vries-Idema J, Veer W, Wilschut J, Huckriede A (2014) Influenza virosomes supplemented with GPI-0100 adjuvant: a potent vaccine formulation for antigen dose sparing. Med Microbiol Immunol 203: 47–55.
- Liu H, Bungener L, Veer J, Coller BA, Wilschut J, et al. (2011) Preclinical evaluation of the saponin derivative GPI-0100 as an immunostimulating and dose-sparing adjuvant for pandemic influenza vaccines. Vaccine 29: 2037–2043.
- Peng S, Wang J, Karanam B, Wang C, Huh W, et al. (2015) Sequential cisplatin therapy and vaccination with HPV16 E6E7L2 fusion protein in saponin adjuvant GPI-0100 for the treatment of a model HPV16+ cancer. PLoS One 10: e116389.
- Sun HX, Xie Y, Ye YP (2009) Advances in saponin-based adjuvants. Vaccine 27: 1787–1796.
- Liu H, Patil HP, Vries-Idema J, Wilschut J, Huckriede A (2012) Enhancement of the immunogenicity and protective efficacy of a mucosal influenza subunit vaccine by the saponin adjuvant GPI-0100. PLoS One 7: e52135.
- Cruz MS, Cabral-Barroso S, Navoni JA, Rocha-Silva-Teles JA, Barbosa-Filho JM, et al. (2016) Effect of hecogenin on DNA instability. Toxicol Reports 3: 539–543.
- Ashraf MF, Aziz MA, Stanslas J, Ismail I, Kadir MA (2013) Assessment of antioxidant and cytotoxicity activities of saponin and crude extracts of chlorophytum borivilianum. Sci World J 2013.
- Chen Y, Miao Y, Huang L, Li J, Sun H, et al. (2014) Antioxidant activities of saponins extracted from Radix Trichosanthis: an in vivo and in vitro evaluation. BMC Complement Altern Med 14: 86.
- Araldi RP, Módolo DG, De-Sá-Júnior P, Consonni SR, Carvalho RF, et al. (2016) Genetics and metabolic deregulation following cancer initiation: A world to explore. Biomed Pharmacother 82: 449–458.
- Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 6: 45–57.
- Dunder R, Quaglio A, Maciel R, Luiz-Ferreira A, Almeida A, et al. (2010) Anti-inflammatory and analgesic potential of hydrolyzed extract of Agave sisalana Perrine ex Engelm Asparagaceae. Rev Bras Farmacogn 20: 376–381.
- Hammuel C, Yebpella GG, Shallangwa GA, Magomya AM, Agbaji AS (2011) Phytochemical and antimicrobial screening of methanol and aqueous extracts of Agave sisalana. Acta Pol Pharm Drug Res 68: 535–539.
- Santos JDG, Branco A (2009) Antimicrobial activity of Agave sisalana. African J Biotechnol 8: 6181–6184.
- Botura MB, Silva GD, Lima HD, Oliveira JVA, Souza TS, et al. (2011) In vivo anthelmintic activity of an aqueous extract from sisal waste (Agave sisalana Perr.) against gastrointestinal nematodes in goats. Vet Parasitol 177: 104–110.
- Barreto SMAG, Maia MS, Benicá AM, Assis HRBS, Leite-Silva VR, et al. (2017) Evaluation of in vitro and in vivo safety of the by-product of Agave sisalana as a new cosmetic raw material: Development and clinical evaluation of a nanoemulsion to improve skin moisturizing. Ind Crops Prod 108: 470–479.
- Araldi RP, Melo TC, Mendes TB, Sá Júnior PL, Nozima BHN, et al. (2015) Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed Pharmacother 72: 74–82.
- Sá-Júnior PL, Câmara DAD, Costa AS, Ruiz JLM, Levy D, et al. (2016) Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine 23: 725–735.
- Sawant S, Fielden M, Black K (2014) Evaluation of genotoxicity testing of FDA approved large molecule therapeutics. Regul Toxicol Pharmacol 70: 87–97.
- Araldi RP, Oliveira D, Silva D, Mendes TB, Souza EB (2013) Análise do potencial mutagênico dos esteróides anabólicos androgênicos (EAA) e da L-Carnitina mediatnte o teste do micronúcleo em eritrócitos policromáticos. Rev Bras Med Do Esporte 19: 448–451.
- Araldi RP, Rechiutti B, Mendes TB, Ito ET, Souza EB (2014) Mutagenic potential of Cordia ecalyculata alone and in association with Spirulina maxima for their evaluation as candidate anti-obesity drugs. Genet Mol Res 13: 5207–20.
- Clark PW, Armentano LE (1993) Effectiveness of neutral detergent fiber in whole cottonseed and dried distillers grains compared with Alfalfa haylage. J Dairy Sci 76: 2644–2650.
- Vigo CLS, Narita E, Marque LC (2004) Influências da variação sazonal e tipos de secagem nas caracterÃsticas da droga vegetal - raÃzes de Pfaffia glomerata (Spreng.) Pedersen (Amaranthaceae). Rev Bras Farmacogn 14: 137–144.
- Flores E, Donins R (1995) Isolation and characterization of a bovine cell line resistant to infection with the pestivirus bovine viral diarrhea virus (BVDV). Virology 208: 565–575.
- Araldi RP, Melo TC, Mendes TB, De-Sá-Júnior P, Nozima BN, et al. (2015) Using the comet and micronucleus assays for genotoxicity studies: A review Biomed Pharmacother 72: 74–82.
- Kuo LJ, Yang LX (2008) Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo 22: 305–59.
- Sharma A, Singh K, Almasan A (2012) Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol Biol 920: 613–626.
- Sedelnikova O, Pilch D (2003) Histone H2AX in DNA damage and repair. Cancer Biol Ther 2: 233–235.
- Ji J, Zhang Y, Redon CE, Reinhold WC, Chen AP, et al. (2017) Phosphorylated fraction of H2AX as a measurement for DNA damage in cancer cells and potential applications of a novel assay. PLoS One 12: 1–18
- Clingen PH, Wu JYH, Miller J, Mistry N, Chin F, et al. (2008) Histone H2AX phosphorylation as a molecular pharmacological marker for DNA interstrand crosslink cancer chemotherapy. Biochem Pharmacol 76: 19–27.
- Manfredi F, Bonito P, Ridolfi B, Anticoli S, Arenaccio C, et al. (2016) The CD8(+) T Cell-Mediated Immunity Induced by HPV-E6 Uploaded in Engineered Exosomes Is Improved by ISCOMATRIXTM Adjuvant Vaccines 4: 42.
- Boulet G, Horvath C, Vanden B, Sahebali S, Bogers F (2007) Human papillomavirus: E6 and E7 oncogenes. Int J Biochem Cell Biol 39: 2006–11.
- Lee SJ, Yang A, Wu TC, Hung CF (2016) Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 27.
- Araldi RP (2015), Bovine papillomavirus: What we know and what we should know, Lambert Academic Publishing, 135.
- Williams V, Filippova M, Filippov V, Payne V, Duerksen-Hughes P (2014) Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J Virol 88: 6751–61.
- Newman MJ, Wu JY, Gardner BH, Anderson CA, Kensil CR, et al. (1997) Induction of cross-reactive cytotoxic T-lymphocyte responses specific for HIV-1 gp120 using saponin adjuvant (QS-21) supplemented subunit vaccine formulations. Vaccine 15: 1001–1007.
- Newman M, Wu J, Gardner B, Munroe K, Lembruno D, et al. (1992) Saponin adjuvant induction of ovalbumin-specific CD8 + cytotoxic T lymphocyte responses. J Immunol 148: 2357–2362.
- Dini I, Tenore CG, Dini A (2009) Saponins in Ipomoea batatas tubers: Isolation, characterization, quantification and antioxidant properties. Food Chem 113: 411–419.
- Huong TTN, Matsumoto K, Kasai R, Yamasaki K, Watanabe H (1998) In vitro antioxidant activity of vietnamese Ginseng saponin and its components. Biol Pharm Bull 21: 978–981.
- Borysiewicz L, Fiander A, Nimako M, Man S, Wilkinson G, et al. (1996) A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347: 1523–1527.
- Meneguzzi G, Cerni C, Kieny MP, Lathe R (1991) Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology 181: 62–9.
- He Z, Wlazlo A, Kowalczyk D, Cheng J, Xiang Z, et al. (2000) Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology 270: 146–61.
- Welters MJP, Kenter GG, Piersma SJ, Vloon APG, Lowik MJG, et al. (2008) Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 14: 178–187.
- Kenter GG, Welters MJP, Valentijn ARPM, Löwik MJG, Berends-van Der Meer DMA, et al. (2008) Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 14: 169–177.
- Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, et al. (2000) A Phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res 6: 3406–3416.
- Lin H, Lin P, Yun Y (2017) A combination of anti-PD-L1 mAb plus LM-LLO-E6 vaccine to supprss tumor growth and metastasis in HPV-infected cancers. ASCO Annu Meet 1–11.
- Jong A, O’Neill T, Khan A, Kwappenberg K, Chisholm S, et al. (2002) Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 20: 3456–3464.
- Peng S, Ji H, Trimble C, He L, Tsai Y, et al. (2004) Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol 78: 8468–8476.
- Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, et al. (2015) Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386: 2078–2088.
- Morrow MP, Kraynyak KA, Sylvester AJ, Shen X, Amante D, et al. (2016) Augmentation of cellular and humoral immune responses to HPV16 and HPV18 E6 and E7 antigens by VGX-3100. Molecular Oncolytics 3: 16025.
- Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, et al. (2009) Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine 27: 431–440.
- Huang C, Peng S, Tsai Y, Boyd D, Hansen T, et al. (2005) Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Thepary 12: 1180–1186.
Citation: Araldi RP, Módolo DG, de Souza JM, de Carvalho RF, dos Santos L, et al. (2017) Analysis of Mutagenic Potential of Therapeutic Vaccine Based on BPV-1 E6 Recombinant Protein Combined with Different Adjuvants. J Vet Med Health 1: 102.
Copyright: ©2017 Araldi RP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 5421
- [From(publication date): 0-2017 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 4413
- PDF downloads: 1008