Combined Effect of Age and Exposure on the Levels of Different Serum Enzymes in Workers of Pesticides Formulation Factories, Pakistan
Received: 18-May-2018 / Accepted Date: 24-Jun-2018 / Published Date: 02-Jul-2018 DOI: 10.4172/2168-9652.1000238
Keywords: Pesticides; Age; Serum enzymes; SGOT; SGPT; GGT; LDH; CPK; ALP; BChE
Introduction
Pesticides being heterogeneous category of chemicals are widely used compounds in our environment for a few decades in pest control, crop production and agriculture [1]. They cover a wide range of compounds including insecticides, fungicides, herbicides, rodenticides, molluscicides and others. These have been found in air, soil, water, human and animal tissues from all over the world. Pesticides uptake occurs mainly through the skin, eyes, by inhalation or ingestion. The fat and water soluble pesticides are absorbed through skin. Sores and abrasions may also facilitate uptake through skin [2]. Absorption resulting from dermal exposure is the most important route of uptake for exposed workers [3]. Occupational exposures occur in mixing of active ingredients of pesticides, loading of equipment and in spraying along with application of insecticides and leads to human poisoning [4].
The use of pesticides in Pakistan was started in 5th decade of 19th century and increased steadily and substantially over the years. Pesticide poisoning is a serious problem in agricultural areas of this country [5,6]. Out of total pesticides used in Pakistan, 88.3% is used in Punjab province followed by Sindh (8.2%) and Khyber Pakhtoon Khan (2.8%). Cotton crop is the major cash crop [7]. Therefore, use of pesticides in cotton crop accounts for about 62% of the total pesticide consumption here. More than 150 types of insecticides, fungicides and rodenticides are being used in Pakistan. Acute sever poisoning is an important clinical problem in Pakistan like other countries of the world [6]. Health implications of pesticide exposure in different factory workers showed considerable variation in enzyme levels of the subjects [8,9]. Most epidemiological studies on pesticides have analyzed the health effects of exposure on human health and the variation in the levels of enzymes have correlated to the influence of pesticides exposure [10].
Many researchers have reported the inhibition of serum Cholinesterase that is widely used as biomarker of acute or chronic exposure to the pesticides. Most studies in the literature regarding the chronic effect of pesticides have mainly focused on imbalance of heart, kidney and liver function enzymes [11]. Acute toxic effects of pesticides are easily recognized, whereas the effects resulting from long-term exposure to low doses are often difficult to distinguish [12]. The pesticides influence the number of enzymes, physiological systems and organs of mammalian system i.e. reproductive, nervous, immune and endocrine systems [13-15]. On the other hand aging is one of the highest risk factors known for most human diseases, including neurodegeneration, cancer, diabetes, and metabolic syndrome [16]. Aging also affects the body’s cells, tissues, and organs, and these changes affect the body fluids and enzyme levels [17]. A number of characteristic aging symptoms are experienced by a majority or by a significant proportion of humans during their lifetimes.
Previously, number of researchers investigated the effect of pesticides on different enzymes of occupationally exposed workers with regard to their age or level of exposure to pesticides independently [10,18].
However, to the best of our knowledge, no study has been carried on enzyme’s level of occupationally exposed workers by taking in account their age and exposure simultaneously. Therefore, in the present study, an effort was made to explore the variation in the levels of different serum enzymes affected by age and exposure and combined effect of both age and exposure in workers of different pesticide formulation factories in Multan, Pakistan.
Methods
This cross sectional study was conducted at Institute of Chemical Sciences after the approval of Board of Advanced Studies and Research (BASR), Bahauddin Zakariya University, Multan, Punjab, Pakistan. Blood samples of workers were collected from different pesticides formulation factories located in Industrial State Multan, Pakistan. All participants were informed about the purpose of the study and gave their written consent to participate. The pesticides formulated in these factories and their World Health Organization [19] classification is given in Table 1.
| Pesticide | Common Name | Chemical Class | WHO Classification |
|---|---|---|---|
| Insecticide | Carbofuran | Carbamate | Ib |
| Triazophos | Organophosphate | II | |
| Chlorpyrifos | Organophosphate | II | |
| Imidacloprid | Neonicotinoids | II & III | |
| Lambdacyhalohrin | Pyrethroid | II | |
| Deltamethrine | Pyrethroid | II | |
| Herbicides | Tribenuron methyl | Triazine | II |
| Fungicide | Difenoconazole | Triazoles | II |
Ib = Highly hazardous; II = Moderately hazardous; III = slightly hazardous (Chemicals and Organization 2010)
Table 1: List of Pesticides Used by the Case Group
Field survey and blood collection
One hundred and eighty male workers potentially exposed to pesticides working on average 42 hours a week were randomly selected from pesticide formulation factories on the basis of their active involvement in mixing, packing and storage of the pesticides. A group of 50 male healthy individuals with same socioeconomic status that were not exposed to pesticides was used as control. The personal history and information regarding the different symptoms of heart and kidney diseases were also obtained. A blood sample of 5 ml from each individual was collected in serum gel tubes (BD Vacutainer®) and the samples were brought to the laboratory immediately. The vials were centrifuged at 2500 rpm for ten minutes at room temperature to separate the serum. A fraction of serum was used for the screening of Hepatitis B and C using Hepatitis Rapid Kits (OraSure Technologies, Bethlehem, PA, USA). The eight workers out of 180 showed positive tests for hepatitis B/C and were excluded from the study. The serum samples of rest of the 172 workers were stored in refrigerator at -20°C for the assay of serum enzymes.
Study design
This cross sectional study comprises the analysis of 222 subjects including 172 pesticide exposed factory workers with 1-10 year of exposure and fifty control subjects. All the subjects were from age 19-58 year. The subjects were categorized in to different groups of exposure. These exposure groups were further categorized in to five groups of age (Table 2).
| Groups | Exposure (Years) |
Age (Years) | Total | ||||
| 19-26 | 27-34 | 35-42 | 43-50 | 51-58 | |||
| 1 | 0 | 30 | 5 | 5 | 5 | 5 | 50 |
| 2 | 1-2 | 6 | 46 | 3 | 3 | 3 | 61 |
| 3 | 3-4 | 4 | 22 | 3 | 3 | 3 | 35 |
| 4 | 5-6 | 7 | 18 | 4 | 3 | 3 | 35 |
| 5 | 7-8 | 4 | 6 | 3 | 3 | 3 | 19 |
| 6 | 9-10 | 7 | 6 | 3 | 3 | 3 | 22 |
| Total | 57 | 103 | 21 | 20 | 20 | 222 | |
Table 2: Study Design to See the Effect of Exposure and Age on All Participants
Enzyme analysis
In the present work, serum enzymes i.e. Butyrylcholinestrase (BChE), Creatine Phosphokinase (CPK) and Lactate Dehydrogenase (LDH), Alkaline Phosphatase (ALP), Serum Glutamic Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), Gamma-Glutamyl Transferase (GGT) were analyzed. The activity of all enzymes was estimated using respective kits and is expressed as U/L. All these measurements were carried in triplicates.
Measurement of ButyrylCholinesterase: Serum Butyrylcholinesterase was measured by colorimetric method using the kit, Randox, UK [20]. The serum butyrylcholinesterase hydrolyses butyrylthiocoline to produce thiocoline and butyrate. The reaction between thiocholine and dithiobisnitrobenzoate (DTNB) gives 2-nitro- 5-mercaptobenzoate, a yellow compound which was measured at 405 nm. The cholinesterase activity of the samples was measured by the proportional decrease in the absorbance.
Measurement of CPK: CPK catalyzes the phosphorylation of ADP to ATP, the catalytic activity is determined from the rate of NADPH formation, measured at 340 nm by following UV-kinetic method as illustrated in kit, Human [21].
Measurement of LDH: Serum LDH was measured by adopting the modified method based on the recommendations of Scandinavian committee on enzymes as explained in LDH kit, Human, Germany [21] and the generation of NAD+ was measured at 340 nm.
Measurement of ALP: Serum ALP was measured by optimized standard method according to the recommendations of the German Clinical Chemistry Association [21], and the absorption of the complex p-nitrophenol was recorded at 405 nm.
Measurement of SGOT, SGPT and GGT: The estimation of transaminases, i.e. SGOT, SGPT and GGT was carried out by the UV-kinetic method according to the recommendations of the expert panel of IFCC (International Federation of Clinical Chemistry) using respective kits, Human, Germany [22,23]. The generation of NAD+ in transaminase reactions for SGOT and SGPT was measured at 340 nm. GGT catalyzes the transfer of a γ-glutamyl group from γ-glutamylp- nitroanilide (GGPNA). The rate of liberation of p-nitroaniline is directly related to the γ-GT activity in the sample and was quantified by measuring the increase in absorbance at 405 nm [23].
Statistical Analysis
The recorded data under continuous variables were segregated according to exposure and age groups. The exposure and age group were considered as two factors. On the basis of the observed data, Two Way Analysis of Variance [24] was conducted with respect to both the factors. In case of Two way ANOVA a P-value<0.05 was considered as significant. Tukey Pairwise Multiple Comparison test was conducted for the main factors and interactions across the seven different enzymes. In case of Multiple comparison test an Adjusted P-value<0.05 was considered as significant when Family Wise Error Rate was 5% (Family wise error level of significance=0.05). The statistical analyses were conducted by using MINITAB version 17.0 and SPSS for Windows version 19.0. For production of few graphs Microsoft Excel 2007 was used.
Results
A sample size of 222 subjects (172 exposed and 50 control individuals) was selected to observe the variation in enzymes levels under the action of age of the workers and exposure of the pesticides in workers of pesticides formulation factories. The changes in enzymatic parameters in studied population are explained below.
Studies on the changes in the levels of serum enzymes
Data was analyzed using different statistical techniques to assess the influence of age, duration of exposure and the combined effect of both age and exposure on the serum levels of different enzymes. The results are explained as follow;
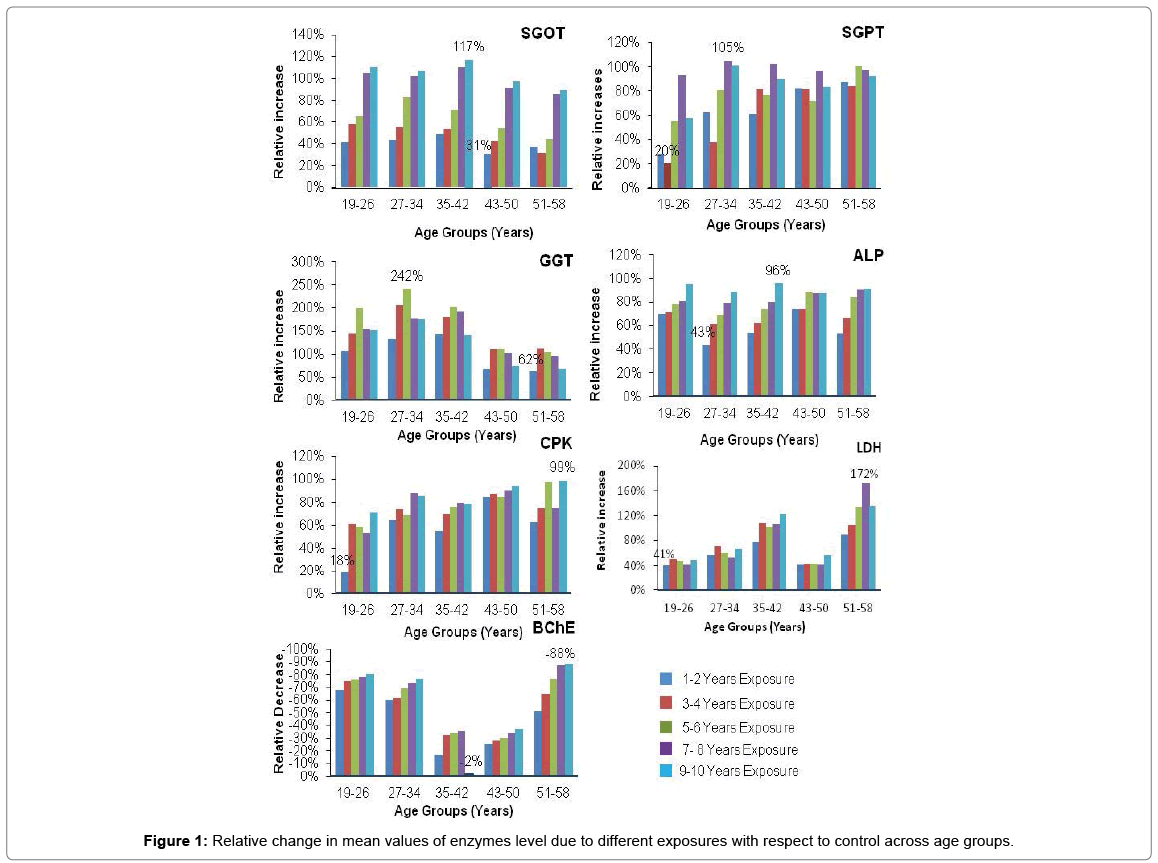
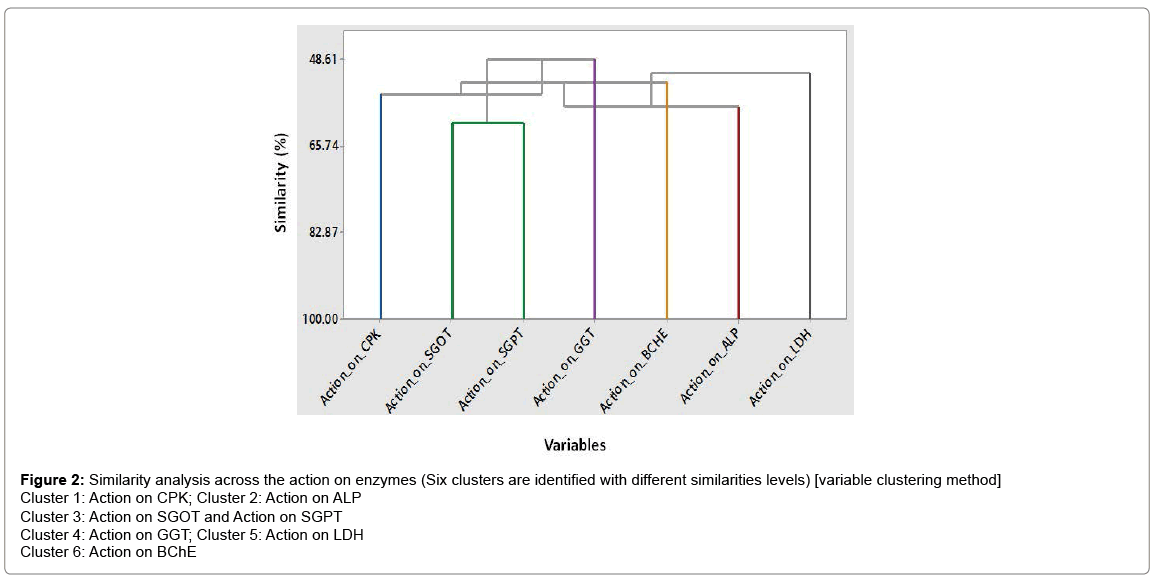
The values for relative change of mean (%) of enzymes level after exposure with respect to control are presented in Figure 1. In case of exposure all enzymes have been observed to vary significantly from that of their respective control levels. A variable cluster analysis was conducted for the seven continuous variables (Enzymes) by following the similarity of observation method and six different clusters were found (Figure 2). Action on BChE variable (cluster 6) has 61.10% similarity in observation with other variables and CPK (cluster 1), ALP (cluster 2), GGT (cluster 4), LDH (cluster 5) have been observed to show 51.33, 58.11, 55.55, 48.61 % similarities respectively from other variables. Action on SGOT and SGPT variables were found to exist in same cluster (cluster 3) with similarity level of 53.17% with other variables. So these analyses indicated towards the similarity of enzyme level changes with respect to all exposure levels and age groups.
Two way ANOVA was conducted by considering exposure and age groups as two factors. ANOVA was conducted on seven different enzymes to find the significant effect of exposure levels, age and combined effect of age and exposure and the results are shown in Table 3. The results showed that exposure levels have significant effect across all the studied enzymes (P<0.05). CPK, SGPT, GGT and BChE were observed to show significant effect with respect to increase in age. The age groups has no significant (P>0.05) effect on the serum levels of ALP, SGOT and LDH. Hence CPK, SGPT, GGT and BChE were observed to show significant effect due to age and exposure independently. While, combined effect of exposure and age had significant effect (P<0.05) only on BChE and the rest of the studied enzymes had no significant effect. Coefficient of determination (R2) values were maximum (67.96%) with BChE enzyme variable and minimum (27.76%) with LDH enzyme variable. R2 explains percentage of dependant variable’s (serum levels of enzymes) variation explained by the linear model (intercept+β1 Exposure+β2 Age group+β3 Exposure*Age group+error), where β1, β2 and β3 are the coefficient of exposure, age group and interaction term respectively. Low R2 value is due to the ordinal nature of the independent variables but still those models have a good fit. These results may not completely acceptable according to biological phenomena of human body. Every person’s enzyme production capacity is varied according to his/her age. The amount of pesticide exposure is also an important factor.
| Factors | P-Values | ||||||
|---|---|---|---|---|---|---|---|
| CPK | ALP | LDH | SGOT | SGPT | GGT | BChE | |
| Exposure | 0.00* | 0.00* | 0.00* | 0.00* | 0.00* | 0.00* | 0.00* |
| Age Group | 0.00* | 0.924 | 0.737 | 0.117 | 0.00* | 0.00* | 0.00* |
| Interaction | 0.69 | 1 | 0.993 | 1 | 0.971 | 0.999 | 0.009* |
| S | 30.00 | 75.15 | 82.85 | 11.38 | 11.99 | 10.60 | 5562 |
| R-sq [in %] | 53.38 | 33.23 | 27.76 | 43.87 | 35.12 | 54.46 | 67.96 |
| R-sq(adj) [in %] | 46.34 | 23.15 | 16.85 | 35.39 | 25.32 | 47.59 | 63.12 |
| R-sq(pred) [in %] | 39.94 | 17.14 | 10.05 | 34.60 | 18.78 | 39.14 | 60.95 |
S=Variance; R-sq= R- Square (Coefficient of determination); R-sq(adj)= R- Square Adjusted; R-sq(pred)= R- Square Predicted
*(P<0.05, Level of significance = 0.05)
Table 3: Two Way ANOVA with interaction of exposure and age on the serum levels of different enzymes of pesticides exposed workers
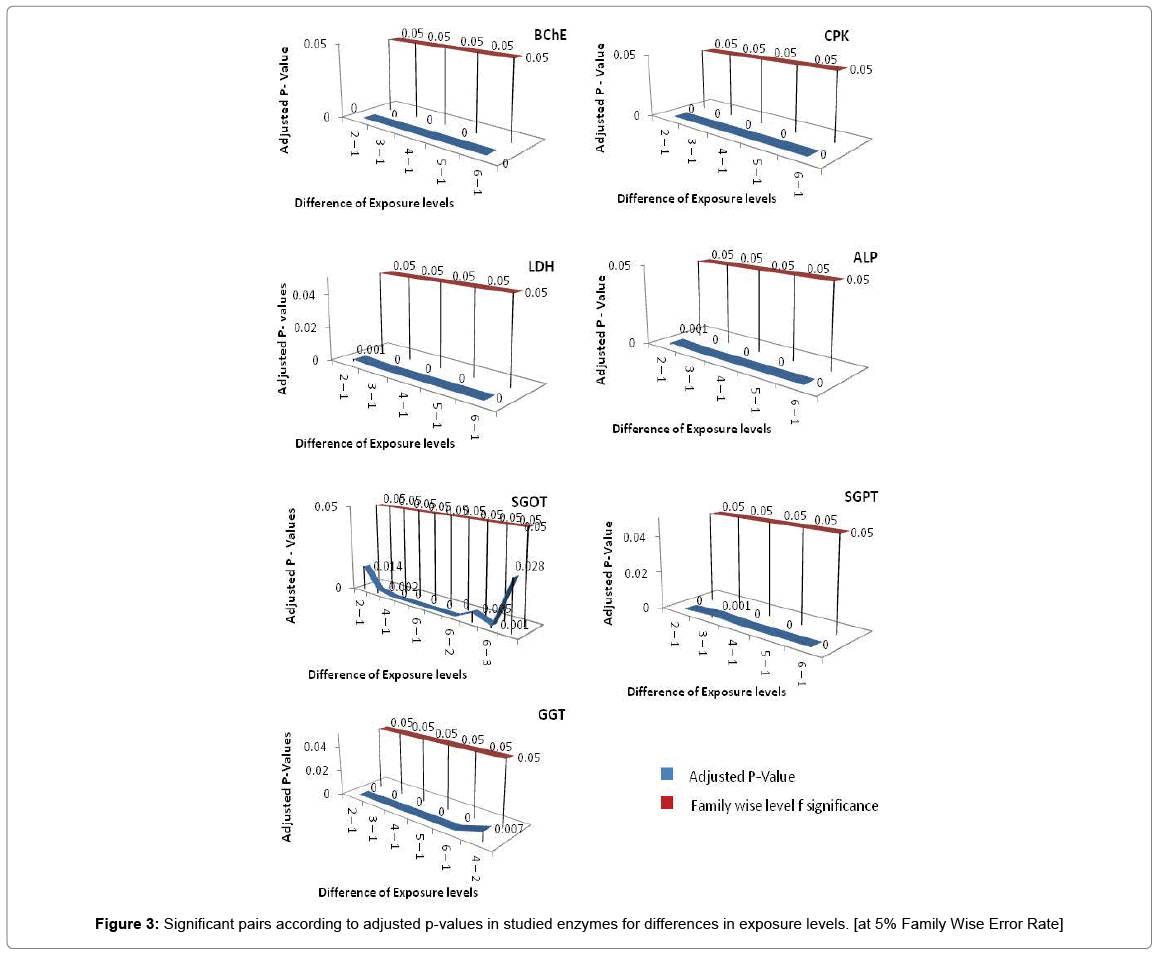
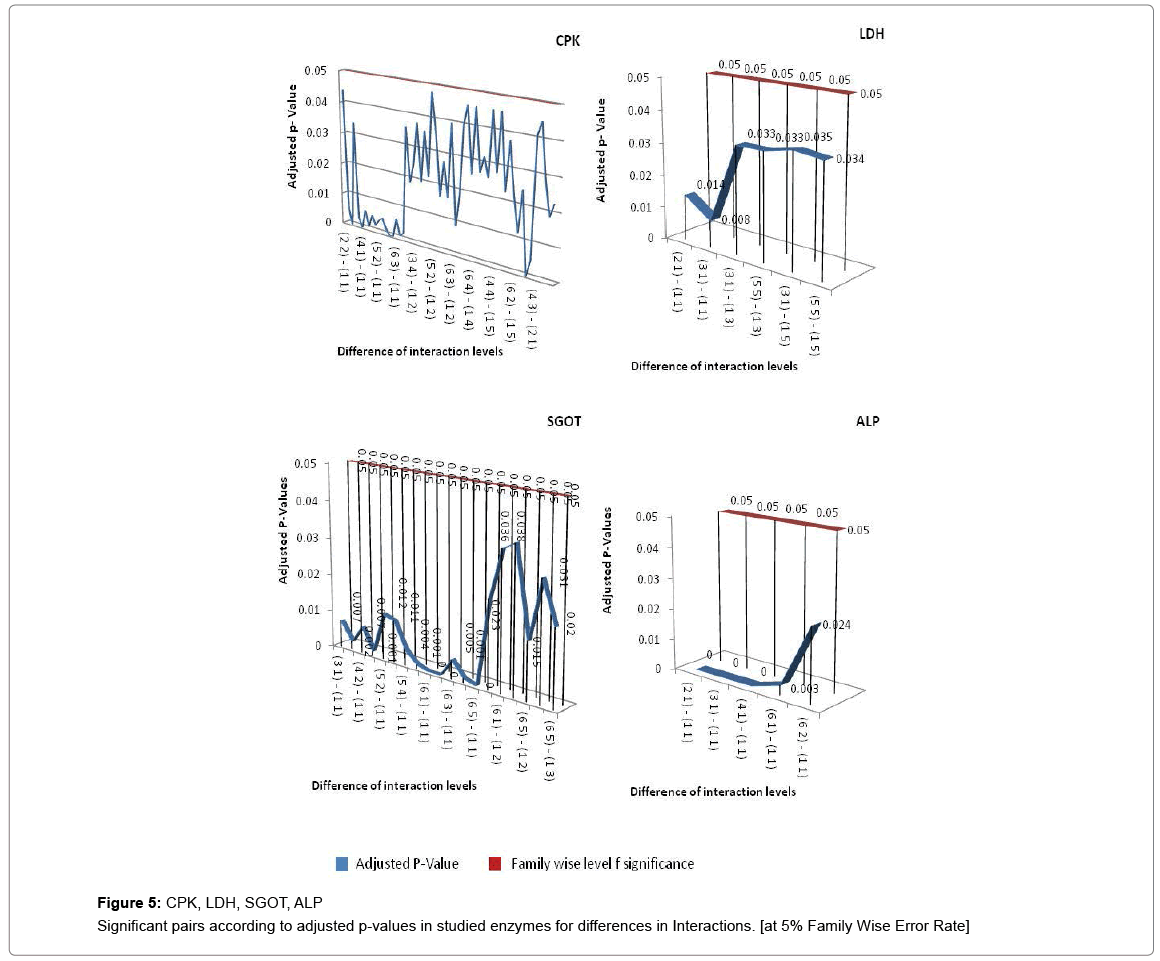
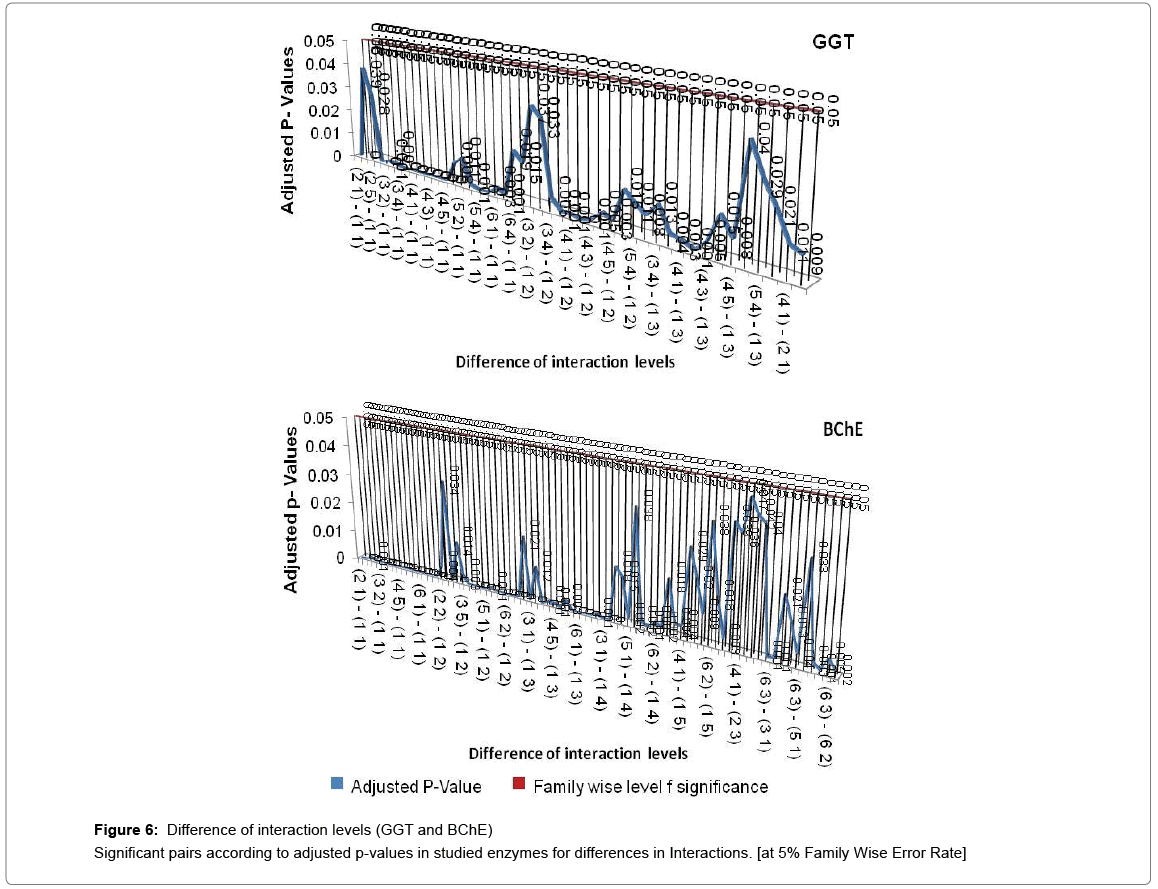
If all exposure levels are supposed to significantly affect the human body enzyme levels, it is again expected to get some significant effect of age and exposure interaction term. Therefore, diagnosis obtained by two way ANOVA was matched with Tukey Multiple Comparison procedure in case of exposure and age group term only. But the two way ANOVA was unable to detect a large amount of significant interaction between exposure and age group in this experimental analysis process and choice of Tukey Multiple Comparison procedure is justified in this regard. Tukey Pair wise Multiple Comparison test was conducted for all studied enzymes with respect to exposure, age group and interaction levels and obtained adjusted P-values were compared with Family wise Error level of significance (0.05) or at 5% Family wise error Rate (FWER). The total observations of multiple comparisons for age, exposure and interaction of age and exposure are expressed in supplementary data (Supplementary Tables S1-S7). The pair showing adjusted P-value less than 0.05 was considered as significant. Only the significant observations were plotted and are expressed in Figures 3-6. Figure 3 shows the results for the effect of exposure on serum levels of all studied enzymes. The significant differences of age group levels for the enzymes BChE, CPK, SGPT and GGT are shown in Figure 4. No significant observation has been found for enzymes LDH, ALP and SGOT with respect to age. In case of interaction, the significant observations of differences in interaction levels for six enzymes i.e. CPK, LDH, SGOT and ALP are shown in Figure 5 and for GGT and BChE, are presented in Figure 6. The enzyme SGPT has not shown any significant observation for differences in interaction levels.
Figure 2: Similarity analysis across the action on enzymes (Six clusters are identified with different similarities levels) [variable clustering method]
Cluster 1: Action on CPK; Cluster 2: Action on ALP
Cluster 3: Action on SGOT and Action on SGPT
Cluster 4: Action on GGT; Cluster 5: Action on LDH
Cluster 6: Action on BChE
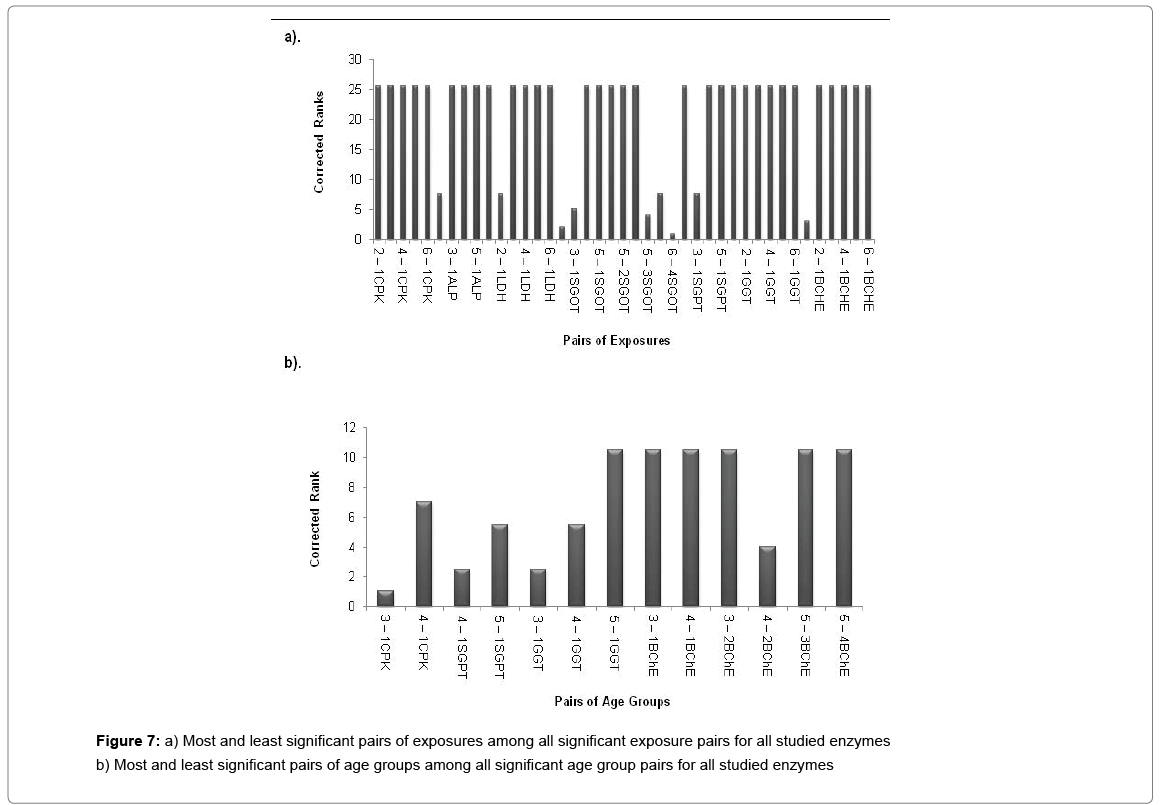
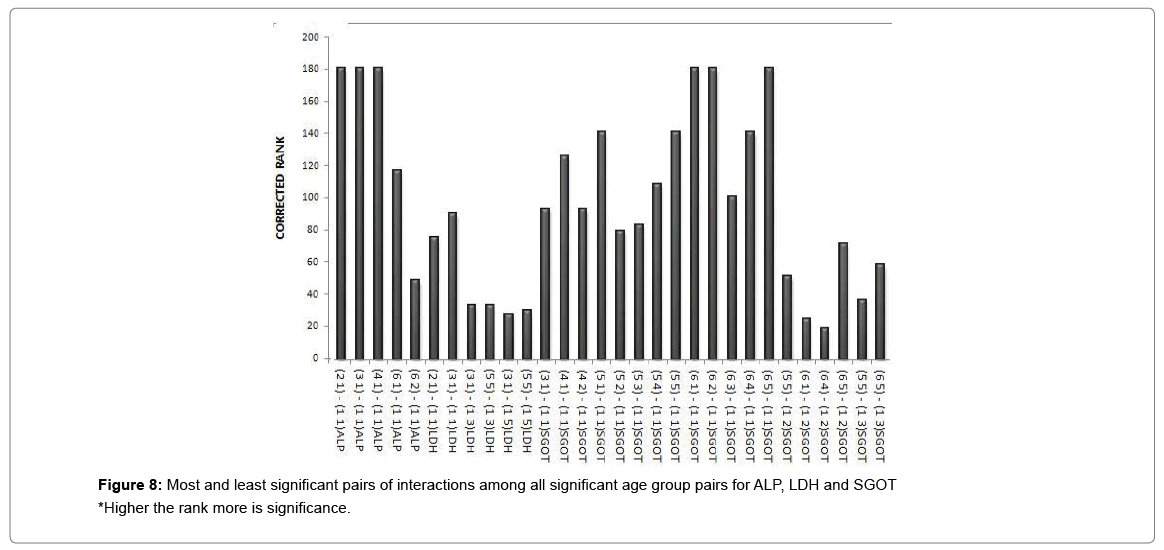
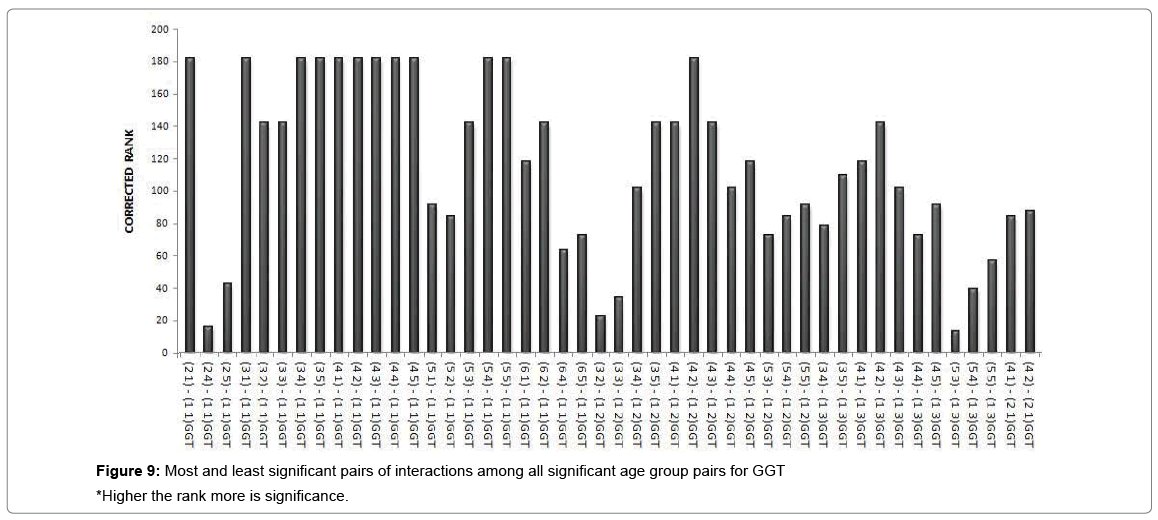
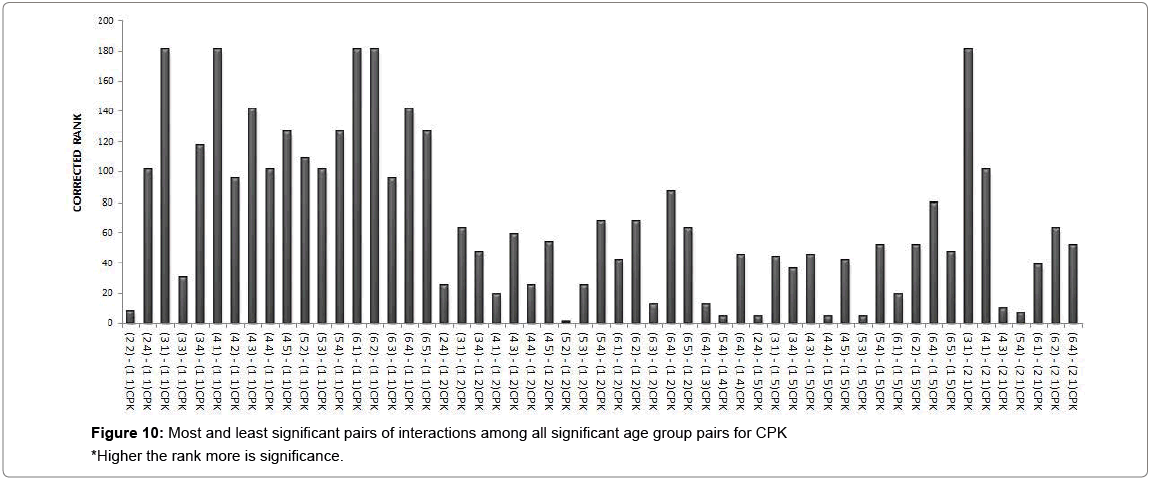
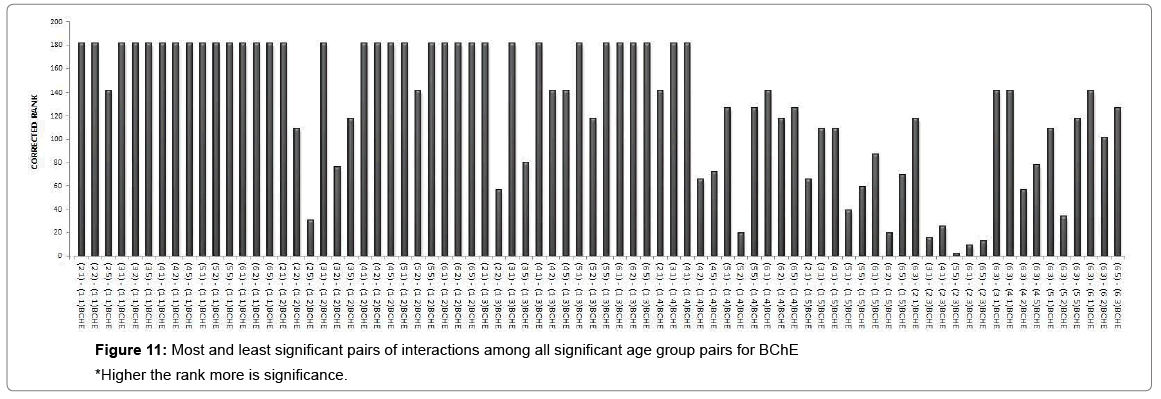
A cluster analysis was conducted for exposure levels, age groups and interaction terms on the basis of significant adjusted P-values. The cluster membership of three variables shows that according to Tukey multiple comparison test at 5% FWER with different enzyme combinations, 41 pairs of exposures, 13 pairs of age groups, 209 pairs of interaction terms are found significant and the details of these pairs are presented in supplementary table (Table S-8). Squared Euclidian distance with centroid linkage method was used for this hierarchical clustering procedure. The Table 4 shows total number of observations of difference of exposure, age and interaction levels across the enzyme types. Exposure term cluster analysis of adjusted P-value gives six different clusters in which cluster 1 contains 32 observations or pairs which are most significant (adjusted P=0.000<0.05) and rest of five clusters also have adjusted P-value <0.005 but their adjusted P-values are greater than 0.000. For age group term cluster analysis of adjusted P-value gives six different clusters in which cluster 5 contains six observations or pairs which are most significant (adjusted P=.000< 0.05) and rest of five clusters also have adjusted P-value <0.005 but their adjusted P-values are greater than 0.000. For interaction term cluster analysis of adjusted P-value gives twelve different clusters in which cluster 3 contains 79 observations or pairs which are most significant (adjusted P= 0.000 to 0.001< 0.05) and rest of eleven clusters also have adjusted P-value <0.005 but their adjusted P-values are greater than 0.001. The ranking of the adjusted P-values are created and a bar plot of corrected rank vs. observations or pairs was produced for exposure, age and interaction. The corrected ranks vs. pairs of exposure and age for all studied enzymes are presented in Figure 7. The results for the adjusted P values for interaction of age and exposure are expressed in Figures 8 and 9 while for the CPK and BChE are expressed in Figures 10 and 11 respectively. This reveals that higher the rank more is significance. Along with these it is also proved that most significant observations are clustered at a same place. Numerically most significant observations are sharing same character.
| Significant In | Exposure* | Age Group* | Interaction* | No. of Enzymes Considered in each Combination |
|---|---|---|---|---|
| CPK, ALP, LDH, SGOT,SGPT,GGT,BChE | 5 | none | None | 7 |
| SGOT ONLY | 5 | none | None | 1 |
| GGT ONLY | 1 | none | 7 | 1 |
| BChE ONLY | None | 4 | 40 | 1 |
| CPK, GGT, BChE | None | 1 | 3 | 3 |
| CPK, SGPT, GGT, BChE | None | 1 | None | 4 |
| SGPT, GGT | None | 1 | None | 2 |
| CPK, BChE | None | none | 7 | 2 |
| CPK, GGT | None | none | 11 | 2 |
| CPK, ALP, LDH, SGOT, GGT, BChE | None | none | 1 | 6 |
| CPK, ALP, SGOT, GGT, BChE | None | none | 3 | 5 |
| CPK, SGOT, GGT, BChE | None | none | 3 | 4 |
| CPK, SGOT, GGT | None | none | 3 | 3 |
| CPK, SGOT | None | none | 2 | 2 |
| CPK, SGOT, BChE | None | none | 2 | 3 |
| CPK, LDH, BChE | None | none | 1 | 3 |
| ALP, LDH, GGT, BChE | None | none | 1 | 4 |
| LDH, BChE | None | none | 1 | 2 |
| LDH, SGOT, GGT, BChE | None | none | 1 | 4 |
| LDH, BChE | None | none | 1 | 2 |
| SGOT, GGT, BChE | None | none | 3 | 3 |
| SGOT, BChE | None | none | 1 | 2 |
| GGT, BChE | None | none | 10 | 2 |
| CPK ONLY | None | none | 19 | 1 |
| TOTAL | 11 | 7 | 120 |
*Significant number of pairs.
Table 4: Significant Number of Pairs According to The Combinations of Different Enzymes across Different factors (based on multiple comparison test result)
Dendrograms of hierarchical clustering showing the position of particular observation or pair for exposure, age and interaction are presented in Supplementary file (Table S8). However, among the all significant values, most significant observations were detected and consolidated according to the name of enzymes. Five pairs of exposures 2–1, 3–1, 4–1, 5–1, 6–1 were found significant across the all studied enzymes at 5% Family wise Error rate. In case of age group, 3–1 showed significance for enzymes BChE, CPK and GGT, 4-1 showed significance in BChE, CPK, SGPT and GGT, 5-1 depicted significance in SGPT and GGT. The age groups 3–2, 4–2, 5–3, 5–4 showed significance for BChE at 5% family wise error rate. In ALP, SGOT, LDH no pair of age group are significant at 5% Family wise error rate. One pair of interaction [(3 1) - (1 1)] is significant across the six enzymes (except SGPT) and three pairs of interactions [(4 1) - (1 1), (6 1) - (1 1), (6 2) - (1 1)] are significant across the five enzymes (except SGPT, LDH) at 5% Family wise Error rate. In this way we get the significant pairs across the most of the number of enzymes and their combinations are given in Table S-1to S-7.
Discussion
An increasing number of human studies in recent years have started to evaluate the potential of pesticides to affect human serum. Pesticide poisoning is also a serious problem in agricultural areas of Pakistan and the use of pesticides has increased by 69% in last twenty years [25]. Pesticides are volatile and get vaporized easily, so when inhaled or come in contact with skin, they affect the human body and cause toxic symptoms like respiratory, neurological, gastrointestinal and skin problems [26,27].
The repeated and excessive use of pesticides promotes toxicological problems in workers. The toxicity of the pesticides is associated with the production of free radicals that damage the body tissues, cause biochemical changes along with liver and kidney damage and results in leaching of enzymes into the blood. A positive correlation of pesticides with the liver enzymes has been reported by many researchers [28-30]. In this study the descriptive analysis reflects that the liver function enzymes (SGOT, SGPT, GGT and ALP) have been affected much in age group 27-34 and 35-42 years under high exposure levels (6-8, 9-10 years). CPK, LDH (related to muscle damage) and Cholinesterase (neurotransmitter) are affected much in age group 51-58 under the higher exposure levels followed by younger age groups. The liver function enzymes like SGOT, SGPT and GGT are percolated out of the cell increasing their level into the bloodstream. Elevated SGOT levels are not only the indication of liver damage but has also been used as a cardiac biomarker [31,32]. SGOT, SGPT and GGT are membrane bound enzymes and are authentic and sensitive test for the detection of hepatic necrosis and liver diseases [33,34]. Increased levels of these enzymes in serum as reported in present study might be due to the leakage of soluble tissue enzymes into the blood as a result of necrosis of the tissues and such observations were also reported in infective hepatitis, acute pancreatic and cardiac disorders in humans [35].
In this cross sectional study, the results were statistically analyzed to find out the combined effect of age and exposure of pesticides on the serum levels of enzymes of the workers. The enzyme’s levels in serum of the subjects from six exposure levels and five age groups were estimated by applying two way ANOVA following the Tukey multiple comparison procedures in case of exposure and age group term only. In case of effect of exposure of pesticides on enzyme levels, we observed the significant difference between control and all exposure levels (1-2, 3-4, 5-6, 7-8, 9-10 years) across the all seven enzymes at 5% FWER. Most of the significant differences between exposure levels are clustered at cluster no. 1 that is showing 32 significant clusters (Table S8a) for all studied enzymes (BChE, CPK, LDH, ALP, SGOT, SGPT and GGT) which shows that these enzymes are affected in a similar pattern under the action of pesticides. Most of the researcher has studied the hepatotoxicity due to pesticides exposure and have reported a positive linear relation of pesticides with the liver enzymes [36,37]. Biochemical effects can appear in case of enzyme inhibition or enzyme induction and proved to be good biomarkers for pesticides damage before the occurrence of adverse clinical health effect [38]. Pesticides have been reported to produce reactive oxygen species (ROS) in both intra- and extra cellular species. Increased levels of ROS may result in significant damage to cell structures and cause impairment of cellular functions [39]. Tissue damage leads to the increased levels of CPK and LDH [40]. So the CPK and LDH serve as cheap and widely available biomarkers for acute organophosphorus poisoning [41]. Most organophosphorus and carbamates irreversibly bind to both AChE and BChE enzymes and inhibits their activities leading to over stimulate and disrupt neurotransmission in both central and peripheral nervous system [42]. While considering the effect of age on serum enzymes, significant difference have been observed between age group 1 (19-26 years) and age group 4 (43-50 years) across the level of enzymes, BChE, CPK, SGPT, GGT enzymes at 5% FWER but no significant difference was observed in level of enzyme ALP, SGOT and LDH. Our findings for the parameters i.e. ALP and SGPT are in accordance with Awad et al. who has reported that no significant difference in ALP serum enzyme level was observed in age groups of 20-60 due to chronic exposure of pesticides and significance difference in SGPT was observed in age groups 30-40 years and 50-60 years by applying t-test [10]. Moreover, they have reported significant differences for the values of SGPT in age groups 30-40 years and 40-50 years in comparison of t-test, but our study doesn’t find significant differences with respect to age in serum levels of SGOT at 5% FWER. The six combinations of age groups are clustered at cluster no. 5 which shows that BChE and GGT lies in the same cluster and are affected in a similar pattern. Cluster 3 and 4 shows SGPT and GGT while CPK lies in cluster 1 and 2 (Table S8b). So in case of the enzymes, ALP, SGOT and LDH, age group does not impact any effect but still exposure levels create an impact on all seven enzyme levels. Creatine Phosphokinase is an enzyme that is found mainly in the heart, brain, and skeletal muscles and the normal range for general CPK varies by age and gender. The peri-operative changes in the serum concentration of CPK levels are related to the heart and muscle damage [43-45]. Human muscles undergo age related degenerative changes that contribute to some of the most common causes of impairment and disability of the middle or older age. Cells lose viability in response to hypoxia, ischemia, exposure to toxic chemicals, and withdrawal of growth factors. Cell death in response to these stimuli can occur within minutes or take many hours to develop. Very rapid onset of cell death typically leads to tissue necrosis [46-48]. SGOT and SGPT are associated with cellular integrity and GGT, ALP are related with some conditions linked to the biliary tract function enzymes [49]. So, the increase in these enzymes level is more or less associated with the tissue necrosis related to the age along with exposure of pesticides. BChE is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters and in humans, it is formed in the liver and found mainly in blood plasma [50]. It scavenges even low doses of organophosphorus and carbamate pesticides and in this way helps the people in protecting from the toxic effects of these poisons [51].
In this study, while considering combined effect of exposure and age on enzymes levels, significant differences have been observed between control and age group 1 (19-26 yr) and exposure level 3 (3-4 yr) and age group 1 (19-26) across the six enzymes except SGPT at 5% FWER (Table S8d, S9e). Most Significant differences of interactions (i.e. 79) are clustered at cluster no. 3 (Table S8c). BChE, GGT, ALP and SGOT fall in the same cluster. Butyrylcholinesterase assays can be used to detect exposure to organophosphorus or carbamate pesticides [52]. Several investigators have shown that enzymatic activities could be used as bioindicators for the toxicity of pesticides and heavy metals in vitro [53]. Butyrylcholinesterase is reliable and widely used biomarker of exposure to pesticides in occupational and clinical toxicology [54]. As a result of study on combined effect of age and exposure on studied enzymes level, it is interpreted that multiple combination of interaction of age and exposure affected the BChE, CPK and GGT. Other enzymes i.e. SGOT, LDH and CPK also observed to be affected under the combined effect of age and exposure while SGPT did not show the significant variation in its level.
Legitimately, due to reluctance of the pesticides factory workers to participate in this study and time constraints, this study resulted in a small sample size. Therefore, it was difficult to establish a causal relationship between pesticide exposure and changes in enzymatic parameters. This is in accordance with Corsini et al. in 2008 [55] and Aroonvilairat et al. in 2015 [56] who mentioned that the appraisal of toxicity of pesticides on the biological systems in workers exposed to several compounds was a very difficult task [56]. According to our study all the studied enzymes BChE, CPK, LDH, ALP, SGOT and GGT except SGPT are significantly affected by pesticides exposure, age of the workers and interaction of age and exposure.
Conclusion
This study concludes that the workers of the pesticide formulation factories were mostly illiterate or having low level of education. They did not observe the precautionary measures for their safety. In Pakistan, peoples are lacking the information regarding affect of pesticides on human health and base-line information needs to be generated so that risk of exposure may be minimized. We observed that in studying the effect of pesticides on pesticides exposed workers, age of the exposed workers is also the important factor. The levels of all enzymes of exposed workers were significantly influenced with regard to their age and duration of exposure. Statistically, it has also been observed that BChE, CPK, LDH, ALP, SGOT and GGT showed significant variation due to the combined of effect of age and exposure. It is therefore, suggested that investigators should take care of both age and duration of exposure of subjects while considering the enzymes as biomarkers for toxicological effects of pesticides.
Acknowledgement
The Scholar, T Rehman is thankful to The Women University Multan, Pakistan for providing the funding under Faculty Development Program for this project.
References
- Damalas CA, Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8: 1402-1419.
- Vega S (1994) Note on the toxicity of pesticides used in tropical crops. Ciencias Ambientales 11: 181.
- Mathur H, Agarwal H, Johnson S, Saikia N (2005) Analysis of pesticide residues in blood samples from villages of Punjab. Centre for Science and Environment Reports, New Delhi, India.
- Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, et al. (2005) Differences between organophosphorus insecticides in human self-poisoning: A prospective cohort study. The Lancet 366: 1452-1459.
- Khan DA, Shabbir S, Majid M, Naqvi TA,et al. (2010a) Risk assessment of pesticide exposure on health of Pakistani tobacco farmers. J Expo Sci Environ Epidemiol 20: 196-204
- Tariq MI, Afzal S, Hussain I, Sultana N (2007) Pesticides exposure in Pakistan: A review. Environ Int 33: 1107-1122.
- Khooharo A (2008) A study of public and private sector pesticide extension and marketing services for cotton crop. phd dissertation, Department of Agricultural Education, Extension & Short Courses, Sindh Agriculture University, Tandojam 42.
- Khan DA, Bhatti MM, Khan FA, Naqvi ST, Karam A (2008) Adverse effects of pesticides residues on biochemical markers in Pakistani tobacco farmers. Int J Clin Exp Med 1: 274.
- Khan MJ, Zia MS, Qasim M (2010b) Use of pesticides and their role in environmental pollution. World Acad Sci Eng Technol 72: 122-128.
- Awad O, El-Fiki S, Abou-Shanab R, Hassanin N, Abd El Rahman R (2014) Influence of exposure to pesticides on liver enzymes and cholinesterase levels in male agriculture workers. Global Nest Journal 16: 1006-1015.
- Jamal F, Haque S, Singh S, Arshad Md (2016) The influence of pesticides on hepatic and renal functions in occupational sprayers of rural malihabad, lucknow (India). Toxicol open access 2: 2.
- Jamal F, Haque S, Singh S, Arshad Md (2016) The influence of pesticides on hepatic and renal functions in occupational sprayers of rural malihabad, lucknow (India). Toxicol open access 2: 2.
- Â Blanco-Munoz J, Morales MM, Lacasana M, Aguilar-Garduno C, Bassol S,et al. (2010) Exposure to organophosphate pesticides and male hormone profile in floriculturist of the state of Morelos, Mexico. Hum Reprod 25: 1787-1795.
- Isman MB (2000) Plant essential oils for pest and disease management. Crop Protection 19: 603-608.
- Ksheerasagar R, Hiremath M, Kaliwal B (2011) Impairment of hepatic biochemical contents and enzymes activities during carbosulfan intoxication in albino mice. Int Multidiscip Res J 1: 6-15.
- Blanco-Munoz J, Morales MM, Lacasana M, Aguilar-Garduno C, Bassol S,et al. (2010) Exposure to organophosphate pesticides and male hormone profile in floriculturist of the state of Morelos, Mexico. Hum Reprod 25: 1787-1795.
- Hassan OM, Metwally ES (2013) Reproductive and thyroid hormones among male agricultural workers exposed to pesticides. The Egyptian Journal of Community Medicine 31: 1-16.
- Dillin A, Gottschling DE, Nystrom T (2014) The good and the bad of being connected: The integrons of aging. Curr Opin Cell Biol 26: 107-112.
- Mohebbi H, Rahmani-Nia F, Yar A, Riasi A, Marandi M (2015) The effects of interval training and age on blood lactate (La) levels and lactate dehydrogenase (LDH) activity in male wistar rats. Par J Med Sci 12: 37-45.
- Knedel M, Böttger R (1967) A kinetic method for determination of the activity of pseudocholinesterase (acylcholine acyl-hydrolase 3.1. 1.8.). Klin Wochenschr 45: 325.
- Arbuckle TE, Lin Z, Mery LS (2001) An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environmental health perspectives 109: 851.
- Varma VR, Varma S, An Y, Hohman TJ, Seddighi S, et al. (2017) Alpha-2 macroglobulin in Alzheimer’s disease: A marker of neuronal injury through the RCAN1 pathway. Mol Psychiatry 22: 13-23.
- Persijn JP, van der Slik W (1976) A new method for the determination of gamma-glutamyltransferase in serum. J Clin Chem Clin Biochem 14: 421-427.
- Szasz G (1969) A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin Chem 15: 124-136.
- Varma VR, Varma S, An Y, Hohman TJ, Seddighi S, et al. (2017) Alpha-2 macroglobulin in Alzheimer’s disease: A marker of neuronal injury through the RCAN1 pathway. Mol Psychiatry 22: 13-23.
- Tariq MI, Afzal S, Hussain I, Sultana N (2007) Pesticides exposure in Pakistan: A review. Environ Int 33: 1107-1122.
- Ali SA, Khan SA, Naaz I, Ali AS (2015) Adverse health effects of pesticide exposure in workers of a pesticide manufacturing factory.
- Fareed M, Pathak MK, Bihari V, Kamal R, Srivastava AK, et al. (2013) Adverse respiratory health and hematological alterations among agricultural workers occupationally exposed to organophosphate pesticides: A cross-sectional study in North India. PLoS One 8: e69755.
- Yassin MM, Al-Shanti TA (2016) Effect of pesticides on kidney function and serum pro-tein profile of farm workers in Gaza Strip. Ann Med Biomed Sci 2: 21-27.
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaiee A (2004) Pesticides and oxidative stress: A review. Med Sci Monit 10: RA141-RA147.
- Karalliedde L, Wheeler H, Maclehose R, Murray V (2000) Possible immediate and long-term health effects following exposure to chemical warfare agents. Public Health 114: 238-248.
- Rahman MF, Siddiqui MK (2003) Biochemical enzyme activity in different tissues of rats exposed to a novel phosphorothionate (RPRâ€V). J Environ Sci Health B 38: 59-71.
- Sahin I, Onbasi K, Sahin H, Karakaya C, Ustun Y, et al. (2002) The prevalence of pancreatitis in organophosphate poisonings. Hum Exp Toxicol 21: 175-177.
- Ali SA, Khan SA, Naaz I, Ali AS (2015) Adverse health effects of pesticide exposure in workers of a pesticide manufacturing factory.
- Janardhan A, Sisodia P (1990) Monocrotophos: Short-term toxicity in rats. Bull Environ Contam Toxicol 44: 230-239.
- Lum G, Gambino SR (1972) Serum gamma-glutamyl transpeptidase activity as an indicator of disease of liver, pancreas, or bone. Clinical Chemistry 18: 358-362.
- Varley H, Van E, Kass I (1980) Practical clinical chemistry. In: William Heinemann Medical Books London, p834.
- Dawson S, Stahl S, Paul N, Barber J, Kenna JG (2012) In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40: 130-138.
- Rathod N, Kshirsagar R (2010) Trace metals content in tissues of freshwater fish Puntius arenatus from Gomai River, North Maharashtra. Bionotes 12: 96.
- Nigg HN, Knaak JB (2000) Blood cholinesterases as human biomarkers of organophosphorus pesticide exposure. Rev Environ Contam Toxicol 163: 29-111.
- Afaf A, Hanan A (2010) Chlorpyrifos (from different sources): Effect on testicular biochemistry of male albino rats. J Am Sci 6: 251-261.
- Asztalos B, Nemcsók J, Benedeczky I, Gabriel R, Szabo A, et al. (1990) The effects of pesticides on some biochemical parameters of carp (Cyprinus carpio L.). Arch Environ Contam Toxicol 19: 275-282.
- Acharya J, Dubey DK, Srivastava AK, Raza SK (2011) In vitro reactivation of sarin-inhibited human acetylcholinesterase (AChE) by bis-pyridinium oximes connected by xylene linkers. Toxicology in Vitro 25: 251-256.
- Baird MF, Graham SM, Baker JS, Bickerstaff GF (2012) Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab 2012: 960363.
- Bhattacharyya K, Phaujdar S, Sarkar R, Mullick OS (2011) Serum creatine phosphokinase: A probable marker of severity in organophosphorus poisoning. Toxicol Int 18: 117.
- Morandi L, Angelini C, Prelle A, Pini A, Grassi B, et al. (2006) High plasma creatine kinase: review of the literature and proposal for a diagnostic algorithm. Neurological Sciences 27: 303-311.
- Bellamy COC, Malcolmson RDG, Harrison DJ, Wyllie AH (1995) Cell death in health and disease: The biology and regulation of apoptosis. Seminars in Cancer Biology 6: 3-16.
- Turan B, Tuncay E, Vassort G (2012) Resveratrol and diabetic cardiac function: Focus on recent in vitro and in vivo studies. J Bioenerg Biomembr 44: 281-296.
- Lockridge O, Masson P (1999) Pesticides and susceptible populations: People with butyrylcholinesterase genetic variants may be at risk. Neurotoxicology 21: 113-126.
- Whitfield J, Pounder R, Neale G, Moss D (1972) Serum γ-glutamyl transpeptidase activity in liver disease. Gut 13: 702-708
- Allderdice P, Gardner H, Galutira D, Lockridge O, LaDu B, et al. (1991) The cloned butyrylcholinesterase (BCHE) gene maps to a single chromosome site, 3q26. Genomics 11: 452-454.
- Eddleston M, Buckley NA, Eyer P, Dawson AH (2008) Management of acute organophosphorus pesticide poisoning. The Lancet 371: 597-607.
- Çelik I, Çamaş H, Arslan O, Ioğlu ÖİK (1996) The effects of some pesticides on human and bovine erythrocyte carbonic anhydrase enzyme activities in vitro. J Environ Sci Health A 31: 2651-2657.
Citation: Rehman T, Hussain M, Shad MA, Ghosh S, Aslam M (2018) Combined Effect of Age and Exposure on the Levels of Different Serum Enzymes in Workers of Pesticides Formulation Factories, Pakistan. Biochem Physiol 7: 238. DOI: 10.4172/2168-9652.1000238
Copyright: © 2018 Rehman T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5582
- [From(publication date): 0-2018 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 4620
- PDF downloads: 962