Mini Review Open Access
Commercializing Biosimilar: Challenges, Strategies and Finding Path to Success
Prashant A Pandya*
Department of Program Management, Clinical Development, Navi Mumbai, India
- *Corresponding Author:
- Prashant A Pandya
Department of Program Management
Clinical Development, Navi Mumbai, India
Tel: +91-9967017172
E-mail: drpandya18@gmail.com
Received Date: August 02, 2017; Accepted Date: August 12, 2017; Published Date: August 21, 2017
Citation: Pandya PA (2017) Commercializing Biosimilar: Challenges, Strategies and Finding Path to Success. Clin Pharmacol Biopharm 6:173. doi: 10.4172/2167-065X.1000173
Copyright: © 2017 Pandya PA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Biosimilars and reference biologics have the same amino acid sequences. Differences in clinically inactive components are generally quite minor. Developments of Bio similar involve a series of complex decision and anyone of which have significant impact on Organization success. Early risk identification allows companies to put preventive measures in place to save development time and maximize return on investment. There are numerous development and regulatory constraints associated with biosimilar development affecting decision of commercialization. It is important to optimize commercialization strategies as regulations are still evolving hence it is vital for the companies to quickly modify biosimilar development strategies matching with the regulatory scenario.
Keywords
Program management; Project management; Biosimilar; Drug development; Strategies
Introduction
Market for the biosimilar is growing in faster pace and development is currently at peak. There is an ever-increasing pressure to reduce healthcare costs as treatment with biologic cost around $1,00,000-4,00,000 a year to patient. There are unique development and commercialization challenges in biosimilar hence requiring detailed clinical development plan including better defined regulatory pathways and information about market access (Figure 1) [1-4].
Biosimilar market
The global biosimilars market is expected to reach USD 14 Billion by 2022 from USD 3.5 Billion in 2016, at a CAGR of 26% during the forecast period.
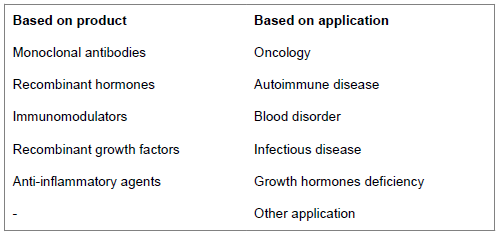
Biosimilar market segment
Below factors influence market potential of biosimilar:
• Physician preference-Since biosimilar are not like simple generics hence it’s difficult to prove that drug is similar enough like originator.
• Patient acceptance.
• Reimbursement from Insurance agency.
• Development cost.
• Cost difference with originator.
• Indication (chronic v acute).
• Sales experience and global tie-ups.
It is not easy to convenience multiple stakeholders to switch from originator to biosimilar unless having unique marketing strategy and significant cost difference.

Commercialization Challenges
Traditional products are created through chemical process due to simple and well-defined structure & having small molecules (Molecular weight ~150 Daltons) hence it is easy to produce identical API unlike biosimilar having large molecule (Molecular weight ~150,000 Daltons having complex structure and structural variation) needs larger clinical trials. Biologics generally derived from the living sources like bacteria, yeast and are typically proteins and antibodies. Below are the key commercialization challenges [4-8].
Funding for product development and commercial manufacturing
It is difficult to obtain adequate funding from Venture Capitalist (VC), Angel investor or Govt. for the product development and commercialization due to competitive market space. Identification of proper funding option is important for the successful commercialization.
Scaling manufacturing to meet commercial requirements
Unlike the manufacturing processes of small-molecule drugs that can be chemically synthesized and easily replicated, a biologic is produced from living organisms and has more complexity and heterogeneity (duplicate text was observed in above two lines, please modify) hence it is difficult to scaling manufacturing. Identification of cost effective raw material and finding effective manufacturing partner (considering geographical proximity) is key for the commercialization success.
Meeting regulatory obligations/compliance of Biosimilar products
Regulations are still evolving hence meeting regulatory obligations and compliance is difficult in biosimilar development unless company adopt integrated strategic plan at the start of program having opinion from experts and stepwise input on regulatory and market to meet obligations.
Other challenges
• The competitive landscape.
• Frequent changes and uncertainty in approval processes.
• A current pricing system that reflects cost rather than value.
• The activities to be performed versus outsourced.
• High technology uncertainty over long time frames and the need for both significant complementary assets and substantial financing.
Strategies and Path to Success
Commercialization of biosimilar encounters series of unexpected challenges as well as opportunities. It requires app. $40-70 million investment and typically priced at around 30% discount in comparison to innovator. Evolving regulatory framework in relation to patient, payer and physician demand careful strategic consideration while implementing commercialization strategies.
Biosimilar sponsors will need to do following:
• Take position on fundamental regulatory issues and develop global strategy.
• Optimizing global, regional and cross-functional collaboration.
• Conduct in-depth pricing analysis and formulate regional strategy.
• Develop patient education series/campaign. Patients must be educated so that they’re willing to switch to a newer product that may not offer the same level of patient support.
• Choose markets with limited or no access to innovator biologic.
• Select country/ region where regulatory agencies are willing to work with companies to bring biosimilar market to reduce healthcare cost.
• Prioritize market entry based on changes in regulatory approvability commercial viability.
Conclusion
The biosimilars market will continue to evolve rapidly and with uncertainty. Successfully developing and marketing a biosimilar presents a unique and contradictory challenge. In biosimilar, commercialization strategies goes beyond target audience team to include prelaunch, customer profiling, data management, program management, scaling manufacturing, data analytics, quality control and more. Outsourcing the commercialization operation can be advantageous in a wide range of biosimilar portfolio.
References
- Lewis G (2014) “Pharma Transformation in Turbulent Times,” presented at DCAT week.
- Fritz T, Lightcap C, Shah K (2012) Manufacturing strategies for biosimilars. Pharma Manufacturing.
- Groves RM, Fowler FJ, Couper MP (2009) Survey methodology (2nd edn.). Hoboken, NJ: John Wiley & Sons.
- Pandya PA (2017) Managing pharmaceuticals and life sciences projects on a global scale: a project management perspective. J Bioanal Biomed 9: 169-172.
- Wang J, Chow SC (2012) On the regulatory approval pathway of biosimilar products. Pharmaceuticals (Basel) 5: 353-368.
- Zelenetz AD, Ahmed I, Braud EL (2011) NCCN Biosimilars White Paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Canc Netw 9: S-1-S-22.
- Pandya P (2017) Managing complex R&D projects-strategies from project management perspectives. Chronicles of Pharmaceutical Science 1: 128-131.
- http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC500226648.pdf
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 3671
- [From(publication date):
September-2017 - Aug 18, 2025] - Breakdown by view type
- HTML page views : 2771
- PDF downloads : 900