Research Article Open Access
Comparative Studies of Chemical Composition, Antioxidant and Antimicrobial Potentials of Essential Oils and Oleoresins Obtained from Seeds and Leaves of Anethum graveolens L.
Sunita Singh1*, SS Das1, G Singh Marina Perroti1, Carola Schuff1 and César A N Catalan2
1Chemistry Department Deen Dayal Upadhyaya Gorakhpur University Gorakhpur, 273009, UP, India
2Faculty of Biochemistry, Chemistry and Pharmacy, Institute of Organic Chemistry, National University of Tucumán, S. M. de Tucumán T4000INI, Argentina
- *Corresponding Author:
- Singh S
Chemistry Department Deen Dayal Upadhyaya Gorakhpur University Gorakhpur, India
Tel: (91)5512270301
Fax: (91)551-2340459
E-mail: oceans.singh@gmail.com
Received Date: October 10, 2016; Accepted Date: January 08, 2017; Published Date: January 18, 2017
Citation: Singh S, Das SS, Perroti GSM, Schuff C, Catalan CAN (2017) Comparative Studies of Chemical Composition, Antioxidant and Antimicrobial Potentials of Essential Oils and Oleoresins Obtained from Seeds and Leaves of Anethum graveolens L.. Toxicol Open Access 3:119. doi:10.4172/2476-2067.1000119
Copyright: © 2017 Singh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
The chemical composition, antioxidant and antimicrobial potentials of essential oils and oleoresins obtained from dill (Anethum graveolens L.) seeds and leaves have been studied. The isolated essential oils and oleoresins were analyzed by gas chromatography coupled with mass spectrometry. The antioxidant activity of essential oils and oleoresins in comparison with synthetic antioxidants were evaluated using different antioxidant assays namely peroxide, thiobarbituric acid values. The antioxidant potentials were further confirmed using other methods such as ferric thiocyanate method in linoleic acid system, scavenging effect (%) on DPPH radical and metal chelating activity. Total phenolic content was also calculated using Folin-Ciocalteau method. Their antimicrobial activity was investigated using various methods on an array of microorganisms. Carvone (47.2%) was the major component in seed essential oil whereas dillapiole (90.2%) was the main component of leave essential oil. The oleoresins were mainly having a major fraction of carvone, dillapiole and oleic acid. Both essential oils have shown excellent antioxidant activity. The seed essential oil showed excellent antimicrobial activity against Aspergillus flavus and Fusarium graminearum in food poisoned method. Significant antibacterial activity was recorded for essential oils against Staphylococcus aureus and Bacillus subtilis while nominal inhibitory effects were observed against Escherichia coli and Pseudomonas aeruginosa.
Keywords
Essential oils; Antioxidant; Total phenolic content; Gaschromatography- mass spectrometry; Antimicrobial activity
Introduction
Herbs and spices have long been used by ancient civilizations for culinary, medicinal and cosmetic uses. With modernization and the development of patent medicines, the use of natural cures and elixirs decrease in popularity. Nowadays, disillusionment with synthetic drugs, artificial additives and their possible side effects [1] has given great rise in popularity to many natural products, be it for culinary, medical or cosmetics purposes.
Anethum graveolens L. (Dill) is a member of Apiaceae family commonly called sowa or vilayti saunf in India. It has been recognized in different system of traditional medicines for the treatment of different diseases and ailments of human beings. The extracts obtained from aerial parts, seeds have antibacterial, antispasmodic, hyperlipidimic, antiulcer, antioxidant, hypolipidemic, genotoxicity, diuretic effect [2]. The diversified uses of dill have attracted many researchers to explore their different potentials, which have been well discussed in literature. The current study was aimed to analyze the difference in chemical composition of essential oils and oleoresins obtained from different part of A. graveolens (seeds and leaves) along with their antioxidant, antimicrobial potentials has also been investigated by different methods. Different samples of same plant have been used to analyse the chemical variance using GC-MS techniques and how these variations affect the antioxidant and antimicrobial properties.
Materials and Methods
Extraction of essential oils and oleoresins
Fresh aerial parts of the dill herb (leaves and seeds) were purchased from the local market of Gorakhpur, Uttar Pradesh, India. A voucher specimen was deposited at the herbarium of the Faculty of Science, Deen Dayal Upadhyaya Gorakhpur University. The leaves and seeds were washed with running water, air dried and then homogenized into fine powder. The dill seed essential oil (DSEO, light yellow colored; yield 2.4%) and dill leaves essential oil (DLEO, green colored; yield 0.9%) was extracted by a hydrodistillation process using a Clevenger’s type apparatus in accordance with the method recommended by the European Pharmacopoeia [3].
The oleoresins (DSET-Dill seeds ethanol oleoresin; DSNH-Dill seeds n-hexane oleoresin; DLET- Dill leaves ethanol oleoresin; DLNHDill leaves n-hexane oleoresin) were extracted from seeds and leaves by using a Soxhlet apparatus. Different reports reveal the utility of more polar solvents for better extraction of components [4]. For this reason, ethanol (polar) and n-hexane (non-polar) have been used for extraction to analyse the difference in chemistry. The essential oil and oleoresins so obtained were poured into bottles and stored in a refrigerator at 4 ± 1°C in the dark for further use.
Reagents
Folin- Ciocalteu (FC) reagent (Qualigens chemicals Ltd. Mumbai, India) and Gallic acid (Qualikems chemicals Ltd. New Delhi, India) were used for the evaluation of total phenolic content. Thiobarbituric acid (TBA), 1,1’-diphenyl-2-picrylhydrazyl radical (DPPH) and linoleic acid are of Acros (New Jersey, USA); BHT, BHA, ethylene diamine tetraacetic acid (EDTA) and propyl gallate (PG) are of sd fine chemicals Ltd. Mumbai, India, Tween 20 and Ferrozine (Merck Pvt. Ltd. Mumbai, India) were used for antioxidant assays. Ampicillin was purchased from Ranbaxy fine chemicals (New Delhi) India. Crude mustard oil was obtained from local oil mill in Gorakhpur. All solvents used were of analytical grade.
Chemical characterization
Gas chromatography-mass spectrometry (GC-MS): Oil samples and oleoresins were analyzed by GC with a gas chromatograph HP 6890, coupled with MS HP 5973. The gas chromatograph has a splitless injector and a capillary column -5MS (5% phenylmethyl siloxane phase, 30 m × 0.25 mm × 0.25 μm) film thickness. The GC conditions include a temperature range of 50 to 300°C. The temperature of the injector was maintained at a temperature of 250°C. The inert gas was helium at a flow of 1.0 mL/min and the volume of injected sample in the split ratio 80:1. The oven temperature is programmed at various temperature namely at 80°C (0 min); 80-300°C (3.5°C/min) and 300°C (5 min). The interphase and ion source temperature were at 280°C and 230°C. The selective mass detector was programmed at 150°C.
Identification of the constituents was based on comparisons of retention index (RI) (determined relatively to the retention times of nalkanes series) and mass spectra with those obtained from authentic standards and/or the NIST and Wiley libraries spectra and relevant literature [5].
Estimation of total phenolic content (TPC)
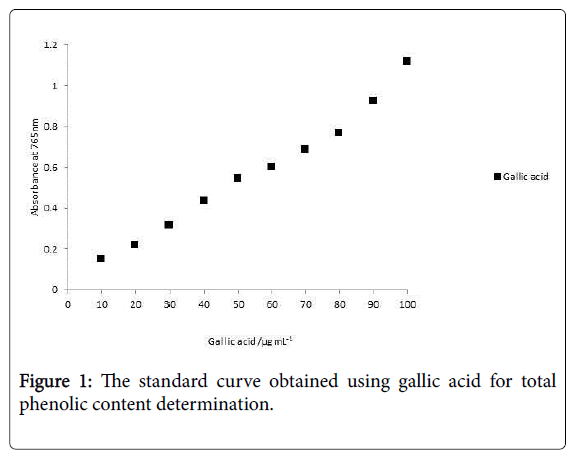
TPC were determined using the Folin- Ciocalteu’s reagent method described by Singleton and Rossi. Gallic acid stock solution (1000 μg.mL-1) was prepared by dissolving 100 mg of gallic acid in 100 mL of ethanol. Various dilutions of standard gallic acid were prepared from this stock solution. Calibration curve (Figure 1) was plotted by mixing 1 mL aliquots of 10-100 μg.mL-1 of gallic acid solutions with 5.0 mL of Folin-Ciocalteu reagent (diluted tenfold) and 4.0 mL of sodium carbonate solution (75 g.L-1). The absorbance was measured after 30 min at 20°C at 765 nm.
Antioxidant assays
Sample preparation: The essential oils and oleoresins were added individually to unrefined crude mustard oil at the concentration of 200 ppm (v/v). Synthetic antioxidants such as BHA, BHT and PG were also added to mustard oil at the same concentration, i.e. 200 ppm (v/v). A control sample was prepared under similar condition without any additive. They were subjected to the Schaal oven test [6] in 100 mL open beakers at 60°C. The antioxidant activity of essential oils and oleoresins in the oxidation of mustard oil was examined by comparing the activity of known antioxidants such as BHA, BHT and PG by the methods reported by Singh et al. [7] for peroxide and TBA values.
Complementary antioxidant assays
Further determination of antioxidant activity of essential oils and oleoresins were determined by the methods reported earlier by Singh et al. [8] namely; ferric thiocyanate method, metal chelating activity and radical scavenging activity on DPPH.
Antimicrobial investigations
All the fungal and bacterial strains for antimicrobial investigations were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh, India.
Antifungal investigations
In order to determine the antifungal efficacy of the volatile oil and oleoresins, the pathogenic fungus Aspergillus niger (1884), Aspergillus flavus (2479), Fusarium monoliforme (1893), Fusarium graminearum (2088) and Penicillium viridicatum (2007) were undertaken. Cultures of each of the fungi were maintained on Czapek dox agar media with adjusting pH 6.0-6.5 and slants were stored at 4°C. The antifungal activity of the volatile oil and oleoresins against fungi were undertaken using inverted petriplate [9] and poison food techniques [10].
Antibacterial investigations
Four pathogenic bacteria Bacillus subtilis [1790], Staphylococcus aureus [3103] (gram positive), Escherichia coli [1672], Pseudomonas aeruginosa [1942] (gram negative) were selected for present study. The agar well diffusion method [10] was employed for the determination of antibacterial activity. Briefly, a suspension of the test microorganism (0.1 ml) was spread on a previously prepared, dried nutrient agar (contains peptone, sodium chloride, beef extract, yeast extract mixed in appropriate composition) plate by using a sterile bent rod. The wells were 10 mm in diameter cut from the agar and different concentration of essential oils or extracts (5 and 10 μl diluted in 1 mL dimethyl sulphoxide) were delivered into them. The control plate without the addition of essential oil or extract containing DMSO was also maintained under the same conditions. After incubating for 24 h at 37°C, all plates were examined for any zones of growth inhibition (in mm).
Statistical analyses
Experimental results were the means ± standard deviation of three parallel measurements (data are not shown). Significant differences between means were determined by Student’s t-test by using a Microsoft Excel (Microsoft Office, India) statistical analysis program and p<0.05 was considered as significant. Standard deviation bars has been provided in the figures.
Result and Discussion
Chemical investigations
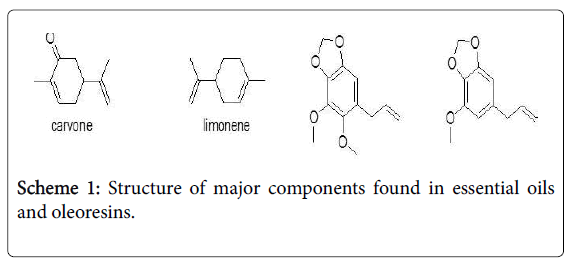
The chemical composition of essential oils and oleoresins were analysed by GC-MS. The identified compounds are listed in Tables 1 and 2 according to their elution order on a Perkin Elmer Elite -5MS capillary column. A total of 23 and 27 components were identified in DSEO and DLEO representing 99.4 and 97.4% of total oil composition obtained respectively. Principle components of DSEO were carvone (47.7%), dillapiole (32.7%), limonene (12.4%), trans-dihydrocarvone (2.7%) and cis-dihydrocarvone (2.1%). Components identified in DLEO were dillapiole (90.2%), myristicin (3.0%) with apiole (1.3%). The major components in oleoresins were oleic acid, Palmitic acid, dillapiole and Stigmast-en-3-β-ol. Structures of some major components found in essential oils and oleoresins are given in scheme 1.
| Compounds | DSEO | DLEO | RI# | IdentificationΦ |
|---|---|---|---|---|
| % MS | % MS | |||
| α−τηυÏÂ?ενε | --- | tr | 925 | MS, RI, co-GC |
| α−πινενε | tr | 0.1 | 928 | MS, RI, co-GC |
| myrcene | tr | tr | 990 | MS, RI, co-GC |
| a-phellandrene | tr | 1.3 | 1004 | MS, RI, co-GC |
| p-cymene | tr | 0.1 | 1019 | MS, RI, co-GC |
| limonene | 12.4 | tr | 1024 | MS, RI, co-GC |
| b-phellandrene | --- | 0.3 | 1025 | MS, RI, co-GC |
| fenchone | tr | --- | 1085 | MS, RI |
| p-cymenene | 0.2 | tr | 1090 | MS, RI |
| menthol | 0.7 | 0.1 | 1180 | MS, RI |
| dill ether | tr | 0.1 | 1184 | MS, RI |
| cis-dihydrocarvone | 2.1 | tr | 1194 | MS, RI |
| trans-dihydrocarvone | 2.7 | tr | 1203 | MS, RI |
| trans-carveol | tr | tr | 1219 | MS, RI, co-CG |
| carvone | 47.7 | tr | 1242 | MS, RI, co-CG |
| trans-carvone oxide | tr | tr | 1276 | MS, RI |
| trans-anethole | tr | tr | 1285 | MS, RI |
| thymol | tr | tr | 1292 | MS, RI |
| carvacrol | tr | 0.1 | 1300 | MS, RI |
| germacrene-D | --- | 0.3 | 1490 | MS, RI |
| trans-b-ionone | tr | 0.1 | 1492 | MS, RI |
| myristicin | 0.9 | 3 | 1520 | MS, RI |
| elemicin | tr | tr | 1559 | MS, RI |
| dillapiole | 32.7 | 90.2 | 1625 | MS, RI |
| selin-11-en-4-a-ol | --- | 0.2 | 1660 | MS, RI |
| ar-turmerone | tr | tr | 1670 | MS, RI |
| apiole | tr | 1.3 | 1680 | MS, RI |
| neocnidilide | --- | 0.2 | 1722 | MS, RI |
| Total | 99.4 | 97.4 | ||
| DSEO-Dill seed essential oil; Dill leaves essential oil trace<0.05; # the retention index was calculated using a homologous series of n-alkanes C8-C18; ΦCo-GC: co-injection with an authentic sample. Percentages are the mean of three runs and were obtained from electronic integrationmeasurements using selective mass detector |
||||
Table 1: Composition of essential oils extracted from dill (Anethum graveolens) seeds and leaves.
| Compounds | DSET | DSNH | DLET | DLNH | RI# | IdentificationΦ |
|---|---|---|---|---|---|---|
| % MS | % MS | % MS | % MS | |||
| limonene | 0.2 | 1.8 | ---- | ---- | 1024 | MS, RI, co-GC |
| (E)-2-decenal | ---- | ---- | 3.6 | ---- | 1189 | MS, RI |
| cis-dihydrocarvone | 0.8 | 0.5 | ---- | tr | 1194 | MS, RI |
| trans-dihydrocarvone | 0.2 | 0.6 | ---- | tr | 1203 | MS, RI |
| carvone | 12.2 | 15.2 | ---- | tr | 1242 | MS, RI |
| (E)-2-undecenal | ---- | ---- | 21.8 | ---- | 1357 | MS, RI |
| myristicin | 0.5 | 0.7 | Tr | tr | 1520 | MS, RI |
| elemicin | 0.2 | 0.2 | 3.3 | tr | 1559 | MS, RI |
| tetradecanal | ---- | ---- | 1.4 | ---- | 1611 | MS, RI |
| dill apiole | 29.6 | 34.7 | 0.8 | 4.9 | 1625 | MS, RI |
| palmitaldehyde | ---- | ---- | 2.7 | ---- | 1720 | MS, RI |
| myristic acid | 0.2 | 0.2 | ---- | ---- | 1739 | MS, RI |
| neophytadiene | ---- | ---- | ---- | 2.5 | 1820 | MS, RI |
| hexahydrofarnesylacetone | ---- | ---- | 1 | ---- | 1848 | MS, RI |
| olealdehyde | ---- | ---- | 1 | ---- | 1907 | MS, RI |
| palmitic acid | 4.6 | 3.5 | 3.7 | 3.9 | 1967 | MS, RI, co-GC |
| ethyl palmitate | 0.3 | tr | Tr | tr | 1980 | MS, RI, co-GC |
| linoleic acid | 8 | 7.9 | Tr | tr | 2124 | MS, RI, co-GC |
| oleic acid | 21.1 | 21.2 | 12.4 | 12.9 | 2128 | MS, RI, co-GC |
| stearic acid | 0.9 | 1.9 | 0.7 | tr | 2157 | MS, RI, co-GC |
| monopalmitin | 0.1 | tr | ---- | 1.3 | ---- | MS |
| monoolein | 3.6 | 2.9 | 5.3 | 7.5 | ---- | MS |
| Plasticizer | 0.9 | ---- | ---- | ---- | ---- | MS |
| 10-nonacosanone | 2.3 | 2.7 | 2.4 | 19.6 | ---- | MS |
| vitamin E | 0.2 | 0.2 | ---- | tr | ---- | MS |
| ergost-5-en-3β-ol | 0.3 | tr | ---- | tr | ---- | MS |
| stigmast-5,22-dien-3-β-ol | 0.9 | 0.6 | 0.9 | 8.5 | ---- | MS |
| 16-hentriacontanone | 7.7 | 0.6 | 24.4 | 3.2 | ---- | MS |
| Tritriacontane | tr | tr | 1 | 8.8 | ---- | MS |
| stigmast-5-en-3-β-ol | 2 | 1.2 | 1.8 | 7.5 | ---- | MS |
| Total | 96.8 | 96.6 | 88.2 | 80.6 | ||
| DSET: Dill Seeds Ethanol Oleoresin; DSNH: Dill Seeds n-hexane Oleoresin; DLET: Dill Leaves Ethanol Oleoresin; DLNH- Dill Leaves n-hexane Oleoresin; tr: Trace <0.05%; # the retention index was calculated using a homologous series of n-alkanes C8-C22; ΦCo-GC: co-injection with an authentic sample. Percentages are the mean of three runs and were obtained from electronic integration measurements using selective mass detector. | ||||||
Table 2: Composition of oleoresins in different solvents extracted by dill (Anethum graveolens) seeds and leaves.
The results obtained from the analysis are in good agreement with the previous reported work [11-13]. It has been previously reported that during the developmental growth of seeds, the conversion of dillapiole to oxygenated terpenes increases [14]. Most of the work reported that carvone as the major component in essential oil of A. graveolens. These contradictory results of leaves and seeds can be reasoned on differences in pedoclimatic conditions, variety, and different agricultural practices [15].
Antioxidant investigations
Amount of total phenolic compounds can be determined using the Folin-Ciocateu reagent. FC assay is not selective; it determines both polyphenols and monophenolics. The absorbance at 765 nm plotted as concentration of standard gallic acid solution is illustrated in Figure 1. The representative regression coefficient (r2) was 0.9911 and the linear regression equation was y=0.0101x+0.0178.
GAE for DSEO and DLEO was found to be 52.38 ± 0.27 and 28.76 ± 1.2 μg/mL whereas the total phenolic content of oleoresins obtained from both seeds and leaves were very low and ranges from 5.6-12.3 μg/mL. Results obtained regarding the total phenolic content was positively correlated with the previous work done [16].The variation in phenolic content [17,18].
The antioxidant activities of spice extracts have been widely studied although their mechanisms are not fully understood. Antioxidants are the compounds which delay autoxidation by inhibiting formation of free radicals or by interrupting propagation of the free radical by one (or more) of several mechanisms: (1) scavenging species that initiate peroxidation, (2) chelating metal ions such that they are unable to generate reactive species or decompose lipid peroxides, (3) quenching •O2- preventing formation of peroxides.
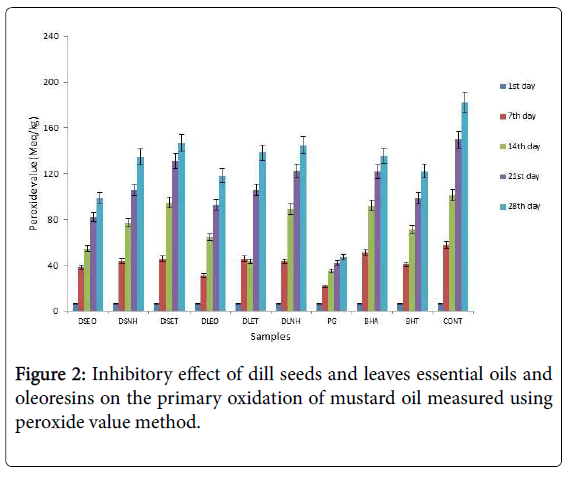
Peroxide values (PVs) were estimated in all samples to determine the extent of peroxide formation due to oxidation of fat (milliequivalents of O2/kg of fat) during the storage period of 28 days (Figure 2). During this time, the peroxide value of blank sample increased to 181.8 meq/kg. The initial peroxide value in all samples was low and increased during the storage. The effectiveness of the additives at concentration of 200 ppm can be put into the following order: PG>DSEO>DLEO>BHT>BHA>DSNH>DSET>DLNH>DLET>Control.
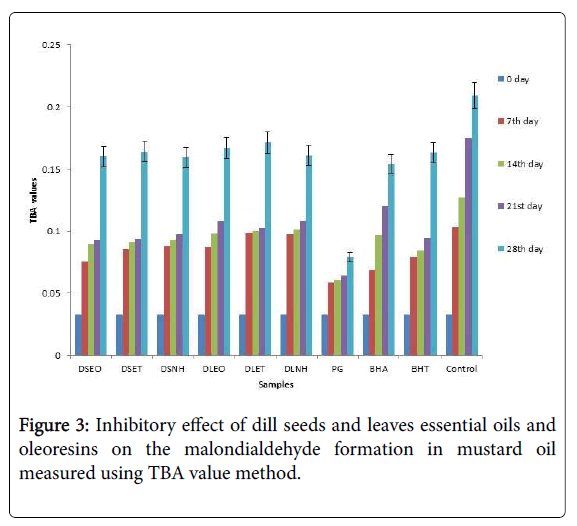
Many secondary products such as alkenes, alcohols, aldehydes and acids were produced during the primary oxidation. In vitro lipid peroxidation was assessed by means of an assay system that determines the production of malondialdehyde and related compounds in the mustard oil [19]. Malondialdehyde is one of the major degradative products of lipid peroxidation and serves as a marker for oxidative stress. The effects of essential oils and oleoresins on malondialdehyde formation for mustard oil in terms of incubation time versus TBA value at 80°C are shown in Figure 3. These results were well supported by the work done by Mousavi et al. [20]. In another study, Murica et al. [21] stated that mint, dill, and ginger could improve the oxidation stability of sunflower, corn and olive oils as well as butter and margarine at 110°C.
The formation of the primary oxidation species, peroxides were quite similar with the secondary oxidation products and the changes of both oxidation characteristics are in good correlation. From the figures it was clear that the inhibition activity of extract was good among the additives and there was a significant difference between the blank and antioxidants at the p<0.05 level.
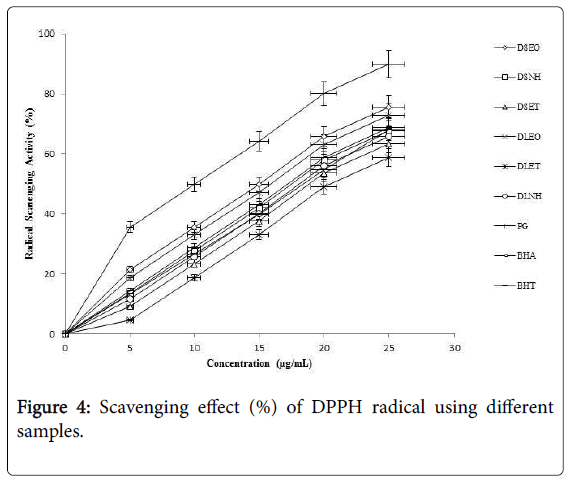
Stable organic radical DPPH has been used widely for determination of antiradical activity for all kind of products. The freeradical scavenging activity of A. graveolens essential oils and oleoresins evaluated using the DPPH method is presented in Figure 4. DSEO and DLEO scavenged the DPPH radicals in a concentration-dependent manner with the maximum scavenging activity of 75% and 67% respectively. PG was found to have maximum scavenging activity at all tested concentrations. There was a significant difference (p<0.05) between the PG and other tested samples.
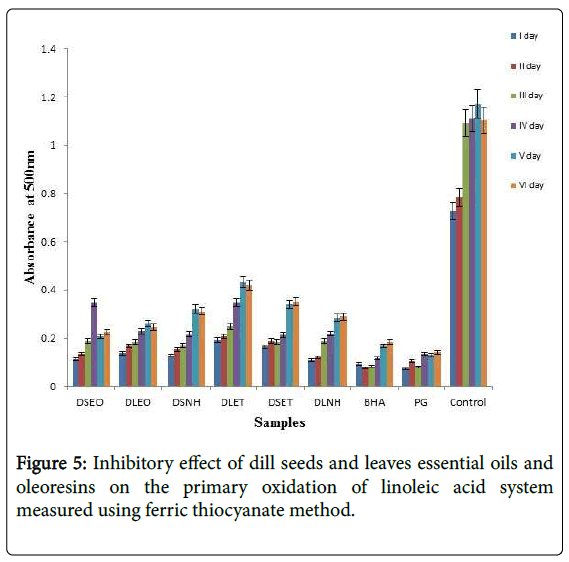
Ferric thiocyanate method is based on the ability of hydroperoxides to oxidize ferrous ions (Fe2+) to ferric ions (Fe3+) in an acidic medium. Ferric thiocyanate is a red-violet complex with absorption spectra at 500-510 nm. In Figure 5, the control gave maximum absorbance up to day 4 and started to decrease since the linoleic acid was already used up and no more hydroperoxides were available to oxidize Fe2+ until this stage [22]. DSEO and DLEO and showed considerable antioxidative properties in the linoleic acid system and were significantly (p<0.05) different from the control. However there was no significant difference (p<0.05) between the antioxidative potentials of extracted oleoresins.
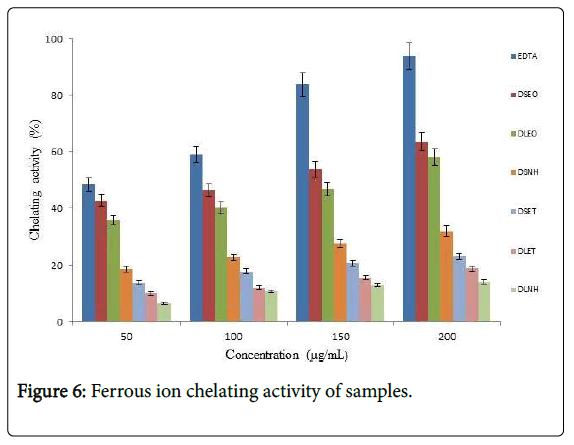
Iron chelating activity also was determined at different concentrations with EDTA as standard compound. The maximum percentage of iron chelating activity of the essential oils and oleoresins is presented in Figure 6. The standard EDTA was observed to be the highest (93.95%) at concentration of 200 μg/mL. DSEO and DLEO had ferrous ion chelating (FIC) activities of 63.56 ± 0.21 and 58.12 ± 1.9 percent at 200 μg/mL respectively.
Ferrozine can quantitatively form complexes with Fe2+. In the presence of other chelating agents, the complex formation is disrupted with the result that the red color of the complex is decreased. Measurement of the rate of color reduction therefore allows estimation of the chelating activity of the coexisting chelator [23]. However, EDTA showed an excellent chelating ability. Iron is the most important lipid oxidation pro-oxidant among the transition metals. Lipid oxidation is accelerated by breaking down of hydrogen peroxide and lipid peroxides by ferrous state of iron to reactive free radicals through the reaction:
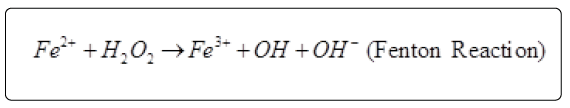
Radicals from peroxides are also produced by Fe3+ ion. However the rate is one tenth of Fe2+ ion. These can lead to lipid peroxidation, modification of protein and damage to DNA. Metal ions could be inactivated by chelating agents and the metal dependent processes could potentially be inhibited [24]. There is no correlation between ferrous ion chelating (FIC) and TPC.
Various studies regarding the antioxidant potentials of A. graveolens L. essential oils and extracts has been done [25] which led to the conclusion that presence of carvone, limonene, dillapiole are responsible for the antioxidant properties of the essential oils. Presence of unsaturated fatty acids in oleoresins also attributed to the antioxidant properties of oleoresins. The role of unsaturated fatty acids as antioxidant is still under consideration and area of research to the scientists [26]. Many researchers have concluded that drying processes has influence on the contents of total phenolics which are directly related to the antioxidant properties of the samples [27]. There was a significant decrease in the components during the drying processes [28] which results in the declined activity of DLEO and its oleoresins. Amin et al. [29] reported that less intensive aquathermal processing of leafy vegetables, such as blanching, causes a decrease of 50% in antioxidant activity. The results obtained for oleoresins in various different antioxidant assays carried out in this study were satisfactory. However, there are no significant (p<0.05) differences between antioxidative activities of DSET, DSNH, DLNH and DLET.
Antimicrobial investigations
The antimicrobial activities of essential oils and oleoresins against the microorganisms examined in the present study and their potency was qualitatively assessed by presence or absence of inhibition zones. In both petriplate and food poisoned method (Tables 3a and 3b), both oils namely DSEO and DLEO showed excellent activity (>80% zone of inhibition) against A. flavus at 10 μL dose. These oils were also found to be highly effective in controlling the growth of A. niger, F. graminearum (>65% zone of inhibition) in the food poisoned method whereas the zone of inhibition were in the range from 40-50% in inverted petriplate method. These oils were less effective against P. viridicatum in both methods.
| Samples | Doses(µl) | AF* | AN* | FM* | FG* | PV* |
|---|---|---|---|---|---|---|
| DSEO | 5 | 71.2 ± 0.50 | 43.6 ± 0.30 | 15.7 ± 1.3 | 39.7 ± 0.14 | 14.7 ± 0.6 |
| 10 | 89.7 ± 0.20 | 63.9 ± 0.36 | 20.3 ± 1.8 | 65.7 ± 0.17 | 17.6 ± 0.7 | |
| DSET | 5 | 17.8 ± 2.4 | 5.7 ± 0.20 | 8.9 ± 0.20 | 10. ± 0.20 | - |
| 10 | 41.9 ± 0.3 | 11.2 ± 0.30 | 13.2 ± 0.30 | 11.3 ± 0.14 | - | |
| DSNH | 5 | 54.5 ± 1.2 | 0.2 ± 0.44 | 4.3 ± 0.17 | 2.4 ± 0.36 | 5.6 ± 0.54 |
| 10 | 39.8 ± 0.1 | 5.5 ± 0.46 | 7.6 ± 0.14 | 9.1 ± 0.41 | 9.7 ± 0.6 | |
| DLEO | 5 | 50.1 ± 2.1 | 19.8 ± 0.20 | 11.2 ± 0.7 | 32.6 ± 1.1 | 18.1 ± 0.6 |
| 10 | 81.2 ± 2.3 | 25.2 ± 0.26 | 16.4 ± 3.6 | 67.1 ± 1.7 | 20.9 ± 0.8 | |
| DLET | 5 | - | - | - | - | - |
| 10 | 21.3 ± 1.2 | 18.9 ± 1.3 | 14.5 ± 1.3 | 34.5 ± 1.4 | - | |
| DSNH | 5 | - | - | - | - | - |
| 10 | 27.8 ± 1.6 | 17.6 ± 1.5 | 11.2 ± 1.7 | 26.7 ± 1.2 | - | |
| *Average of three replicates; AN*: Aspergillusniger; AF*: Aspergillusflavus; FM*: Fusariummonoliforme; FG*: Fusariumgraminearum; PV*: Penicilliumviridicatum | ||||||
Table 3a: Antifungal activity (% zone of inhibition*) of essential oils and oleoresins of dill seeds and leaves using food poisoned method.
| Samples | Doses(µl) | AN* | AF* | FM* | FG* | PV* |
|---|---|---|---|---|---|---|
| DSEO | 5 | 43.6 ± 0.30 | 45.7 ± 1.3 | 71.2 ± 0.50 | 39.7 ± 0.14 | 34.7 ± 0.6 |
| 10 | 85.9 ± 0.36 | 70.3 ± 1.8 | 89.7 ± 0.20 | 65.7 ± 0.17 | 87.6 ± 0.7 | |
| DSET | 5 | - | 8.9 ± 0.20 | 10.0 ± 0.20 | - | 9.9 ± 0.36 |
| 10 | 11.2 ± 0.30 | 13.2 ± 0.30 | 11.3 ± 0.14 | 21.5 ± 2.1 | 13.7 ± 0.40 | |
| DSNH | 5 | - | - | - | - | - |
| 10 | 5.5 ± 0.46 | 7.6 ± 0.14 | 4.5 ± 1.2 | 9.1 ± 0.41 | 5.6 ± 0.54 | |
| DLEO | 5 | 19.8 ± 0.20 | 11.2 ± 0.7 | - | 21.3 ± 1.5 | |
| 10 | 25.2 ± 0.26 | 16.4 ± 3.6 | 20.1 ± 2.1 | 45.6 ± 1.3 | 20.9 ± 0.8 | |
| DLET | 5 | - | - | - | - | - |
| 10 | - | - | - | - | - | |
| DSNH | 5 | - | - | - | - | - |
| 10 | - | - | - | - | - | |
| *Average of three replicates; AN*: Aspergillusniger; AF*: Aspergillusflavus; FM*: Fusariummonoliforme; FG*: Fusariumgraminearum; PV*: Penicilliumviridicatum | ||||||
Table 3b: Antifungal activity (% zone of inhibition*) of essential oils and oleoresins of dill seeds and leaves using inverted plate method.
The oleoresins extracted from seeds and leave showed very weak antifungal activity due to its viscous nature. The results obtained for oleoresins in the food poisoned method were satisfactory (zone of inhibition upto 40%). The activity against A. flavus species was in good agreement with the reported work [30,31]. Singh et al. [8] have already reported lower antimicrobial efficacy of acetone extracts of ginger, which could be due to the lower volatility.
The antibacterial activity was examined by Agar well diffusion method (Table 4). Only DLEO and DSEO are able to inhibit the growth of tested bacteria. The diameter obtained for zone of inhibition was highest against the gram positive bacteria. At 10 μL, both DSEO and DLEO were found to be highly effective in controlling the growth of S. aureus and B. subtilis where the zone of inhibition was found in between 13-25 mm. There was nominal zone of inhibition found against the E. coli and P. aeruginosa . The efficiency of essential oils against S. aureus and B. subtilis has been reported occasionally in the past [7,32-34], these results being in contrast with other studies where inefficiency of DSEO and DLEO against the gram positive bacteria was reported [35]. The oleoresins showed very weak antibacterial activity in agar well diffusion method against the tested bacterial strains. Absence of Carvone component is responsible for weak activity of oleoresins.
| SAMPLES | Doses (µl) | Diameter of inhibition zone ( mm#) | |||
|---|---|---|---|---|---|
| BS | SA | EC | PA | ||
| DSEO | 5 | 13.2 ± 1.3 | 17.8 ± 1.7 | 5.6 ± 1.8 | 4.7 ± 1.3 |
| 10 | 15.6 ± 1.6 | 20.3 ± 2.3 | 7.5 ± 1.6 | 8.9 ± 1.5 | |
| DLEO | 5 | 11.3 ± 0.9 | 12.3 ± 1.4 | 4.4 ± 1.4 | 3.4 ± 1.7 |
| 10 | 12.4 ± 0.6 | 14.7 ± 0.3 | 5.7 ± 1.2 | 4.9 ± 1.1 | |
| DSET | 5 | - | - | - | - |
| 10 | 2.4 ± 1.5 | 3.4 ± 1.9 | - | - | |
| DLET | 5 | - | - | - | - |
| 10 | 3.4 ± 1.7 | 3.7 ± 1.5 | - | - | |
| DSNH | 5 | - | - | - | - |
| 10 | 4.6 ± 0.6 | - | - | - | |
| DLNH | 5 | - | - | - | - |
| 10 | - | - | - | - | |
| Ampicillin | 1000 ppm | 15.3 ± 2.1 | 18.4 ± 0.9 | - | - |
| 3000 ppm | 18.6 ± 1.0 | 28.9 ± 2.1 | - | 12.6 ± 1.3 | |
| #Average of three replicates; BS: Bacillus subtilis; SA: Staphylococcus aureus; EC: Escherichia coli; PA: Pseudomonas aeruginosa | |||||
Table 4: Antibacterial activity of dill essential oils and its oleoresins against a few bacterial species using agar well diffusion method.
Only a few studies have reported the antifungal properties of essential oil of dill. Our results confirm earlier reports that essential oils and solvent extracts from these plants have proven antifungal effects. These extracts were consistently found to be effective on fungal growth by inhibition of sporangial production. In a separate study done by YS Kwon [36], dillapiole has antimicrobial property. Volatile oil obtained from A. graveolens exhibited a broad range of antifungal activity, inhibiting some nail infecting fungi such as A. niger, A. flavus, A. fumigatus, A. ustus, Candida albicans, Epidermophyton flooccosum, Microsporum canis, M. audouini. M. nanum, M. gypseum, Rhizopus nigricans, Trichophyton tonsurons and T. violaceum [37].
Most studies investigating the action of whole essential oils against food spoilage organisms and food borne pathogens agree that, generally, essential oils are slightly more active against gram-positive than gram-negative bacteria. That gram-negative organisms are less susceptible to the action of antibacterial is perhaps to be expected, since they possess an outer membrane surrounding the cell wall which restricts diffusion of hydrophobic compounds through its lipopolysaccharide covering [38].
Carvone, limonene, dillapiole and myristicin constitute the terpenoids class which is basically terpenes that undergo biochemical modifications via enzymes that add oxygen molecules and move or remove methyl groups. The increased activity of terpenoids as compared to terpenes can be attributed to the linked functional moieties [39]. As far as non-phenolic components of essential oils are concerned, the type of alkyl group has been found to influence activity [40]. The antimicrobial activity of most terpenoids is linked to their functional groups, and it has been shown that the hydroxyl group of phenolic terpenoids and the presence of delocalized electrons are important for antimicrobial activity [41].
It has been already established that essential oils and oleoresins are complex mixtures of components that show usually higher activities than their isolated components; their final activities are due to the combined effects of several minor components. Thus, they contain multifunctional components that exert their activities through different mechanisms [42].
Conclusion
To the best of our knowledge, this is the first study to provide comparative data on the antimicrobial and antioxidant activities of the essential oils and oleoresins from aerial parts of A. graveolens L. The results obtained in this study showed that the essential oils obtained from seeds and leaves may be a potential source of natural antioxidants and antimicrobial agents. These in vitro assays indicate that DSEO has a significant source of antioxidant which might be useful in preventing the progress or various oxidative stresses. The results showed that different parts of same plant utilized for extraction may have significant effect on the fraction of major components in the essential oils and oleoresins. Hence, more queries will be addressed in future studies to explore the potential of A. graveolens L. seeds bioactive compounds as chemo preventive and therapeutic agents. However, further studies need to be conducted to understand the mechanism of the activity and obtain more information on the safety and toxicity of the oils.
Acknowledgement
We acknowledge profound gratitude to Head, Chemistry Department Deen Dayal Upadhyaya Gorakhpur University, Gorakhpur, India for providing laboratory facilities. The authors also wish to thanks the University Grants Commission and Department of Science & Technology for providing financial assistance to Sunita Singh (UGC-SRF) and Dr. Gurdip Singh (Emeritus Scientist) respectively.
References
- Aidi Wannes W, Mhamdi B, Sriti J, Ben Jemia M, Ouchikh O, et al. (2010) Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem Toxicol 48: 1362-1370.
- Kaur GJ, Arora DS (2010) Bioactive potential of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi belonging to the family Umbellifereae -Current status. J Med Plants Res 4: 87-94.
- Dapkevicius A, Venskutonis R, van Beek TA, Linssen JPH (1998) Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric 77: 140-146.
- Maisonneuve SA, Ruffine, Sainte (1983) European Pharmacopoeia 1 p.v.4.5.8.
- Adams RP (2007) Identification of essential oil components by Gas chromatography/mass spectrometry, 4th ed. Allured publishing, Carol Stream, Illinios, USA.
- Bandoniene D, Gruzdiene D, Venskutonis PR (2001) Antioxidant activity of sage extracts in rapeseed oil irradiated with UV-rays. Nahrung 45: 105-108.
- Singh G, Maurya S, Catalan C, de Lampasoma MP (2005) Studies on essential oils, Part 42: Chemical, antifungal and antioxidant and sprout suppressant studies of ginger oil and its oleoresin. Flav Frag J 20:1-6 .
- Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, et al. (2008) Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem Toxicol 46: 3295-3302.
- Alvarez-Castellanos PP, Bishop CD, Pascual-Villalobos MJ (2001) Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochem 57: 99-102.
- Ramdas K, Suresh G, Janardhanan, Masilamani S (1998) Antifungal activity of 1,3-disubstituted symmetrical and unsymmetrical Thioureas. Pestic Sci 52: 145-151.
- Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV, Damianova ST (2003) Composition, quality control, and antimicrobial activity of the essential oil of long-time stored dill (Anethum graveolens L.) seeds from Bulgaria. Journal of Agricultural and Food Chem 51: 3854-3857.
- Singh G, Maurya S, Lampasona MP, Catalan C (2005) Chemical constituents, antimicrobial investigations and antioxidative potentials of Anethum graveolens L. Essential oil and acetone extract: Part 52. J Food Sci 70: 208-215.
- Sharapov FS, Wink M, Gulmurodov IS, Isupov SJ, Zhang H, et al. (2013) Composition and Bioactivity of the Essential oil of Anethum graveolens L. from Tajikistan. Inter J Med Aromatic Plants 3: 125-130.
- Vineeta, Kewalanand, Vandana, Vishwanath (2013) Effect of irrigation, FYM levels and storage conditions on oil constituents of European Dill (Anethum graveolens L.) Ind J App Pure Biol 28:157-164.
- Djenane D, Yanguela J, Montanes L, Djerbal M, Roncales P (2011) Antimicrobial activity of Pistacia lentiscus and Satureja montana essential oils against Listeria monocytogenes CECT 935 using laboratory media, efficacy and synergistic potential in minced beef efficacy and synergistic potential in minced beef. Food Cont 22: 1046-1053.
- Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N (2005) Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem 92: 491-497.
- Galoburda R, Kruma Z, Ruse K (2012) Effect of Pretreatment Method on the Content of Phenolic Compounds, Vitamin C and Antioxidant Activity of Dried Dill. World Acad Sci Engineer Tech 64: 1075-1079.
- Hajimehdipoor H, Adib N, Khanavi M, Mobli M, Amin GR, et al. (2012) Comparative study on the effect of different methods of drying on Phenolic Content and Antioxidant activity of some Edible Plants. Intern J Pharmac Sci Res 3: 3712-3716.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44-84.
- Mousavi RS, Mahasti P, Nateghi L, Mahalati H (2013) The stabilizing effect of Dill extract on sunflower seed oil. J Food Bio Tech. Islamic Azad University, Sci Res Branch 3: 81-86.
- Murica MA, Egea I, Romojaro F, Parras P, Jimenez AM, et al. (2004) Antioxidant evaluation in dessert spices compared with common food additives. Influence of irradiation procedure. J Agric Food Chem 52: 1872-1881.
- Jayaprakasha GK, Singh RP, Sakariah KK (2001) Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 73: 285-290.
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H (2000) Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem 48: 180 -85.
- Finefrock AE, Bush AI, Doraiswamy PM (2003) Current status of metals as therapeutic targets in Alzheimer's disease. J Am Geriatr Soc 51: 1143-1148.
- Rathore SS, Saxena SN, Saxena R, Tilak R (2013) Analysis of medicinally important compounds and anti-oxidant activity in fixed and essential oil of dill (Anethum graveolens L.) genotypes. Intern J Seed Sci 3: 12-15.
- Nunzio MD, Valli V, Bordoni A (2011) Pro-and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostagland Leuk Essen Fatty Acids 85: 121-127.
- Ciz M, Cizova H, Denev P, Kratchanova M, Slavov A, et al. (2010) Different methods for control and comparison of the antioxidant properties of vegetables. Food Cont 518-523.
- Kamel SM, Thabet HA, Algadi EA (2013) Influence of Drying Process on the Functional Properties of Some Plants. Chem Mater Res 3: 1-8.
- Amin I, Norazaidah Y, Emmy Hainida KI (2006) Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem 94: 47-52.
- Tian J, Ban X, Zeng H, He J, Chen Y, et al. (2012) The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One 7: e30147.
- Abd E-Khalek HH (2013) Chemopreventive potential of some plant essential oils against Aspergillus flavus and Aspergillus ochraceus growth and mycotoxin production. Int J Microbiol Immunol Res 1: 037-046.
- Nevas M, Korhonen AR, Lindström M, Turkki P, Korkeala H (2004) Antibacterial efficiency of Finnish spice essential oils against pathogenic and spoilage bacteria. J Food Prot 67: 199-202.
- Dahiya P, Purkayastha S (2012) Phytochemical analysis and antibacterial efficacy of dill seed oil against multi-drug resistant clinical isolates. Asian J Pharmac Clinic Res 5: 62-64.
- Bor MD, Tofana M, Suharoschi R, Rotar A (2013) A study regarding the antibacterial activity of some commercial essential oils on food-borne pathogenic and spoilage bacteria. J Agroalimen Proc Tech 19: 314-318.
- Abed KF (2007) Antimicrobial activity of essential oils of some medicinal plants. Saudi J Biolog Sci 14: 53-60.
- Kwon YS, Choi WG, Kim WJ, Kim WK, Kim MJ, et al. (2002) Antimicrobial constituents of Foeniculum vulgare. Arch Pharm Res 25: 154-157.
- Fernandez-Ocana AM, Gomez-Rodriguez MV, Velasco-Negueruela A, Camacho-Simarro AM, Fernandez-Lopez C, et al. (2004) In vivo antifungal activity of the essential oil of Bupleurum gibraltarium against plasmopara halstedii in sunflower. J Agric Food Chem 52: 6414-6417.
- Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol 94: 223-253.
- Bassole IHN, Lamien-Meda A, Bayala B, Tirogo S, Franz C, Novak J, et al. (2010) Composition and antimicrobial activities of Lippia amultiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molec 15: 7825-7839.
- Dorman HJ, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88: 308-316.
- Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10: 813-829.
- Koroch AR, Juliani HR, Zygadlo JA (2007) Bioactivity of essential oils and their components. Flav Frag Chem 87-115.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 15644
- [From(publication date):
March-2017 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 14517
- PDF downloads : 1127