Cortical Auditory Evoked Potential and Its Relationship with Speech Perception Score of Children Using Hearing Aids
Received: 31-Aug-2018 / Accepted Date: 20-Nov-2018 / Published Date: 27-Nov-2018 DOI: 10.4172/2161-119X.1000356
Abstract
The obligatory evoked cortical potential measurement mainly used to check maturation of the brain structure. The advancement of digital technology made possible to fit hearing aids even severe loses. Improved SNR, directional microphone other feature made hearing aids uses popular among children. The selection and fitment procedure of optimum level of hearing aids mainly relay on parental feedback. Therefore present study aimed to find out the relationship between behaviour speech perception ability and objective measure of cortical evoked P1 peak. 87 hearing aids users were recruited in the study with mean age of 8.61 years and SD 1.62 years. Intelligent Hearing System 3.36 Smart Evoked Potential clinical instrument was used for recording free field evoked potential. Hindi Speech perception test was used to measure speech perception skill. Results indicated that children with good speech perception showed significant inverse relationship with latency of P1 peak. This study discussed use of P1 peaks latency to measure speech perception ability and auditory cortex maturation and functionality.
Keywords: Auditory cortical evoked potential; Auditory brainstem response; Speech perception skill; Hearing aids; Amplification device
Introduction
Cortical evoked potentials are being used for mainly measuring maturation of auditory structure of brain [1-5]. The clinical utility of cortical evoked potentials is found to be limited due to time consuming measurement procedure. The Hearings Impaired (HI) children rehabilitation mainly done by the amplification devices such as Hearing Aids (HA) or cochlear implant [6-9]. The Audiological measure among the young children is challenging task as they lack in correct behavious response [9-15]. Audiologist are using objective test such as ABR, OAE, IA for measurement of auditory responses from young children [6-7]. One of the challenging tasks for audiologist is measurement of utility of amplification devices in young children. Accurate threshold of audibility helps to programme the amplification devices at optimum level. These devices plays important role in the rehabilitation process of young children. The rehabilitation programme requires continue fine tuning of HA.
The speech signal contains information about the fundamental frequency, the first formant F1 and sometimes the second formant, F2 [6]. The presence of fundamental frequency helps to indicate the presence of voiced sounds (e.g., vowels) and therefore one could easily discriminate between voiced and voiceless sounds [6,15]. Temporal spectrum changes in fundamental frequency also gives information about prosodic features of sentence, i.e. one could be able to tell whether a sentence is a statement or a question.
Similarly, F1 frequency information also helpful for discrimination vowels such as vowels/i, u/and/a, ae/. Finally, assuming that the temporal details in the waveform are preserved, the individual should be able to discriminate among the consonant sets /s, f, θ, f/,/b, d, g, p, t, k/ and /w, r, 1, j/ which have different waveform characteristics [16]. The limited high frequency responses are seen with the HA, which hampers the speech perception ability. The modern amplification system has advance technological modification in many features to improve speech perception such as directional microphone to improve signal to noise ratio [6].
The measurement in speech perception ability helps therapist to track rehabilitation programme [17-19]. Two approaches have been followed in development of speech perception battery for children. One approach followed at the Central Institute for the Deaf (CID) assumes that children acquire speech perception abilities in a hierarchical manner starting from simple detection through spoken word comprehension. The outcome of this testing is used to categorize the children’s speech perception abilities and determine auditory training goals [20]. The advantage of this approach is that it require less time. Second approach by Kirk et al. [21] in which they administered a battery of tests that evaluate a range of speech perception abilities and are then assigned scores for each test in the battery.
In India Early Speech Perception Test by Geer and Moog is being most commonly used. The test has been adapted by several research institutes in several regional languages [20,22]. The utility of Aided auditory evoked potential are been long standing interest among the researcher. Aided ABR response been studied by several researchers to check function of amplification devices [23-28]. ABR measurement mainly used brief stimulus i.e. clicks and tone pips. These stimuli have several limitations for measuring HA function. The brief duration of stimulus was found to be problematic to activate HA circuit [24- 25]. Study reported that brain stem has limited role in the speech perception ability and need to evaluate higher cortical structure [27]. Further researcher studied cortical responses among young children and found that P1 response can be used as Biomarker for auditory maturation [1-5].
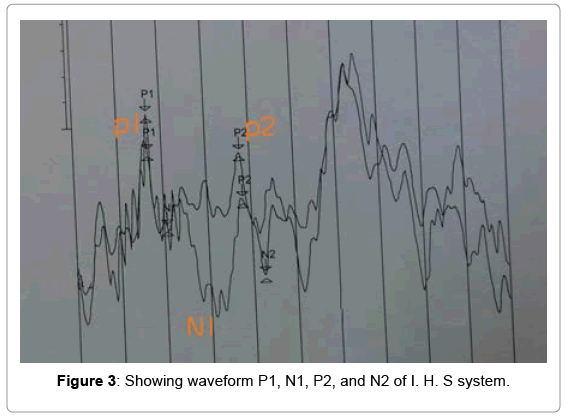
The obligatory cortical evoked potential response commonly uses P1, N1 and P2. The origin of P1 potential is thought to be from primary cortex and thalamus [29-32]. These peaks can be measured in young children, it didn’t required sedation or sleep to obtain the auditory response. Cortical evoked potential can be used for measurement of behavious threshold in young children and difficult to test population. Obligatory potential peaks are objective and seen reliable responses [33]. Several researchers have given importance to P1 peaks which reflect sum of the synaptic transmission delays throughout the central auditory nervous system [32].
Indian audiological clinical setups are using two methods to verification of HA fitment. These include (REIG) Real Ear Insertion Gain and prescriptive target gain is used to ensure that access to the speech information is at optimum level. These methods are requires frequency specific hearing threshold information which can be mainly obtainable in adults. In children hearing information or frequency specific responses obtained by ABR, ASSR response only. HA performance in young children mainly checked by the parental reports/ feedback form or questionnaire response to ensure that HA not causing loudness discomfort. Therefore the present study aimed to find out the relationship of speech perception skill and auditory cortical evoked potential’s amplitude and latency.
Methodology
87 HI children using HA with no associated disability (such as CP, ADHD etc.) had participated. The mean of age of HA users group was 8.61 years with standard deviation 1.62. Children with HI using HA more than 2 years were included in the research study. The detail procedure was explained to all participants and parent and informed consent was obtained. Intelligent Hearing System 3.36 Smart EP clinical evoked potential instrument was used for measurement of cortical auditory evoked potential.
Before measurement of evoked potential detail demographic data and case history information was collected. The basic pure tone thresholds were measured from 250 to 8000 Hz via air conduction; similarly, bone conduction thresholds were also measured from 250 to 4000 Hz. The pure tone threshold was collected using modified Hughson and Westlake Procedure. Impedance audiometry results were recorded using GSI Tympstar instrument. Tympanometry test was conducted by 226 Hz probe tone at 85 dB SPL and acoustic reflex test was done at tone of 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz ipsilaterally and contralaterally. Transient Evoked Otoacoustic Emissions (TEOAE) was measured using click stimulus at 85 dBSPL in both ears.
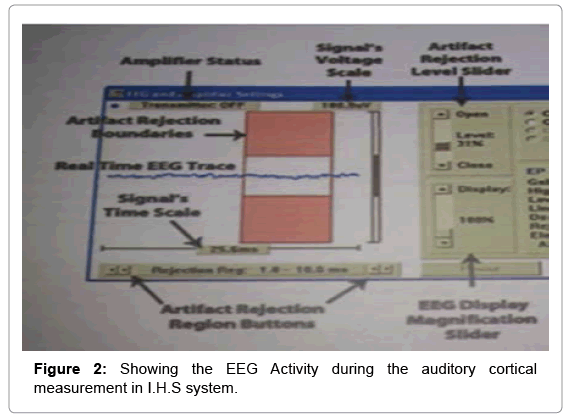
All the testing was performed in recommended test environment and with standardized test protocol. The subjects were seated in a reclining wooden chair in an electrically shielded and acoustically treated room (ANSI 3X 76). Silver chloride (AgCl) electrodes were placed at the recording sites, after cleaning those sites with Neuro Prep abrasive gel. Surgical adhesive tape was used to hold the electrodes firmly in recording sites. The standard and well accepted auditory evoked potential protocol was used throughout cortical potential acquisitions. Subjects were asked to remain quietly seated with minimum body movement accompanied with parent. The prevention of electric interference was controlled by insuring that all possible other electric instrument such mobile, I-pad etc not to bring inside testing chamber. EAC characteristic was measured using Phoenix EAC HA analyzer instrument 12.32 version.
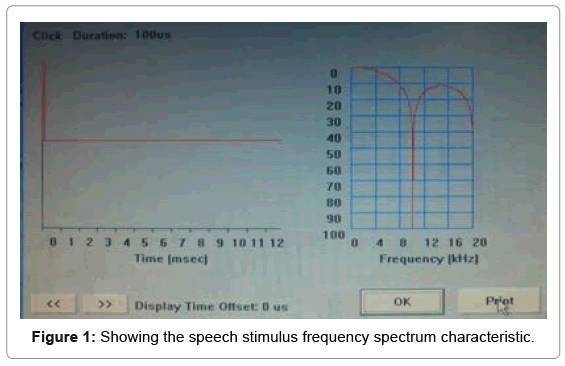
Before the test conduction, it was also insured that HA at most comfortable level. Cortical evoked potential and speech perception measurement minimum test duration was 30-45 mins each. The speech stimuli were presented approximate normal conversational level i.e. at 70 dB SLP (which was measured at the client’s head). The stimulus presentation was via a loudspeaker placed at 45 azimuth degree angle. The subject was allowed to watch a silent cartoon on tablet screen. McArthur et al. [34] reported that present of low level of silent mobile or tablet did not significantly effect on the P1 N1 P2 peaks of cortical potential. This arrangement helps us to keep subject engaged without interfering with the stimulus. Speech stimulus/ba/100 ms (20 ms rise/fall, 20 ms plateau tones) was used for cortical evoked potential measurement. Inter-stimulus interval was kept at 1125 ms. EEG filter setting was kept high pass filter 1 Hz and low pass filter 30 Hz. 512 numbers of sweeps were collected for cortical recording.
The dual channel system was used to record evoked potential. The first channel’s active electrode was placed at Cz position, connected to the preamplifier input +ve and reference electrode was placed on the test ear lobule connected to the preamplifier -ve. The common/ground electrode was placed at FPz position.
The second channel was used to monitor eye blink artifacts. The active electrode (i.e. preamplifier input +ve) was placed on the non-test ear side supra-orbital position and -ve input at infra orbital position [35]. The second channel artifact rejection level was adjusted to include the ocular movement amplitude and eye blink. The subtraction procedure was used to remove eye blink effect between channel one and channel two.
Amplitude marking: Auditory cortical evoked waveforms are occurring within 50-300 ms after the presentation acoustic stimulation. The auditory cortical potential peaks are denoted as P1, N1, P2, N2 [36-38]. The peak identification was done on the basis of amplitude difference between the 0.0 uV point to the maximum positive value. Similarly, latencies and amplitudes were calculated based on the difference P1/N1 between negative and following positive peak vice versa. The validity of peak marking was done by two independent observers who had clinical experience more than 5 years in auditory evoked potential measurement (Figures 1-3).
Speech perception skill measurement
Speech perception skill was measured by using early speech perception test [20]. As most of subjects were local language user, the early speech perception test was adopted in Hindi and Marathi language [39].
Early Speech perception test having three sections, first section contains 12 items, which were for assessing syllabic length perception i.e. mono-syllable, bi syllable and tri-syllable words. Second section consists of 12 bi-syllable words and last section having 12 monosyllable words items. The test was administered in a quiet room with minimum visual and audible distractions. The adequate light in the test room was maintained for good visibility of picture plates of the test. The test conduction was done by live voice of the researcher.
Seating arrangement
Subject and tester were seated next to each other with the tester’s chair slightly behind that of subject’s chair to avoid any visual cues. Tester was seated on the side of good residual hearing ear.
Results
The speech perception ability reflects normal speech and language development. In the case of young children, speech perception ability measurement is challenging task for audiologist and speech therapist. The current research is attempted to study speech evoked cortical potential in children using HA and their speech perception ability. The present study only used P1 peaks to study relationship with speech perception recommend by previous literature [2-5].
Descriptive analysis: The collected data was analyzed in SPSS 16 and means, standard deviations were obtained. Test of normality was applied to collected data sample to check distribution of sample.
As above Tables 1 and 2 showing only the HA user P1 latency was having normal distribution pattern. Other all component doesn’t follow normal distribution pattern (significance value are greater than 0.005). Therefore further analysis was done by non-parametric test.
| Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Minimum | Maximum | Mean | Std. Deviation | Skewness | Kurtosis | |||
| Statistic | Statistic | Statistic | Statistic | Statistic | Statistic | Std. Error | Statistic | Std. Error | |
| HA P1 latency | 87 | 64.50 | 130.50 | 105.14 | 22.97432 | -0.436 | 0.258 | -1.447 | 0.511 |
| HA N1 Latency | 87 | 106.00 | 191.50 | 153.81 | 23.32994 | -0.401 | 0.258 | -.837 | 0.511 |
| HA P1 amplitude | 87 | 0.70 | 3.50 | 2.2729 | 0.44609 | -0.093 | 0.258 | 1.452 | 0.511 |
| HA N1 amplitude | 87 | 1.90 | 3.88 | 2.9944 | 0.54873 | -0.460 | 0.258 | -1.088 | 0.511 |
| Speech perception score | 87 | 22.00 | 72.00 | 51.1149 | 14.01198 | -0.005 | 0.258 | -1.181 | 0.511 |
| HA users age | 87 | 6 | 13 | 8.63 | 1.618 | 0.572 | 0.258 | -0.251 | 0.511 |
Table 1: Showing descriptive Analysis results of Hearing aids user group.
| Kolmogorov-Smirnov | ||
|---|---|---|
| Statistic | Sig. | |
| HA P1 Latency | 0.242 | 0.000 |
| Amplitude HA P1 | 0.128 | 0.032 |
| Speech Perception HA | 0.122 | 0.053 |
Table 2: Showing test of normality results of HA users.
The value of Table 3 shows that speech perception score and auditory cortical evoked potential P1 latency has inverse relationship i.e. -0.728. Similarly, value of Table 4 indicates that speech perception score is directly relationship with auditory cortical evoked potential P1 amplitude i.e. 0.513. Considering correlation value one can say that 0.72 indicating strong relationship i.e. latency of P1 and speech perception strongly related.
| Correlations | Spearman’s rho | |||||
|---|---|---|---|---|---|---|
| Speech perception HA | Cortical potential HA P1 Latency |
Speech perception HA | Cortical potential HA P1 Latency |
|||
| Speech perception score HA | Pearson Correlation | 1 | -0.754** | Correlation Coefficient | 1.000 | -0.728** |
| Sig. (2-tailed) |
0.000 | Sig. (2-tailed) |
0.000 | |||
| N | 87 | 87 | N | 87 | 87 | |
| Cortical potential HA P1 Latency |
Pearson Correlation | -0.754** | 1 | Correlation Coefficient | -0.728** | 1.000 |
| Sig. (2-tailed) | 0.000 | Sig. (2-tailed) | .000 | |||
| N | 87 | 87 | N | 87 | 87 | |
| Note: **Correlation is significant at the 0.01 level (2-tailed). | ||||||
Table 3: Statistical correlation test result of Auditory evoked cortical potential P1 Peak latency and Speech perception score of HA users.
| Correlations | Spearman’s rho | |||||
|---|---|---|---|---|---|---|
| Speech perception HA | Cortical potential HA P1 Amplitude |
Speech perception HA | Cortical potential HA P1 Amplitude |
|||
| Speech perception score HA | Speech perception HA | Cortical potential amHAp1 | Spearman’s rho | Speech perception HA | Cortical potential amHAp1 | |
| Pearson Correlation | 1 | 0.532** | Correlation Coefficient | 1.000 | 0.513** | |
| Sig. (2-tailed) | 0.000 | Sig. (2-tailed) | 0.000 | |||
| Cortical potential HA P1 Amplitude |
N | 87 | 87 | N | 87 | 87 |
| Pearson Correlation | 0.532** | 1 | Correlation Coefficient | 0.513** | 1.000 | |
| Sig. (2-tailed) | 0.000 | Sig. (2-tailed) | 0.000 | |||
| Note: **Correlation is significant at the 0.01 level (2-tailed). | ||||||
Table 4: Statistical correlation test result of Auditory evoked cortical amplitude P1 Peak latency and Speech perception score of HA users.
Discussion
In the present research P1 latency and amplitude were measured and statistical analysis preformed. The P1 latency of auditory cortical evoked potential has shown significant relationship compared to P1 amplitude. The present study has only invested interest on P1 peaks of auditory cortical evoked potential for statistical analysis. There are various possible advantages of measuring P1 peak of the auditory cortical evoked potentials. The P1 peak was even observable in newborn.
Infants P1 latency is seen around 200-300 ms post-auditory stimulation. The maturation of auditory cortex can be seen by P1 rapid reduction in latency approximately 100 ms at first two years of life. This systematic reduction in latency value has been quantified with 95% confidence intervals. This is providing normative data for central auditory development by using P1 latency [1,2,40-43]. P1 and N1 components of cortical potentials are the obligatory potential (i.e. Auditory attention to stimuli are not required for elicitation of these cortical potentials). These peaks P1, N1 are found to be automatically in response to acoustic stimulus. In the present study P1 latency of auditory cortical evoked potential has shown significant relationship with the speech perception ability. Sharma and colleagues have conducted large-scale research studies to examining cortical development in congenitally HI children fitted with a cochlear implant at different ages [1,2,40,41].
Similar results were reported that good speech perception ability found to be children having early age auditory management and normal cortical functioning [2,42]. Sharma et al. examined P1 latencies in 245 congenitally HI children fit with a cochlear implant [3]. The study result showed that children who received stimulation via an implant early in childhood (i.e. less than age 3.5 years) showed nearly normal P1 morphology and latency. On the other side children who fitted cochlear implant more than age 7 years had abnormal cortical response in terms of latency and wave morphology. Sharma et al. studies individual developmental trajectories for the P1 peak latency and amplitude response after cochlear implantation in 231 children [41]. All children showed delayed P1 latencies prior to implantation, children implanted under age 3.5 years showed near normal P1peak response latencies after implantation. Though children implanted after age 7 years also showed latency decreases over time, their developmental trajectories were abnormal, with P1 latencies never reaching near normal limits even implant usage [44].
The similar result has been replicated in the present study i.e. good speech perception ability reflect normal functioning of auditory cortical structures. Latency of P1 peaks of cortical potential showed inverse relationship with the speech perception ability. The child with poor speech perception showed significant prolongs P1 Latency of cortical potential. Good speech perception ability reflects normal function of structure of cortex such as thickness of Heschl’s Gyrus [45,46]. Children with better speech perception shows region-dependent decreases in gray matter of CSF volume occur, concurrent with white matter CSF volume increases, possibly providing a cortical structural basis for developmental changes reported for the P1 and N1 peaks of cortical potentials [47-51].
The finding was reported by Sharma et al. [52] while studding P1 Peak among children with Auditory Neuropathy Spectrum Disorder. They reported that cortical evoked potential are not only recordable in children with Auditory Neuropathy Spectrum Disorder, but they were also highly correlated with speech perception ability. P1 peak latency, amplitude and morphology of cortical potential served to divide a group of 21 children with Auditory Neuropathy Spectrum Disorder into three distinct categories. They divided Children with ANSD in to Children with normal P1 peak latency, amplitude and wave morphology; Children with normal P1 peak morphology, but delayed latency and decreased amplitude; Children with abnormal P1 peak response. IT MAIS speech perception score were compared among the three groups. It was reported that children with normal P1 peak latency responses showed superior auditory skill development over those who had delayed or abnormal P1 peak responses. Similarly, children who received early auditory intervention with HA were more likely to show near normal P1 peak responses and good (speech perception ability) behavioral outcome.
The absence of appropriate auditory stimulation during early age (a short sensitive period), early in childhood negatively affects cortical maturation. Lack of appropriate cortical maturation is correlated with abnormalities in speech and language development and speech perception ability. Children with early implanted showed the better speech perception skills as compared to late implanted. Cortical potential reflects the activation of auditory cortical areas and better behaviour outcome i.e. speech perception ability.
Conclusion
The P1 peak cortical potential can be non-invasively recorded without any behavioral response from the subject and therefore, represents an objective measurement technique for assessing the central auditory development in infants and young children. The maturational status of the central auditory pathways may serve as a bio-marker of the effectiveness of early intervention of auditory management. Children early fitted appropriately with amplification or electrical stimulation ought to show, normal development of the central auditory system. Similar finding reported by present research study P1 peak of cortical potential represent cortical development correlated with the speech perception ability.
References
- Sharma A, Dorman MF, Spahr AJ (2002) Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport 13: 1365-1368.
- Sharma A, Dorman M, Spahr A, Todd NW (2002) Early cochlear implantation in children allows normal development of central auditory pathways. Ann Otol Rhinol Laryngol 189: 38-41.
- Sharma A, Kraus N, McGee TJ, Nicol TG (1997) Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephal Clin Neurophysiol 104: 540-515.
- Sharma A, Martin K, Roland P, Bauer P, Sweeney MH (2005) P1 latency as a biomarker for central auditory development in children with hearing impairment. J Am Acad Audiol 16: 564-573.
- Sharma A, Campbell J, Cardon G (2015) Developmental and crossmodal plasticity in deafness: Evidence from the P1 and N1 event related potentials in cochlear implanted children. Int J Psychophysiol 95: 135-144.
- Hall J, Mueller HG (1998) Audiology desk reference: Audiologic management, rehabilitation and terminology. San Diego, CA: Singular.
- Martin FH, Clark JG (2009) An introduction to audiology. Boston, MA: Allyn and Bacon.
- Katz J, Robert F, Burka RD, Medwetsky L (2002) Handbook of clinical audiology. Lippincott: William and Wilkins.
- Kirk KI, Miyamoto RT, Lento CL, Ying E, O'Neill T, et al. (2002) Effects of age at implantation in young children. Ann Otol Rhinol Laryngol Suppl 189: 69-73.
- Kirk KI (2000) Challenges in the clinical investigation of cochlear implant outcomes. In: Niparko J (Eds.) Cochlear Implants: Principles and Practices. Baltimore: Lippincott, Williams and Wilkins, 225-259.
- Kirk K, Pisoni D, Miyamoto R (2000) Lexical discrimination by children with cochlear implants: effects of age at implantation and communication mode. In: Waltzman S, Cohen N (Eds.) Cochlear implants. New York: Thieme, 252-254.
- Northern JL, Downs MP (2001) Hearing in Children. (5th Edition). Baltimore, MD: Lippincott, Williams and Wilkins.
- Alpiner JG, McCarthy PA (2000) Rehabilitative audiology: Children and adults. Baltimore, MD: Lippincott, Williams and Wilkins.
- Katz J, Medwetsky L, Burkard R, Hood L (2009) Handbook of clinical audiology. Baltimore: Lippincott, Williams and Wilkins Publishers.
- Dorman MF, Smith L, Parkin J (1993) Loudness balance between acoustic and electric stimulation by a patient with a multichannel cochlear implant. Ear Hear 14: 290-292.
- Estabrooks W (1998) Auditory-verbal ages and stages of development in (Levels I-VIII) in cochlear implants for kids. AG Bell: Washington, DC.
- Estabrooks W (2006) Auditory-verbal therapy and practice. Washington, DC.
- Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL (1998) Language of early and later-identified children with hearing loss. Pediatrics 102: 1161-1171.
- Moog JS, Geers AE (1990) Early speech perception test. Central Institute for the Deaf, Missouri.
- Kirk KI, Pisoni DB, Osberger MJ (1995) Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear Hear 16: 470-481.
- The list of test/therapy materials (2018) All India Institute for Speech and Hearing.
- Beauchaine KA, Gorga MP, Reiland JK, Larson LL (1986) Application of ABRs to the hearing-aid selection process: preliminary data. J Speech Hear Res 29: 120-128.
- Brown E, Klein AJ, Snydee KA (1999) Hearing-aid-processed tone pips: Electroacoustic and ABR characteristics. J Am Acad Audiol 10: 190-197.
- Gerling IJ (1991) In search of a stringent methodology for using ABR audiometric results. Hear J 44: 28-30.
- Gorga MP, Beauchaine KA, Reiland JK (1987) Comparison of onset and steady-state responses of hearing aids: Implications for use of the auditory brainstem response in the selection of hearing aids. J Speech Hear Res 20: 130-136.
- Hecox KE (1983) Role of auditory brain stem response in the selection of hearing aids. Ear Hear 4: 51-55.
- Kileny P (1982) Auditory brainstem responses as indicators of hearing aid performance. Ann Otol Rhinol Laryngol 91: 61-64.
- Erwin RJ, Buchwald JS (1987) Mid latency auditory evoked responses in the human and the cat model. Electroencephalogr Clin Neurophysiol 40: 461-467.
- McGee T, Kraus N (1996) Auditory development reflected by middle latency response. Ear Hear 17: 419-429.
- Liégeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P (1994) Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalograph Clin Neurophys 92: 204-214.
- Eggermont JJ, Ponton CW, Don M, Waring MD, Kwong B (1997) Maturational delays in cortical evoked potentials in cochlear implant users. Acta Otolaryngol. 117: 161-163.
- Sharma A, Glick H, Campbell J, Biever A (2013) Central auditory development in children with hearing loss: Clinical relevance of the P1 CAEP biomarker in hearing-impaired children with multiple disabilities. Hearing Balance Commun 11: 2-10.
- McArthur GM, Bishop DVM, Proudfoot M (2003) Do video sounds interfere with auditory event-related potentials? Behav Res Methods Instru Comp 35: 329-333.
- Ventura LM, Alvarenga Kde F, Costa Filho OA (2009) Protocol to collect late latency auditory evoked potentials. Braz J Otorhinolaryngol 75: 879-883.
- Hall J (2007) New handbook of auditory evoked responses. Pearson: Boston.
- Hood LJ, Berlin CI (1986) Auditory evoked potentials. The university of Michigan.
- Jacobson JT (1994) Introduction to audiologic rehabilitation. Allyn and Bacon: Pearson Educational.
- Zheng Y, Soli SD, Wang K, Meng J, Meng Z, et al. (2009) Development of the mandarin pediatric speech intelligibility (MPSI) test. Int J Audiol 48: 718-728.
- Sharma A, Dorman MF, Spahr AJ (2002c) A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear 23: 532-539.
- Sharma A, Gilley PM, Dorman MF, Baldwin R (2007) Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol 46: 494-499.
- Sharma A, Dorman MF (2006) Central auditory development in children with cochlear implants: Clinical implications. Adv Otorhinolaryngol 64: 66-88.
- Gilley PM, Sharma A, Dorman MF (2008) Cortical reorganization in children with cochlear implants. Brain Res 1239: 56-65.
- Sharma A, Dorman MF (1999) Cortical auditory evoked potential correlates of categorical perception of voiceonset time. J Acoust Soc Am 106: 1078-1083.
- Fu M, Zuo Y (2011) Experience-dependent structural plasticity in the cortex. Trends Neurosci 34: 177-187.
- Liem F, Zaehle T, Burkhard A, Jäncke L, Meyer M (2012) Cortical thickness of supratemporal plane predicts auditory N1 amplitude. Neuroreport 23: 1026-1030.
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174-8179.
- Group BDC (2012) Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI study of normal brain development. Cereb Cortex 22: 1-12.
- Lenroot RK, Giedd JN (2006) Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30: 718-729.
- Muftuler LT, Davis EP, Buss C, Head K, Hasso AN, et al. (2011) Cortical and subcortical changes in typically developing preadolescent children. Brain Res 1399: 15-24.
- Nie J, Li G, Shen D (2013) Development of cortical anatomical properties from early childhood to early adulthood. Neuroimage 76:216-224.
- Sharma A, Cardon G (2015) Cortical development and neuroplasticity in auditory neuropathy spectrum disorder. Hear Res 330: 221-232.
Citation: Bhimte SL, Rangasayee R (2018) Cortical Auditory Evoked Potential and Its Relationship with Speech Perception Score of Children Using Hearing Aids. Otolaryngol (Sunnyvale) 8: 356. DOI: 10.4172/2161-119X.1000356
Copyright: © 2018 Bhimte SL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4096
- [From(publication date): 0-2018 - Dec 10, 2025]
- Breakdown by view type
- HTML page views: 3132
- PDF downloads: 964