Creation of an Accurate Artificial Neural Network Prediction Model of Radiologist Reported CT Features for Colorectal Anastomotic Leaks
Received: 25-Feb-2019 / Accepted Date: 24-Mar-2019 / Published Date: 31-Mar-2019 DOI: 10.4172/2167-7964.1000307
Abstract
Objective: As colorectal anastomotic leaks (AL) often present with non-specific clinical features, Computed Tomography (CT) scans are commonly used to aid in diagnosis. Aim was to define radiologist reported features in CT scans following colorectal resection as diagnostic factors for clinical AL detection.
Methods: Consecutive patients identified with a clinically confirmed post-operative AL. Control group (matched 2:1 ratio) selected from patients who were scanned with a clinical suspicion of an AL, though eventually disproved and who did not require re-operation. Four gastrointestinal radiologists reviewed CT scans, blinded to clinical outcome. Radiologists assessed for the overall impression of a radiological AL and presence of the adjunct leak features. A leak prediction model was constructed with multivariate logistic regression with outcome classified as clinical AL.
Results: 18 patients with confirmed AL, 36 matched control patients. No significant difference in the sensitivity/specificity between the radiologists in accuracy of leak detection, with overall correct diagnosis of clinical AL 81.4%. Radiological Leak, abnormal bowel wall appearance and ileus were significant predictors (P<0.05) within regression model. The prediction model produced an overall sensitivity 85.2%, specificity 80.2% and ROC curve area of 87.3%.
Conclusion: Individual radiologist reported CT features have been used to create a risk prediction model that improves diagnostic accuracy of AL over general radiological impression alone.
Keywords: Anastomotic leak; Colorectal; Artificial neural network; Imaging; Computed tomography
Introduction
Anastomotic leaks (AL) are the largest cause of early postoperative mortality following colorectal surgery [1,2]. Mortality following colorectal resection with an anastomosis rises from 1%-2% in patients without a leak [3,4] to 12-27% in those with a confirmed leak [3,5-7] and accounts for up to a third of post-operative deaths [6,8]. Delays to diagnosis of AL have been associated with further increases in mortality [9,10] with earlier treatment being shown to improve outcomes [11]. AL is also associated with worse oncological outcomes [12,13] three-fold increase in hospital length of stay [14], poorer bowel function [15] and reduced quality of life [16].
A clinical and academic conundrum is in the precise definition of an AL, as it incorporates a wide spectrum of clinical presentations from patients who are clinically well with radiological evidence of a leak, to those patients with profound septic shock secondary to faecal peritonitis [17]. All of these patients technically have the same underlying pathological condition - an AL caused by a degree of dehiscence of the anastomosis; however their clinical pictures and outcomes are likely to be vastly different [18]. This has led to a plethora of definitions of AL [19-21], with most studies making a distinction between leaks that cause clinical sequelae versus those that are solely detected upon radiological investigation with no overt signs or symptoms [22]. The term clinically important anastomotic leak has been used to define this former group [22].
Radiological studies are often performed in the early postoperative period with the aim of assisting the clinician diagnose a potential AL in patients with non-specific clinical signs [23]. In a survey of colorectal surgeons, the majority responded that they radiologically confirmed their clinical suspicions of an AL in over 80% of patients, prior to any intervention [24]. This is with the aim of both excluding other diagnoses and also determining the extent and effect of a potential leak. There is a significant crossover in terms of presentation with other common post-operative complications such as an infected collection, superficial wound infection, chest infection or ileus - all of which require tailored management [25].
Computed Tomography (CT) scan is the current gold standard in imaging post-operative patients with suspected colorectal AL [22,26]. This has largely superseded other modalities such as contrast enema. It has been recognised that both imaging modalities can ‘over’- diagnose an AL; It was found that if routine imaging is performed on all patients post-resection, there can be radiological evidence of an AL in up to 44% with no corresponding clinical sequelae [27] (and in whom no treatment was required). At present, when a leak is suspected radiologically, correlation with laboratory and clinical findings is used to determine further management. The reported sensitivity of CT scans in the diagnosis of AL ranges from 47%-85% [28,29]. In addition to detection of a leak, cross sectional imaging also has the advantage of revealing alternative or additional post-operative complications - such as collections, wound infections and chest sepsis.
It has been previously reported that a false negatively reported CT of a patient with an AL is associated with a significant negative impact for the patient in terms of both mortality and morbidity. Marres et al. found that a false negative report was associated with a 24 hour delay to intervention and a 10 times increase in mortality [10]. Clinician decision making tools, using their own reported patient-level findings are gaining traction as ways to augment the clinician and improve patient outcomes [30,31]. This could aid both radiologists and surgeons in deciding which patients may require urgent re-operation, as opposed to those who may be more suited to conservative management or other interventional options.
During this study we have used the term anastomotic leak (AL) to refer to those patients in whom the anastomotic leak is associated with septic sequelae and who required return to theatre within 30 days of their index operation, with anastomotic dehiscence to some degree confirmed during re-operation.
Aims
The primary aim of this study was to define the key features identified by radiologists on CT scans in determining an AL following colorectal resection and anastomosis. The secondary aim would be to utilise these factors to establish their relative importance to improve the accuracy of clinical leak detection. This study aims augment radiologists in identifying those patients who would require operative re-intervention as a result of their AL.
Methods
A study was constructed to identify and compare radiologist identified CT features of a post-operative AL following a colorectal resection and anastomosis. Patients were identified from a prospectively maintained hospital database from January 2005- December 2011.
Two groups of patients were compared; Subject group - patients who had a confirmed AL; Control group - patients in whom an AL was suspected but was subsequently discounted. The control group was selected from patients in whom an AL was suspected but in whom this was subsequently excluded and the patient improved without intervention, potentially including patients with radiological but not clinical AL, as it is this distinction that the study is designed to investigate. Due to the relatively low incidence of AL following colorectal resection, patients with confirmed leaks were matched in 1:2 ratios with the control group. Control patients were matched to the subject group in age (+/- 5 years) and day of post-operative scan.
A proforma was constructed (Appendix 1) with individual radiological features related to a potential leak. Features were selected from a literature review of radiological features associated with AL [17,20,22,23,27,32-35] with potential factors divided into features of a radiological leak and adjunct features aimed at differentiating a clinically important AL (Table 1). As well as identification of individual radiological features, the radiologists were also asked to provide an overall judgement if there was an AL present.
| Leak Features | Adjunct Features |
|---|---|
| Local Collection | Ileus |
| Abscess Cavity | Small bowel obstruction |
| Stricture | Abnormal bowel wall |
| Free air | Abnormal mesentery |
| Disseminated contamination | |
| Disrupted staple line | |
| Radiological leak |
Table 1: Radiological Features selected for investigation into clinical AL predictors.
CT studies were anonymised, leak and control scans were randomised, and the radiologists were blinded to the clinical outcome. Included with each study, the radiologists were provided with a standardised clinical information set similar to that provided on routine requests of diagnostic scans; patient age, initial operation details and post-operative day that the scan was conducted.
Each one of four specialist gastro-intestinal radiologists (3 consultants (AH, PP, SR), 1 post-FRCR senior fellow (DB)) individually reviewed the CT images of all patients. During review, each feature was marked as either present or absent. A final judgement was given of if they felt an AL was present (termed a radiologists diagnosed leak (RL)) along with indication of their confidence in that overall diagnosis on a 1-10 scale (1 indicating not confident and 10 very confident). This final judgement of a RL was used as the comparator against clinical outcomes and modelled predicted outcomes.
Data was collated within a secure spread sheet in Excel (Microsoft Excel™, Microsoft, Seattle, WA, USA) with statistical analysis conducted using IBM SPSS v19 (IBM Corp., New York, NY, USA). Univariate analysis was performed to identify differences between the subject and control group; chi-squared Fisher’s exact test for categorical data and Student T-test for continuous variables.
Regression analysis for relative risk
A predictive model (with clinical AL as the binomial outcome) was constructed using a randomly selected 90% proportion of cases using IBM SPSS v19. Binomial logistical regression was employed to assess significant factors and inter-variable relationships (using feedforward regression). p-value of 0.05 was considered cut off for statistical significance, unless otherwise specified. The regression model was then tested on the out-of-group 10% subset to test for validity.
Artificial neural network analyses
A classifier ANN was trained using the feed-forward net NeuroShell Classifier, which has the ability to have variable hidden layers, and is a multi-layer perceptron equipped with a genetic training strategy (NeuroShell Classifier, Ward Systems Group Inc., Frederick, Maryland, USA). Due to relatively higher clinical importance of missing a clinical leak when compared to that of misdiagnosing a non-leak patient with a leak, the output optimisation goals were set during training to reflect this. Higher weights were placed upon correctly identified leaks and penalties upon missed cases within a fitness coefficient matrix.
A process of backward variable selection was applied [36] wherein variables with the least impact upon the network (those with the least weightings) were sequentially removed until further removal of variables was detrimental to the overall predictive function of the model. These variables were then used as the base function create the final neural network with multiple networks created and trained with varying penalties or rewards placed upon incorrect and correct predictions respectively. Using a custom input method of outcome penalties, each of the four outcomes could be relatively prioritised - for example, the network was relatively more harshly penalised for missing a leak (false negative) and more neutrally treated for over-predicting a leak (false positive). Each network was trained until there were one hundred iterations without improvement at which point the network was considered optimised and training halted, with the nodal weights set. Iterations were limited to only one hundred iterations without network alteration to prevent over fitting of the network. Repeated iterations of the network were created (with the aim of over one hundred) with an initial goal of sensitivity to leak detection and secondary goal of specificity. Iterative network continued until specificity was optimised without compromising sensitivity. An optimised generalizability function was applied to the final network post-creation to reduce the effects of any over-fitting. The trained ANN was then applied to the 30% out of set data with classification compared with known clinical outcomes, producing the ANN sensitivity, specificity with a receiver operating characteristic area under the curve (AUC) for overall accuracy.
Ethics
The predictions of the ANN were not revealed to the patient’s clinical team, and there was no modification in routine patient care. Following discussion with the hospital local clinical research & audit team, ethics approval was deemed not required due to absence of patient intervention.
Results
During the study period there were 17 patients who had a confirmed AL in whom a post-operative CT scan was available for analysis. 34 control patients were selected, who had undergone a post-operative scan with a clinical suspicion of an AL, matched for age and timing of imaging study. Seven control patients were subsequently excluded from analysis as the image files were found to be of insufficient quality. There was 98.7% data set completion.
There was no significant difference in the baseline features between leak and control groups in terms of age, day of scan or location of anastomosis (Table 2).
| Feature | Leak Subjects | Control subjects | Significance |
|---|---|---|---|
| Age (years) | 66.7 (17.1) | 60.2 (9.3) | p=0.32 |
| Day of scan | 7.00 (3.65) | 5.97 (5.27) | p=0.15 |
| Location of anastomosis Small Bowel Right Left Pelvic |
1 5 4 7 |
4 5 4 16 |
p=0.23 |
Table 2: Comparative features between leak and control subjects-results presented as Mean and (standard deviation) for Age and Day of Scan. Frequency used within location of anastomosis.
There was only a 7% inter-observer error between observers (Kappa = 0.79) with no significant differences in the accuracy, sensitivity or specificity between the four radiologists of their overall judgement if an AL was present (RL) (Table 3). The presence or absence of a RL correctly correlated with an AL in 81.4% of cases. Aggregated performance to correctly identify an anastomotic leak produced; Sensitivity 86.15%, Specificity 78.57%, Negative predictive value 70.0%, Positive predictive value 90.72%, Receiver operating characteristic Area under curve 0.862.
| Observer | AL/RL | No AL/RL | AL/No RL | No AL/No RL |
|---|---|---|---|---|
| Radiologist 1 | 14 | 5 | 2 | 23 |
| Radiologist 2 | 13 | 3 | 4 | 25 |
| Radiologist 3 | 14 | 6 | 2 | 22 |
| Radiologist 4 | 15 | 10 | 1 | 18 |
| All radiologists | 56 | 24 | 9 | 88 |
Table 3: RL predicted outcomes, per radiologist against final AL diagnosis.
Univariate Analysis
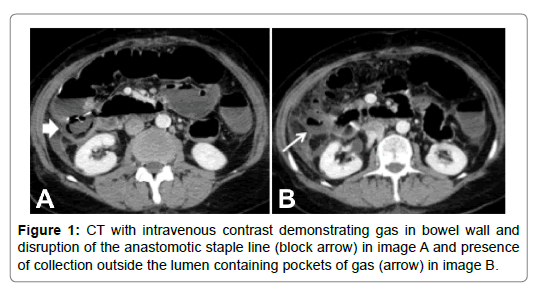
Univariate analysis is shown in Table 4. Free air, disseminated contamination, disrupted staple line, radiological leakage of contrast, abnormal bowel wall (demonstrated in Figure 1) and abnormal mesentery were all found to have significant correlation with an AL on univariate analysis and were therefore used as predictors in multivariate analysis.
| Radiological Feature | Feature rated as present | No AL | AL | Univariate P value |
|---|---|---|---|---|
| Local Collection | No Yes |
94 17 |
48 17 |
0.112 |
| Abscess Cavity | No Yes |
111 0 |
64 1 |
0.369 |
| Stricture | No Yes |
111 0 |
65 0 |
N/A |
| Free air | No Yes |
104 7 |
49 16 |
0.001 |
| Disseminated contamination | No Yes |
106 5 |
35 30 |
<0.001 |
| Disrupted staple line | No Yes |
93 16 |
34 28 |
<0.001 |
| Radiological leak of contrast | No Yes |
87 25 |
8 57 |
<0.001 |
| Ileus | No Yes |
76 35 |
39 26 |
0.325 |
| Small bowel obstruction | No Yes |
98 14 |
56 9 |
0.819 |
| Abnormal bowel wall | No Yes |
96 15 |
32 29 |
<0.001 |
| Abnormal mesentery | No Yes |
69 43 |
15 50 |
<0.001 |
Table 4: Univariate analysis of radiological features for leak prediction.
Multivariate Analysis
A multivariate analysis was then performed to assess the impact of radiological assessment of CT scans in the identification of patients with an AL (Table 5). This was conducted incorporating the co-variates of age, location of anastomosis and day of scan with the significant variables at univariate analysis; free gas, disseminated contamination, a disrupted staple line, radiological leak of contrast, abnormal bowel wall and an abnormal mesentery. Backwards stepwise regression (based on likelihood ratio) maintained abnormal bowel wall, collection, overall impression of a leak and ileus (along with the constant) as significant predictors. Containing these variables, the model reached a χ2 (df 5, N=165) = 85.4, p<0.001, indicating that the model was able to distinguish between patients with an AL and those without with significant accuracy. The model explained between 41.7% and 57.2% of the variance in AL based upon the CT scan alone (Cox & Snell R2 & Nagelkerke R2 0.417 & 0.572 respectively), and correctly classified in 85.4% of cases. Overall impression of a leak (OR 50.69, p<0.001), local collection (OR 0.24, p=0.011) and ileus (OR 3.56, p=0.013) remained significant within the model on multivariate analysis, with abnormal bowel wall trending towards significance (OR 2.62, p=0.053), but still conferring benefit within the model. The value of the model to correctly select for anastomotic leaks had a sensitivity of 81.97%, specificity 86.24%, negative predictive value 76.92%, positive predictive value 89.52% and receiver operating characteristic area under curve 0.841.
| Multivariate logistic regression model and outcomes | |||
| Maintained variables within the model | |||
| Variable | OR | p-value | |
| Overall impression of leak | 50.69 | <0.001 | |
| Local Collection | 0.24 | 0.011 | |
| Ileus | 3.56 | 0.013 | |
| Abnormal Bowel Wall | 2.62 | 0.053 | |
| Model results on out of training sample | |||
| Actual No Leak | Actual Leak | ||
| Predicted No Leak | 94 | 11 | |
| Predicted Leak | 15 | 50 | |
| Overall Outcomes | |||
| Overall Correct | 85.40% | ||
| Overall Incorrect | 14.6% | ||
| Sensitivity (for Leak) | 81.97% | ||
| Specificity (for Leak) | 86.24% | ||
| Negative Predictive Value | 76.92% | ||
| Positive Predictive Value | 89.52% | ||
| Receiver Operating Characteristic | 0.8410 | ||
Table 5: Multivariate logistic regression model and outcomes.
A correct clinical leak prediction was associated with a significantly higher level of confidence: 7.09/10 when incorrect vs. 8.50/10 when correct; T-test (F=0.4.86, df = 174) p=0.02.
Artificial Neural Network
A random seed was used to select 70% of cases from the original dataset to be presented the ANN for training. All available variables and co-variables were included in the initial ANN build. Utilising over one hundred rounds of backwards variable selection, the gene optimised ANN maintained 9 input variables; Age, side of operation, small bowel obstruction, overall impression of a leak, abnormal mesentery, ileus, collection, disrupted staple line and abnormal bowel wall. These factors were then used to train one hundred-twenty-three parallel optimised versions of the ANN for AL selection, with minor alterations in the penalties for incorrect answers, and in ANN structure. This second round of optimisation continued until sensitivity was maximised, with secondary consideration given to maintaining specificity, with further adjustments leading to deterioration in sensitivity. Within the final optimised model, the optimisation grid allowed for neutral scoring of a true negative, -5 penalties for a false negative, -1 penalty for a false positive and neutral scoring for a true positive. Internal validation was conducted on the remaining 30% out of set group to test for sensitivity, specificity, negative predictive and positive predictive values as well as to calculate the Receiver Operating Characteristics Area under the Curve. The variables maintained with the ANN in decreasing order of importance within the model were; Age, location of anastomosis, disrupted staple line, small bowel obstruction, prediction of clinically important leak, abnormal bowel wall, collection, abnormal bowel mesentery and ileus.
The internal validation set consisted of 55 cases, 24 of whom had a clinically verified anastomotic leak and 31 without a verified leak. The optimised neural network identified all 24 cases with an anastomotic leak, 28 non-leak patients and over-predicted in 4 cases, classifying them as having a leak incorrectly (Table 6). This resulted in an overall correct prediction in 92.72% of cases; sensitivity (for a leak) of 100.0%, specificity 87.1%, negative predictive value 100.0%, positive predicted value 85.7% and Receiver Operating Characteristic Area under Curve of 0.9704.
| Network training and outcomes | ||
| Outcome penalties | ||
| Clinical No Leak | Clinical Leak | |
| Predicted no Leak | 0 | -5 |
| Predicted Leak | -1 | 0 |
| Variable Weights within the model | ||
| Parameter | Weight | |
| Age | 0.675 | |
| Location of anastomosis | 0.116 | |
| Disrupted Staple line | 0.065 | |
| Small Bowel Obstruction | 0.051 | |
| Radiologist prediction of clinical leak | 0.046 | |
| Abnormal Bowel Wall | 0.030 | |
| Collection | 0.011 | |
| Abnormal Mesentery | 0.005 | |
| Ileus | 0.003 | |
| Network results on out of training sample | ||
| Actual No Leak | Actual Leak | |
| Predicted No Leak | 27 | 0 |
| Predicted Leak | 4 | 24 |
| Overall Outcomes | ||
| Overall Correct | 92.73% | |
| Overall Incorrect | 7.27% | |
| Sensitivity (for Leak) | 100.0% | |
| Specificity (for Leak) | 87.10% | |
| Negative Predictive Value | 100.0% | |
| Positive Predictive Value | 85.71% | |
| Receiver Operating Characteristic | 0.9704 | |
Table 6: Final neural network structure trained to detect clinically relevant anastomotic leaks following colorectal resection.
Discussion
AL accounts for up to a third of post-operative deaths in colorectal resection surgery patients [6]. Long term quality of life is adversely affected following a clinical AL, as well as; increased rates of tumour recurrence, presence of a permanent stoma and overall poorer bowel function. Earlier diagnosis of AL (on or before post-operative day 5) is associated with improved patient outcomes [1,37]. Within this early post-operative period, significant clinical overlap exists between AL and alternative complications - with accurate diagnosis important to differentiate and guide effective management [25]. CT imaging is the imaging modality of choice within the majority of healthcare institutions [22,38], usually with ready access both during and out of routine office hours.
A higher level of accuracy in radiological reporting of clinical AL was seen in patients with right sided anastomoses in comparison to pelvic anastomoses (92-95% versus 72-84%). This result is mirrored within similar published studies an accurate correlation declines with decreasing distance between anastomosis and anal verge [29]. Degree of confidence in the diagnosis of a radiological AL positively correlated with a correct diagnosis of clinical AL. The confidence in diagnosis was significantly higher when there was a correct correlation of radiological and clinical AL - suggesting that in clinical practice there is value in the surgeon and radiologist discussing the imaging study; and for the radiologist to clearly indicate their level of confidence in the likelihood of a significant AL.
Whilst most surgeons request cross sectional imaging to assist in the diagnosis of a clinical AL [24], the reported sensitivity is from 47%-85% [28,29] with an over-diagnosis of a radiological leak in up to 44% in clinically well patients [27]. Kornmann et al. performed a systematic review on the value of CT in the diagnosis of anastomotic leaks following colorectal surgery [38]. Following review, they included 221 abdominal scans for analysis and found an aggregated sensitivity of 68% (95% confidence interval 59%-75%).
Within our study the radiologists diagnosis of a radiological leak correctly correlated with a clinical AL in 81.4% of cases. This accuracy was increased to 85.4% with application of the logistic regression model and further to 92.73% with the neural network model (Table 7).
| Clinical | Regression Model | Neural Network | |
|---|---|---|---|
| Overall accuracy | 81.40% | 85.40% | 92.73% |
| Sensitivity | 86.15% | 81.97% | 100.0% |
| Specificity | 78.57% | 86.24% | 87.10% |
| Negative Predictive Value | 70.00% | 76.92% | 100.0% |
| Positive Predictive Value | 90.72% | 89.52% | 85.71% |
| ROC AUC | 0.862 | 0.841 | 0.970 |
Table 7: Comparison of analysis technique performance upon outcomes.
Systematic assessment of post-operative CT studies, using a standardised proforma, enabled the reliable identification of individual radiological features as demonstrated by the low level of inter-observer variability within this study. Doeksen found that there was a 10% intraobserver error on assessment of CT scans (28), in comparison to the 7% inter-observer error within our study.
Gervaz et al. created a scoring system to identify patients who incurred a clinically significant anastomotic leak (requiring reoperation) following colorectal resection [39]. In this study of 74 patients, 17 on whom went on to have re-operation confirming the condition, two independent radiologists were asked to independently re-review CT imaging performed with an aim of confirming or excluding an anastomotic leak. When clinical and radiological factors were combined into a multiple regression model they found the remaining significant factors were; white blood cell count >9 × 109/L, >5 mm depth of pneumoperitoneum either adjacent to the anastomosis or within the mesentery as well as >500 mls of free fluid within the abdomen. In patients who had all three features present the incidence of a relevant anastomotic leak in this group was 100%, 31% with two factors, 5.9% with one factor, 0.0% with no factors.
Although this study was able to correctly identify patients with a high risk for anastomotic leak, in order to create a group in which all leaks were detected, this would identify 93.2% of patients, and if aiming to identify 94.1% of leaks, this group would still include 47.3% of the cohort, of whom less than half (42.8%) would have a leak.
This study illustrates a challenge of multiple logistic regressions where the statistical balance of a false positive and false negative are equally treated, whereas in real world scenarios the clinical ramifications of delay in diagnosing a leak outweighs those of a false positive. Our logistic regression model ran into similar difficulties which, whilst it had an overall correct result in 85.4%, it missed 11 of 61 leaks (sensitivity 81.97%) and over predicted 15 of 109 non leaks (86.24%).
Within clinical applications, it is beneficial to confer relatively more importance to missed positives or negatives, depending on the clinical situation; within this clinical setting, patients can suffer significant deleterious effects if an anastomotic leak is missed, as opposed to the lesser effects of over-investigating/treating those patients who would have recovered without intervention. Kornmann et al. found that in a study of 524 patients, 97 had a post-operative CT to aid in the diagnosis of a leak. The overall mortality in patients who had a confirmed anastomotic leak was 21.1% (n=12); this is in comparison to a mortality rate of 62.5% of patients who eventually had a confirmed diagnosis of a leak, but in whom the scan was initially reported as ‘no leak’ [37]. This threefold increase in mortality reflects both the increasing mortality of anastomotic leaks with a delay in diagnosis, and our reliance upon imaging to guide our treatment options when considering re-operation. They found that 33.3% of patients in whom the scan was reported as having no leak, were eventually diagnosed with an anastomotic leak at re-operation. Whilst a low number of missed anastomotic leaks can still give a good statistical outcome (the accuracy for CT scan in this study for anastomotic leaks was 74%, sensitivity 59%, specificity 88%), within the group of missed positives confers a significant clinical deleterious outcome.
Artificial neural networks, which work on a more fluid modelling system than logistic regression, have the benefit of being able to be modelled on the relative importance of the outcomes. Our neural network was strongly penalised for missing an anastomotic leak, with a more neutral feedback for over-predicting a non-leak into the leak category. This non-symmetrical weighting system enabled our neural network to preferentially select out anastomotic leaks and with repeated training to reach a sensitivity of 100% within a separate dataset (out of set group) of new data. This unstable balance will always be at the expense of some loss of specificity, however we were still able retain a specificity of 87.10%, on a par with those of the logistic regression model of 86.24% and radiologist assessment of 78.57%. All three methods (radiologist/LR/ANN) had similar positive predictive values of 90.72%, 89.52% and 85.71% respectively, however there was a significant improvement in negative predictive value of 70.0%, 76.92% and 100.0%.
The focus of this study was in the correct identification of clinical AL using CT imaging. Whilst we have created a model that improves diagnostic ability beyond that of sole radiological interpretation, a negative result does not preclude that the patient has an alternative complication that could require intervention.
Conclusion
We have shown that individual CT features can be used to create an effective risk prediction model for leak detection. This study represents the first artificial neural network guided interpretation of CT scans to aid in the diagnosis of anastomotic leaks following colorectal surgery. The adaptive nature of neural networks has proved to be particularly suited to this setting, outperforming either logistic regression or radiological assessment alone.
While the neural network model may provide a higher degree of accuracy in leak detection using identification of individual radiological features, it should be taken together with the degree to which the radiologist is confident that these features are present. Future work to validate this prediction model prospectively may enable an increase in early detection of anastomotic leaks, reduced missed diagnosis and overall improve patients’ outcomes.
References
- Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, et al. (2002) Factors associated with clinically significant anastomotic leakage after large bowel resection: Multivariate analysis of 707 patients. World J Surg 26: 499-502.
- Nikolian VC, Kamdar NS, Regenbogen SE, Morris AM, Byrn JC, et al. (2017) Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surg 161: 1619-1627.
- Isbister WH (2001) Anastomotic leak in colorectal surgery: a single surgeon’s experience. ANZ J Surg 71: 516-520.
- McNair AG, Whistance RN, Forsythe RO, Macefield R, Rees J, et al. (2016) Core outcomes for colorectal cancer surgery: a consensus study. Plos Med 13: e1002071.
- Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, et al. (2008) Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: A prospective monocentric study. Int J Colorectal Dis 23: 265-270.
- Snijders HS, Wouters MWJM, Van Leersum NJ, Kolfschoten NE, Henneman D, et al. (2012) Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol 38: 1013-1019.
- Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H (2014) Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: A nationwide cohort study. Ann Surg 259: 930-938.
- Shekarriz H, Eigenwald J, Shekarriz B, Upadhyay J, Shekarriz J, et al. (2015) Anastomotic leak in colorectal surgery: are 75Â % preventable? Int J Colorectal Dis 30: 1525-1531.
- Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P (1999) Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg 189: 554-559.
- Marres CCM, van de Ven AWH, Leijssen LGJ, Verbeek PCM, Bemelman WA, et al. (2017) Colorectal anastomotic leak: delay in reintervention after false-negative computed tomography scan is a reason for concern. Tech Coloproctol 21: 709-714.
- Ortega-Deballon P, Radais F, Facy O, D’Athis P, Masson D, et al. (2010) C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg 34: 808-814.
- Sauven P, Playforth MJ, Evans M, Pollock AV (1989) Early infective complications and late recurrent cancer in stapled colonic anastomoses. Dis Colon Rectum 32: 33-35.
- Dupré A, Slim K (2012) Anastomotic leakage after colorectal surgery: Can it be detected earlier and more easily?. J Visc Surg 149: e287-e288.
- Frye J, Bokey EL, Chapuis PH, Sinclair G, Dent OF (2009) Anastomotic leakage after resection of colorectal cancer generates prodigious use of hospital resources. Color Dis 11: 917-920.
- Kiely JM, Fazio VW, Remzi FH, Shen B, Kiran RP (2012) Pelvic sepsis after IPAA adversely affects function of the pouch and quality of life. Dis Colon Rectum 55: 387-392.
- Khan AA, Wheeler JMD, Cunningham C, George B, Kettlewell M, et al. (2008) The management and outcome of anastomotic leaks in colorectal surgery. Color Dis 10: 587-592.
- Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208: 269-278.
- Thornton M, Joshi H, Vimalachandran C, Heath R, Carter P, et al. (2011) Management and outcome of colorectal anastomotic leaks. Int J Colorectal Dis 26: 313-320.
- Bruce J, Krukowski ZH, Al-Khairy G, Russell EM, Park KG (2001) Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 88: 1157-1168.
- Tytherleigh MG, Bokey L, Chapuis PH, Dent OF (2007) Is a minor clinical anastomotic leak clinically significant after resection of colorectal cancer? J Am Coll Surg 205: 648-653.
- Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, et al. (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the international study group of rectal cancer. Surg 147: 339-351.
- Power N, Atri M, Ryan S, Haddad R, Smith A (2007) CT assessment of anastomotic bowel leak. Clin Radiol 62: 37-42.
- Nicksa GA, Dring RV, Johnson KH, Sardella WV, Vignati PV, et al. (2007) Anastomotic leaks: What is the best diagnostic imaging study? Dis Colon Rec 50: 197-203.
- Adams K, Papagrigoriadis S (2013) Little consensus in either definition or diagnosis of a lower gastro-intestinal anastomotic leak amongst colorectal surgeons. Int J Colorectal Dis 28: 967-971.
- Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240: 205-213.
- Hirst NA, Tiernan JP, Millner PA, Jayne DG (2014) Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis 16: 95-109.
- Lim M, Akhtar S, Sasapu K, Harris K, Burke D, et al. (2006) Clinical and subclinical leaks after low colorectal anastomosis: A clinical and radiologic study. Dis Colon Rectum 49: 1611-1619.
- Doeksen A, Tanis PJ, Wüst AFJ, Vrouenraets BC, Lanschot JJB, et al. (2008) Radiological evaluation of colorectal anastomoses. Int J Colorectal Dis 23:863-868.
- Khoury W, Ben-Yehuda A, Ben-Haim M, Klausner JM, Szold O (2009) Abdominal computed tomography for diagnosing postoperative lower gastrointestinal tract leaks. J Gastrointest Surg 13: 1454-1458.
- den Dulk M, Noter SL, Hendriks ER, Brouwers MAM, van der Vlies CH, et al. (2009) Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol 35: 420-426.
- Thornblade LW, Flum DR, Flaxman AD (2018) Predicting future elective colon resection for diverticulitis using patterns of health care utilization. eGEMs (Generating Evid Methods to Improv patient outcomes) 6: 1.
- DuBrow RA, David CL, Curley SA (1995) Anastomotic leaks after low anterior resection for rectal carcinoma: evaluation with CT and barium enema. Am J Roentgenol 165: 567-571.
- Zissin R, Gayer G (2004) Postoperative anatomic and pathologic findings at CT following colonic resection. Sem Ultrasound CT MRI 25: 222-238.
- Tang CL, Yeong KY, Nyam DC, Eu KW, Ho YH, et al. (2000) Postoperative intra-abdominal free gas after open colorectal resection. Dis Colon Rectum 43: 1116-1120.
- Akyol AM, McGregor JR, Galloway DJ, George WD (1992) Early postoperative contrast radiology in the assessment of colorectal anastomotic integrity. Int J Colorectal Dis 7: 141-143.
- Mofidi R, Duff MD, Madhavan KK, Garden OJ, Parks RW (2007) Identification of severe acute pancreatitis using an artificial neural network. Surg 141: 59-66.
- Kornmann VN, Van Ramshorst B, Smits AB, Bollen TL, Boerma D (2014) Beware of false-negative CT scan for anastomotic leakage after colonic surgery. Int J Colorectal Dis 29: 445-451.
- Kornmann VN, Treskes N, Hoonhout LHF, Bollen TL, Van Ramshorst B, et al. (2013) Systematic review on the value of CT scanning in the diagnosis of anastomotic leakage after colorectal surgery. Int J Colorectal Dis 28: 437-445.
- Gervaz P, Platon A, Buchs NC, Rocher T, Perneger T, et al. (2013) CT scan-based modelling of anastomotic leak risk after colorectal surgery. Color Dis 15: 1295-1300.
Citation: Adams K, Hansmann A, Bosanac D, Peddu P, Ryan S, et al. (2019) Creation of an Accurate Artificial Neural Network Prediction Model of Radiologist Reported CT Features for Colorectal Anastomotic Leaks. OMICS J Radiol 8:307. DOI: 10.4172/2167-7964.1000307
Copyright: © 2019 Adams K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3817
- [From(publication date): 0-2019 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 2861
- PDF downloads: 956