Research Article Open Access
Effect of a Botanical Mouth Rinse on Dental Plaque Formation: A Randomized, Double-blinded, Placebo-controlled Trial
William Z Levine1*, Noah Samuels2 and Ray C Williams3
1Jerusalem Perio Center, Jerusalem, Israel
2Tal Center for Integrative Medicine, Institute of Oncology, Sheba Medical Center, Tel Hashomer, Israel
3Stony Brook University School of Dental Medicine, Stony Brook, New York, USA
- *Corresponding Author:
- William Z Levine
Jerusalem Perio Center
101 Derech Hevron St.
Entrance A Jerusalem 93480, Israel
Tel: +972-2-563-3250
Fax: +972-2-5661508
E-mail: perio@bezeqint.net
Received Date: July 21, 2014; Accepted Date: August 04, 2014; Published Date: August 10, 2014
Citation: Levine WZ, Samuels N, Williams RC (2014) Effect of a Botanical Mouth Rinse on Dental Plaque Formation: A Randomized, Double-blinded, Placebo-controlled Trial. J Oral Hyg Health 2:150. doi:10.4172/2332-0702.1000150
Copyright: © 2014 Levine WZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Background and Purpose: Current approaches for controlling dental plaque are limited by technical difficulties and side effects (e.g., staining, ulceration). PeriActive mouth rinse (Izun Pharmaceuticals Corporation, New York, NY) contains botanical compounds with anti-bacterial, anti-inflammatory and tissue reparatory properties. This study examined the effect of the PeriActive rinse on plaque formation and gingival inflammation in an experimental gingivitis model.
Methods: Healthy volunteers with mild-to-moderate gingivitis were randomized and blinded to either PeriActive or a color/taste-matched water-only rinse. Treatment was self-administered t.i.d. for two weeks, during which participants abstained from any oral hygiene practice (brushing, flossing, etc.). Gingival index (GI), plaque index (PI), and number of bleeding sites (as determined by bleeding-upon-probing) were measured at baseline, and again at the end of the study period.
Results: A total of 54 participants completed the full study protocol (PeriActive, n=23; water-only controls, n=31). GI values increased (i.e., worsened) more significantly among water-only controls when compared to the PeriActive treatment group (p<0.001 vs. p=0.182; between-group Δ progression, p=0.004), as did PI scores (p<0.001 vs. p=0.005; between-group Δ progression, p=0.002) and number of bleeding sites (p=0.001 vs. p=0.304; betweengroup Δ progression, p=0.001). No significant adverse effects were reported in either group.
Conclusions: PeriActive mouth rinse is a safe and effective anti-bacterial and anti-inflammatory rinse, which significantly reduces the progression of gingivitis and dental plaque formation and the number of spontaneous bleeding sites in an experimental gingivitis model. Further research is needed in order to confirm and extend these findings.
Keywords
Herbal; Mouth rinse; Gingivitis; Periodontitis; Bleedingon- probing
Introduction
Dental plaque is an important etiological factor in the development of gingival and periodontal inflammation [1]. Plaque formation is the product of bacteria and bacterial byproducts of periodontal pathogens, which form a biofilm above and below the gingival margin. This, in turn, stimulates a chronic inflammatory response which results in decreased tissue repair capabilities and subsequent periodontal bone destruction [2-5]. Current therapies for plaque reduction include mechanical means for removing plaque, such as tooth brushing and flossing, as well as regular dental visits for scaling and root planning procedures [6]. Tooth brushing with fluoride-containing dentifrice is considered the most important method for removing dental plaque [7]. However, this method is limited by the presence of areas in the oral cavity which are difficult to reach. In addition, patients often exhibit inadequate skill and motivation, with reduced adherence to the treatment regimen [8]. Oral rinses are effective for both preventing plaque development as well as reducing inflammation [9]. The use of anti-microbial mouth rinses, based on components such as cetylpyridinium chloride (CPC) or essential oils (EO) and chlorhexidine (CHX) have been shown to further enhance plaque removal when used in conjunction with mechanical debridement techniques [10,11] Oral rinses are particularly useful for patients with severely inflamed gums who may not brush properly because of pain and discomfort, and for patients with physical and/ or cognitive disabilities. However, the use of rinse components such as CHX can be accompanied by a number of side effects such as staining of the teeth and tongue, ulcerations and a burning sensation [12-15]. While these side effects may not be considered to be severe from a medical perspective, they may be regarded as an aesthetic detriment and may contribute to reduced adherence to a regular oral hygiene regimen [8]. Traditional essential oil (EO) mouthwashes contain ethanol, which can have a negative effect on the surfaces of composite restorations [16], and they have been linked to the development of oropharyngeal cancer [17,18].
PeriActive™ (Izun Pharmaceuticals Corporation, New York, NY) is a botanical-based mouth rinse comprised of the herbs Centella asiatica, Echinacea purpurea, and Sambucus nigra which was developed for the treatment of gingivitis. The herbal components of the rinse are prepared in accordance with the guidelines of the U.S. homeopathic pharmacopoeia (HPUS), and formulated into a film-forming rinse. The present prospective, double-blinded, randomized controlled trial evaluated the effect of PeriActive in an experimental gingivitis model, in which patients were instructed to refrain from oral hygiene for a period of two weeks. During this period, subjects were instructed to use either the PeriActive or a comparable water-only rinse. Absolute and delta increases in gingival index (GI) and plaque index (PI) scores, as well as the number of spontaneously bleeding sites, were compared between the two groups. The anti-bacterial and anti-inflammatory effects of the PeriActive botanical components are discussed.
Materials and Methods
Participants and study medications
Subjects participating in the study were healthy volunteers who were examined in a specialized periodontal clinic located in Jerusalem, Israel. Participants aged 14-75 years with at least 24 teeth and clinicallydiagnosed mild-to-moderate gingivitis were eligible for inclusion. Subjects with severe periodontitis (i.e., periodontal pockets of >5 mm in three or more areas) were excluded, as were those with a chronic medical condition (e.g., diabetes, cardiovascular disease, chronic inflammatory illness); those taking either anti-inflammatory or antibiotic medications; pregnant women, and smokers. Participation in the study was voluntary, with participants receiving a reasonable remuneration for their time and trouble. Following an in-depth explanation of the study proceedings and potential adverse events, all eligible candidates were asked to sign a written informed consent form. The study protocol was approved by the Institutional Review Board at the Shaare Zedek Medical Center in Jerusalem, and was registered at ClinicalTrials.gov (NCT00885599).
PeriActive is a proprietary mixture of extracts from the following herbs: Centella asiatica, Echinacea purpurea, and Sambucus nigra. This combination of herbal components was selected following extensive pre-clinical research (unpublished data). The ingredients in PeriActive are formulated in an alcohol-free solution which contains flavorants and a preservative. The water-based rinse was color- and flavorcoordinated with the PeriActive rinse, with the same preservative as the study rinse.
Experimental design
The experimental gingivitis model, as described by Loe et al., requires study subjects to refrain from any type of oral hygiene whatsoever, this for a set period of time [19]. In the present study, following induction to the study protocol, subjects were randomly allocated to either PeriActive rinse treatment or water-only rinse treatment, using the Moses-Oakford assignment algorithm. The primary study outcome was the severity of gingival inflammation and extent of plaque distribution, as measured by gingival index (GI) and plaque index (PI) [20], and a count of the number of sites of spontaneous bleeding, as determined by bleeding-upon-probing. Adverse events believed to be related to the study treatments were noted. In order to standardize the outcome measurements and minimize inter-observer variability, the FDA Guidance for Industry protocol guidelines were followed [21] For this purpose, one of the study coordinators was responsible for calibrating three licensed and practicing dentists to serve as examiners. The calibration process consisted of training and then testing the accuracy of examiner measurements of GI and PI on 20 patients, verifying that identical scores were assigned to each outcome (inter-observer variation of ≤ 10%). Examiners were monitored throughout the study proceedings, with periodic retraining by the study coordinator. Each participant was examined at the various stages of the study by the same examiner, enabling a more accurate comparison of baseline and final outcome parameters. Upon study induction, all study subjects underwent a full dental prophylaxis treatment with scaling and tooth polishing. Baseline measurements (GI, PI and number of bleeding sites) were taken at this point. Participants were then instructed to abstain from any form of dental hygiene , including tooth brushing, dental flossing, use of breath mints or any mouth rinse other than the designated study rinse. Participants received a detailed explanation on the proper use of the study rinses, which were to be swished (15 ml each time) for 60 seconds, and then expectorated. This was to be repeated three times daily, for a period of 14 days. Unmarked bottles containing 250 ml each of study rinse were given to each patient, each with a 15 mL-dose cap which was to be filled at each administration of the rinse. Both participants and examining dentists were blinded as to the contents of the bottles of rinse.
On day 14 of the study, participants were re-examined for study outcomes (GI, PI, number of bleeding sites, and adverse effects), and then given full dental prophylaxis and instructed to return to their full oral hygiene regimen. Throughout the study period subjects were instructed to record the time and dose of the self-administered study rinse in a study log. Adverse effects were recorded as well, and were graded on a three-point scale (mild, moderate, serious), and reported to the study monitor. Participants were asked to hand in their treatment logs, as well as all bottles of rinse at the end of the study period. Adherence to the study treatment regimen was established based on the log records as well as the residual volume of rinse in the returned bottles.
Data collection and statistical analysis
Data were entered into a Microsoft Excel 2003 worksheet and transferred to SPSS version 16 (SPSS, Inc., www.spss.com) for statistical analysis. The outcome measures GI and PI were calculated both as mean values at the beginning and end of each phase (± SD), as well as calculating the absolute difference (Δ=final-baseline mean GI, PI and number of bleeding sites). A positive difference indicated a worsening of plaque formation and inflammation. Data met the conditions for normalcy as determined by Goodness-of-Fit Test (Kolmogorov- Smirnov test). The paired t-test was used to determine whether there were significant differences between mean baseline and final values. In addition, a single t-test assuming equal variance was used in order to compare the PeriActive rinse treatment to the water-only rinse control, using a one-tailed t-test. A p-value of less-than or equal-to 0.05 was considered statistically significant.
Results
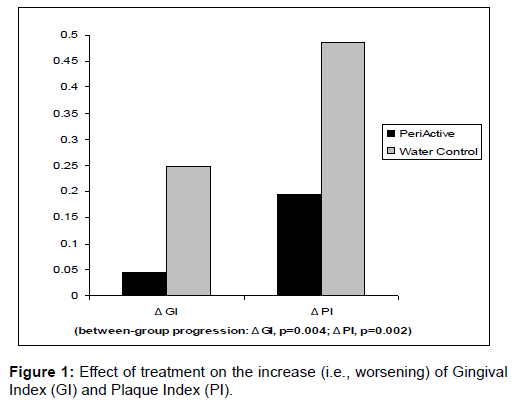
A total of 59 subjects were enrolled, with 54 completing the twoweek study protocol: 23 in the PeriActive treatment group (17 male, 16 female; mean age 18.3 ± 2.83 years) and 31 in the water-only control group (22 male, 9 female; mean age 17.5 ± 1.71 years). Only one patient in the PeriActive group was a mild smoker (<5 per day), with no smokers in the water-only control group (Figure 1).
Study outcomes
Baseline GI and PI values were similar in both study groups, and reflected minimal to mild gingival disease (Table 1). GI scores increased significantly more (indicating a worsening of gingival inflammation) in the water-only treatment group (p<0.001) when compared to the PeriActive treatment group (p=0.182; between-group Δ progression, p=0.004). PI scores also increased more significantly in the water-only group (p<0.001) than in the PeriActive group (p=0.005; betweengroup Δ progression, p=0.002). The number of bleeding sites (Table 2) increased more significantly in the water-only rinse group (p=0.001) than in the PeriActive-treated subjects (p=0.304; between-group Δ of progression, p=0.001). No serious adverse events were noted in either group of the study, though mild symptoms were noted in 15 of the water-only treated group, compared to 7 in the PeriActive treated group (Table 3).
| Water Control | PeriActive | |
|---|---|---|
| n | 31 | 23 |
| GI Scores | ||
| At baseline | 0.666 ± 0.223 | 0.731 ± 0.338 |
| After 2 weeks | 0.915 ± 0.318 | 0.777 ± 0.367 |
| p value | <0.001 | 0.182 |
| PI Scores | ||
| At baseline | 0.558 ± 0.257 | 0.586 ± 0.251 |
| After 2 weeks | 1.044 ± 0.391 | 0.781 ± 0.313 |
| p value | <0.001 | 0.005 |
‡lower p values indicate a greater worsening of the outcome parameter
Table 1: Effect of Treatment‡ on Gingival Index (GI) and Plaque Index (PI) Scores ± SD.
| Water Control | PeriActive | |
|---|---|---|
| n | 31 | 23 |
| At baseline | 12.35 ± 9.35 | 16.35 ± 16.36 |
| After 2 weeks | 22.71 ± 15.41 | 19.00 ± 18.49 |
| p value | 0.001 | 0.304 |
| Δ no. of bleeding sites | -10.35 ± 13.19 | -2.65 ± 11.10 |
(between-group Δ of progression, p=0.014)
‡as determined by bleeding-upon-probing
Table 2: Effect of Treatment‡ on the number of spontaneous bleeding sites ± SD.
| Adverse Event | PeriActive | Water Control |
|---|---|---|
| Altered taste | 1 | 4 |
| Discoloration | 3 | 4 |
| Increased sensitivity | 2 | 0 |
| Burning sensation | 1 | 0 |
| Oral ulceration | 0 | 2 |
| Halitosis | 0 | 1 |
| Gum laceration | 0 | 3 |
| Pain, discomfort | 0 | 1 |
| Total | 7 | 15 |
Table 3: Reported adverse events associated with the study rinses.
Discussion
This prospective, interventional, double-blinded, randomized, placebo-controlled trial set out to examine the effects of the botanical compound-based PeriActive mouth rinse on gingival inflammation and plaque formation. For this purpose, an experimental gingivitis model was used, in which study subjects were instructed to abstain from any dental hygiene other than the study rinses for a period of two weeks. The PeriActive rinse was found to significantly reduce the progression of gingival inflammation and plaque build-up, as measured by percentage increase of GI and PI scores, as well as the number of spontaneous bleeding sites at the end of the study period. PeriActive was found to be safe and well-tolerated by participants. The three herbal components of the PeriActive rinse are considered safe, and are commonly used for a number of medical conditions. These compounds have been shown to exhibit both anti-inflammatory and anti-microbial properties. The component Centella asiatica (Centella), used in the treatment of wounds [22-24], has been shown to increase collagen production and stimulate cell growth by increasing DNA, protein, and hexosamine levels in granulation tissue [25] This leads to an increase of collagen cross-linking, with increased tensile wound strength and re-epithelialization [26,27]. Centella has also been shown to reduce ß-glucuronidase activity, which reflects a decrease in gingival inflammation [28] Patients with subgingival implants undergoing treatment with a biodegradable chip containing Centella and Punica granatum demonstrated reduced GI scores at 3 and 6 months [29]. In vitro studies have shown significant anti-bacterial effects with this herb as well [30]. The herbal component Sambucus nigra (elderberry), has been shown to have immuno-modulatory effects on a number of pro-inflammatory cytokines, reducing the pro-inflammatory effects of periodontal bacteria as well as bacterial and viral activity [31,32]. Elderberry reduces cytokine production, neutrophilic oxidative burst and integrin activation resulting from the periodontal pathogens Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans [29]. Elderberry has also been shown to activate NFκB, believed to be responsible for atherosclerotic changes associated with chronic periodontal inflammation [32]. Echinacea purpurea (purple coneflower) is the third PeriActive component, and has been shown to inhibit the pro-inflammatory cytokines interferon-γ and TNF-α [33-36]. The herb has also been shown to reverse stress-delayed wound healing in mice [37]. Echinacae exhibits anti-viral, antibacterial and anti-cytotoxic effects [35,36], and is commonly used for the treatment and prevention of respiratory tract infections. The present study has a number of limitations, the most important of which is the small size of the sample, all of whom were healthy participants. Thus, the findings need to be confirmed in large, randomized controlled studies, and in patients with chronic periodontal inflammation. The study model, in which participants abstain from all dental hygiene other than the use of the study rinses, creates a situation in which there is increased food and material alba buildup, with accelerated plaque formation and gingival inflammation. Also, patient self-reporting may not accurately reflect adherence to treatment, and residual volumes do not take into account factors such as spillage or deviation from protocol. Finally, the individual contribution of each of the botanical components was not examined. Nevertheless, the reduced increase of GI and PI scores, as well as the number of spontaneous bleeding sites with the use of the PeriActive rinse, when compared to the water-only control rinse, indicates that this product may offer a safe and beneficial effect in the treatment of gingival inflammation and plaque formation.
Conclusion
The botanical mouth rinse PeriActive was found to be a safe and beneficial treatment for reducing the increase in gingival inflammation and plaque formation in an experimental gingivitis model in which patients refrained from mechanical cleaning. The effects of the study rinse may reflect anti-bacterial and anti-inflammatory effects of the botanical components of the rinse. Future research is needed to confirm and extend these findings.
Disclosure
Dr Levine is Chief Executive Officer, and Drs. Samuels and Williams have provided consulting services for Izun Pharma, Israel. The study medications were provided free of charge by Izun Pharma, Israel, who funded the statistical analysis of the data as well. PeriActive™ is a product of Izun Pharmaceuticals Corporation, New York, NY.
References
- Timmerman MF, van der Weijden GA (2006) Risk factors for periodontitis. Int J Dent Hyg 4: 2-7.
- Williams RC, Paquette DW (2000) Understanding the pathogenesis of periodontitis: a century of discovery. J IntAcadPeriodontol 2: 59-63.
- Ebersole JL (2003) Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000 31: 135-166.
- Potempa J, Banbula A, Travis J (2000) Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000 24: 153-192.
- Madianos PN, Bobetsis YA, Kinane DF (2005) Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J ClinPeriodontol 32 Suppl 6: 57-71.
- Van Dyke TE (2008) The management of inflammation in periodontal disease. J Periodontol 79: 1601-1608.
- Wilkins EM (2004) Clinical Practice of the Dental Hygienist. (9thEdn), Lippincott Williams & Wilkins, 2004 Philadelphia USA.
- Haps S, Slot DE, Berchier CE, Van der Weijden GA (2008) The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: a systematic review. International Journal of Dental Hygiene 6:290-303.
- Moran JM (2008) Home-use oral hygiene products: mouthrinses. Periodontol 2000 48: 42-53.
- Barnett ML (2003)The role of therapeutic antimicrobial mouthrinses in clinical practice. Control of supragingival plaque and gingivitis. Journal of the American Dental Association 134: 699–701.
- Paraskevas S, van der Weijden GA (2006) A review of the effects of stannous fluoride on gingivitis. J ClinPeriodontol 33: 1-13.
- Ciancio SG, Mather ML, Bunnell HL (1975) Clinical evaluation of a quaternary ammonium-containing mouthrinse. J Periodontol 46: 397-401.
- Bonesvoll P, Gjermo P (1978)A comparison between chlorhexidine and some quaternary ammonium compounds with regard to retention, salivary concentration and plaque-inhibiting effect in the human mouth after mouth rinses. Archives of Oral Biology 23: 289–294.
- Ashley FP, Skinner A, Jackson PY, Wilson RF (1984) Effect of a 0.1% cetylpyridinium chloride mouthrinse on the accumulation and biochemical composition of dental plaque in young adults. Caries Res 18: 465-471.
- Ashley FP, Skinner A, Jackson P, Woods A, Wilson RF (1984) The effect of a 0.1% cetylpyridinium chloride mouthrinse on plaque and gingivitis in adult subjects. Br Dent J 157: 191-196.
- Penugonda B, Settembrini L, Scherer W, Hittelman E, Strassler H (1994) Alcohol-containing mouthwashes: effect on composite hardness. J Clin Dent 5: 60-62.
- Smigel K (1991) High-alcohol mouthwashes are under scrutiny. J Natl Cancer Inst 83: 751.
- Llewelyn J (1994) Oral squamous cell carcinoma. Mouthwashes may increase risk. BMJ 308: 1508.
- LOE H, THEILADE E, JENSEN SB (1965) EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol 36: 177-187.
- Löe H (1967) The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 38: Suppl:610-616.
- Food and Drug Administration. Gingivitis: Development and Evaluation of Drugs for Treatment or Prevention at https://www.fda.gov/Cder/guidance/ 5146dft.htm#_Toc98216094. [Accessed: June, 2014]
- Shukla A, Rasik AM, Dhawan BN (1999) Asiaticoside-induced elevation of antioxidant levels in healing wounds. Phytother Res 13: 50-54.
- Cesarone MR, Incandela L, De Sanctis MT (2001) Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centellaasiatica: a clinical prospective randomized trial with a microcirculatory model. Angiology 52(suppl 2):S49-S54.
- Punturee K, Wild CP, Kasinrerk W, Vinitketkumnuen U (2005) Immunomodulatory activities of Centellaasiatica and Rhinacanthusnasutus extracts. Asian Pac J Cancer Prev 6: 396-400.
- Lu L, Ying K, Wei S, Fang Y, Liu Y, et al. (2004) Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int J Dermatol 43: 801-807.
- Arpaia MR,Ferrone R, Amitrano M (1990)Effects of Centellaasiatica extract on mucopolysaccharide metabolism in subjects with varicose veins. International Journal of Clinical Pharmacology Research 10:229-233.
- Shetty BS, Udupa SL, Udupa AL, Somayaji SN (2006) Effect of Centellaasiatica L (Umbelliferae) on normal and dexamethasone-suppressed wound healing in Wistar Albino rats. Int J Low Extrem Wounds 5: 137-143.
- Sastravaha G, Gassmann G, Sangtherapitikul P, Grimm WD (2005) Adjunctive periodontal treatment with Centellaasiatica and Punicagranatum extracts in supportive periodontal therapy. J IntAcadPeriodontol 7: 70-79.
- Zaidan MR, Noor Rain A, BadrulAR, Adlin A, Norazah A (2005) In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine 2:165-70.
- Harokopakis E, Albzreh MH, Haase EM, Scannapieco FA, Hajishengallis G (2006) Inhibition of proinflammatory activities of major periodontal pathogens by aqueous extracts from elder flower (Sambucusnigra). J Periodontol 77: 271-279.
- Hajishengallis G, Sharma A, Russell MW, Genco RJ (2002) Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol 7: 72-78.
- Krawitz C, Mraheil MA, Stein M, Imirzalioglu C, Domann E, et al. (2011) Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complementary and Alternative Medicine 11:16.
- Mishima S, Saito K, Maruyama H, Inoue M, Yamashita T, et al. (2004) Antioxidant and immuno-enhancing effects of Echinacea purpurea. Biol Pharm Bull 27: 1004-1009.
- Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA (2006) Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. IntImmunopharmacol 6: 1214-1221.
- Woelkart K, Marth E, Suter A (2006) Bioavailability and pharmacokinetics of Echinacea purpurea preparations and their interaction with the immune system. International Journal of Clinical Pharmacology Therapeutics 44:401-408.
- Sharma SM, Anderson M, Schoop SR, Hudson JB (2010) Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce): dual actions against respiratory bacteria. Phytomedicine17:563-568.
- Zhai Z, Haney DM, Wu L, Solco AK, Murphy PA, et al. (2009) Alcohol extract of Echinacea pallida reverses stress-delayed wound healing in mice. Phytomedicine 16: 669-678.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 18560
- [From(publication date):
September-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 13651
- PDF downloads : 4909