Research Article Open Access
Eggmass Structure and Parasitism of Thaumetopoea wilkinsoni (Lepidoptera: Thaumetopoeidae) in Lebanon
| 1FAO, Viale delle Terme di Caracalla, 00154 Rome, Italy Jenny Nasr1, 2Department of Agricultural Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, P.O. Box 11-0236, Riad El Solh 1107 2020, Lebanon Efat Abou-Fakhr Hammad2, 3Le Rieufroid, F. 84340, Malaucène, France Guy Demolin3 and 2Department of Agricultural Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, P.O. Box 11-0236, Riad El Solh 1107 2020, Lebanon 4Department of Agriocultural Sciences, Faculty of Agricultural and Food Sciences, Holy Spirit University of Kaslik, P.O.Box 110236, jounieh, Lebnaon Nabil Nemer2,4* |
||
| Corresponding Author : | Nabil Nemer Department of Agricultural Sciences Faculty of Agricultural and Food Sciences Holy Spirit University of Kaslik P.O.Box 110236, jounieh, Lebnaon E-mail: nabil.nemer@usek.edu.lb |
|
| Received January 31, 2013; Accepted August 24, 2013; Published August 27, 2013 | ||
| Citation: Nasr J, Hammad EAF, Demolin G, Nemer N (2013) Eggmass Structure and Parasitism of Thaumetopoea wilkinsoni (Lepidoptera: Thaumetopoeidae) in Lebanon. Adv Crop Sci Tech 1:110. doi: 10.4172/2329-8863.1000110 | ||
| Copyright: © 2013 NASR J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
The pine processionary moth, Thaumetopoea wilkinsoni, is the most damaging pest of pine trees in Lebanon and other countries of the Mediterranean region. It spreads in Lebanon on Pinus halepensis, P. brutia and P. canariensis. The study assesses the importance of egg parasitoids of the pine processionery moth. The egg masses collected from two locations of different altitudes were placed in the environmental chamber at a constant temperature of 22 ± 2.5°C. Emergence of the parasitoids was recorded daily in the laboratory and the peak of emergence was determined for each parasitoid species. The most widely spread species in Lebanon were identified as Baryscapus servadeii and Ooencyrtus pityocampa. A significant correlation was determined between length of the egg mass and the number of eggs/egg mass for a given row number. The impact of egg parasitoids varied from 10-16%. The followup on parasitoid emergence showed different peak emergences for the two major egg parasitoids, O. pityocampae and B. servadeii and explained the coincidence between egg deposition by T. wilkinsoni and emergence of the adult parasitoids.
| Keywords | |
| Pine processionery moth; Baryscapus servadeii; Ooencyrtus pityocampa; Egg parasitoid | |
| Introduction | |
| The pine processionary moths (PPM), Thaumetopoea wilkinsoni Tams and Thaumetopoea pityocampa Denis & Schiffermuller (Lepidoptera: Thaumetopoeidae) are the most important enemy of pine trees in the Mediterranean basin attacking different species of pine and cedar. The thermal requirement of the insect allows its spread in the Mediterranean basin where the annual insulation period is superior to 1800 hours and the mean minimum temperatures in January are superior to – 4°C [1]. T. wilkinsoni known as the pine processionary moth of Cyprus was first found in Cyprus, described by [2] as a new species, and investigated by Wilkinson in 1926 [3]. T. wilkinsoni was also reported to exist in Lebanon, Syria and southwest Turkey. The PPMs T. wilkinsoni and T. pityocampa are called winter processionary caterpillars because they undergo their development at the end of summer till the beginning of spring and use the solar energy radiation for their winter nests [4]. These species have the same pheromone (11-yne-13Z- hexadecene acetate), the same climatic requirements [1,5] and can be crossed with each other [4]. In a schematic way, T. pityocampa corresponds to the geographic races existing in Mediterranean Europe and North Africa and T. wilkinsoni corresponds to those living in the oriental part of the Mediterranean basin and part of Turkey. However, recent molecular studies indicate the existence of strong genetic differentiation between the two species that became separated before the quaternary ice ages [6-8]. | |
| In the 1960s, it was estimated that 10,000 ha of pine forests were infested by this pest in Lebanon whereas in Syria 35,000 ha were attacked [9]. Lately, we witnessed a serious ecological imbalance that has favored the spread of the PPM. | |
| The insect biological cycle extends one or more years and is divided into two phases taking place at two different levels of the forest ecosystem. The first aerial level occurs on the pine tree as the egg stage and the five larval instars; the other underground level as prepupa, pupa, and adult. Adult emergence takes place from end of June till beginning of August depending on altitude and latitude [1]. | |
| As for most of defoliating Lepidoptera, the eggs of PPM, are subject to the action of numerous natural enemies more particularly that of parasitoids that have a significant incidence in the control of host populations [10,11]. The highest incidence of egg parasitoids was found in egg masses on Pinus halepensis Miller followed by those on Pinus pinaster Aïton and in decreasing order on Pinus pinea Linnaeus and on Pinus insignis D.Don which are as the pine species least preferred by egg parasitoids [12]. | |
| The main objective of the study is to identify the parasitoids of the PPM in Lebanon and explore the various criteria concerning egg masses and parasitoid emergence. The study also aims to explain the coincidence of egg parasitoid emergence with the presence of T. wilkinsoni egg masses in the field. | |
| Materials and Methods | |
| Field collection and measurement of PPM egg masses morphological characteristics | |
| Scheduling for egg mass collection was initiated by monitoring for emergence of adult moths. Monitoring of male adult moths emergence by pheromones (INRA, Versailles) was performed in two locations: Qnat (North Lebanon, alt.: 1175m; lat. North: 34°15’, long. East 3553’) and Jeita (alt.: 310m; lat. N: 33°57’; long. E: 35°37’). Traps were placed during mid-June in Qnat, while at Jeita they were placed in mid-July; capsules were changed once every month. The traps were located in open areas of Pinus brutia Tenore forests or areas overlooking the road or on solitary trees. Traps with three trapped males were removed from Qnat and Jeita on July 20 and September 4, 1999, respectively, in order to allow for egg laying. Collection of egg masses started in Qnat and in Jeita in August and October 1999, respectively. The egg mass collection continued till February 2000. During the first month of egg sampling following the male emergence, egg masses were collected weekly and only once per month afterwards. The total number of egg masses collected is 146 egg masses from Jeita and 61 egg masses from Qnat. | |
| The egg masses collected from the two locations were placed in the environmental chamber in the Entomology laboratory at the American University of Beirut, at a temperature of 22 ± 2.5ºC with an adjusted photoperiod of 12L: 12D. The dates of egg hatching were recorded and graphed in relation to the date of the first males emerging in the field. | |
| The eggs were then conditioned for parasitoid emergence. The scales covering the egg masses were removed. Various criteria related to the egg masses were assessed. The length, number of eggs and number of rows per egg mass were determined by counting them on 61 and 146 egg masses collected from Qnat and Jeita respectively. Frequency of number of eggs per egg mass was also determined for the two locations. | |
| Identification, emergence and abundance of the egg parasitoids of the PPM | |
| The randomly collected egg masses in Jeita and Qnat were used to identify species of egg parasitoids of the PPM and to determine percentage parasitism. Egg masses were collected every week from the two locations even after egg hatching. Four egg masses were collected from Qnat region in May 1999 to evaluate the presence of overwintering parasitoids. Emerging parasitoids from collected egg masses were identified to the family level. Further identification to the species level was performed by sending random specimens to the Natural History Museum in London. | |
| The emerging parasitoids from collected egg masses in the environmental chamber were counted every 48 hours to determine the rate of emergence of each species. The scales on each egg mass were removed during mid-April 2000 by rolling the egg masses smoothly on a sticky tape for counting the parasitized eggs versus hatching eggs of the PPM. Recognition of the two types of eggs depended on diameter of the egg opening; the parasitized eggs had a smaller diameter compared to normally hatching eggs. These openings may be seen by the naked eye or with the help of a magnifying lens. Relative abundance of each parasitoid species was determined by dividing the number of adults of a given species by the total number of emerging parasitoids per egg mass from the sampling date of egg masses till April 17, 2000. | |
| Statistical analysis | |
| Correlation analysis was used to determine the relationship between egg number and length of egg mass for a given number of rows. The correlation coefficient was determined along with the regression equation and the regression coefficient [13]. | |
| Results | |
| Characteristics of the egg masses of the PPM | |
| The collected egg masses were found to be deposited on two needles of P. Brutia and P. halepensis in the form of a cylindrical mass. Egg masses deposited on three or four needles amounted to only three out of the total number of egg masses (207) collected in the two locations. During our sampling in the two locations, egg masses have been observed to remain more than one year on the tree host after eclosion. | |
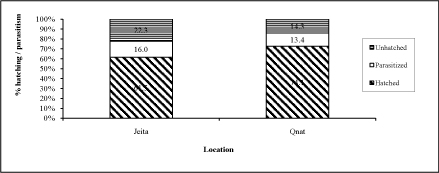
| Eggs collected from Qnat and Jeita started hatching in the laboratory on Sept. 19 and Oct. 31, respectively. The average percent hatchability into first instars per egg mass was found to range between 61.5 and 74.1% in the two locations (Figure 1). However, there were 14.3-22.3% of unhatched eggs, that could be dead due to natural mortality or they could have late emerging parasitoids. | |
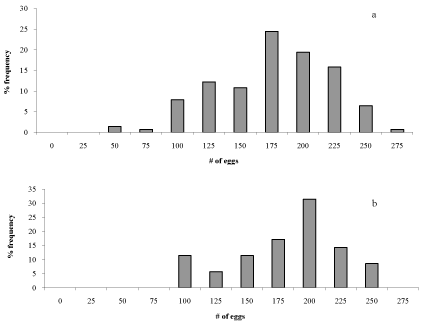
| The period between male emergence and egg hatching observed in the laboratory was on the average 65 and 61 days for Jeita and Qnat respectively. The distribution frequency of the number of eggs in egg masses was determined for the two locations (Figure 2). The highest frequency for the number of eggs in egg masses was found to be between 175 and 200 eggs per egg mass in both Qnat and Jeita. The average number of eggs per egg mass was 188 and 209 eggs for Jeita and Qnat, respectively. There was significant correlation between the number of eggs/egg mass and the length of the egg mass. The number of rows observed was comprised of 6-8 rows for egg masses deposited on pine needles (Table 1). | |
| Emergence and abundance and of the egg parasitoids | |
| Emergence of parasitoids from collected egg masses took place from May 1999 till April 2000. They were recognized by the small openings versus large openings in eggs that contained hatched larvae of the PPM. Three species of hymenopterous parasitoids were identified as: Baryscapus servadeii Graham (Hym., Eulophidae), Ooencyrtus pityocampae Mercet (Hym., Encyrtidae) and Anastatus bifasciatus Geoffroy (Hym., Eupelmidae) by the Natural History Museum in London. The total number of parasitoids that emerged till April 17, 2000 was 527 and 1710 from Qnat and Jeita, respectively. Among the adult parasitoids that hatched from the egg masses collected from Qnat and Jeita starting May 1999 till April 2000, the most abundant species was B. servadeii followed by O. pityocampae (Table 2). | |
| The parasitoids exploited a large number of the collected egg masses (Table 3). O. pityocampae was comparable in its ability to exploit egg masses by itself as B. servadeii in Qnat. However, in Jeita, the two species shared a higher number of egg masses. | |
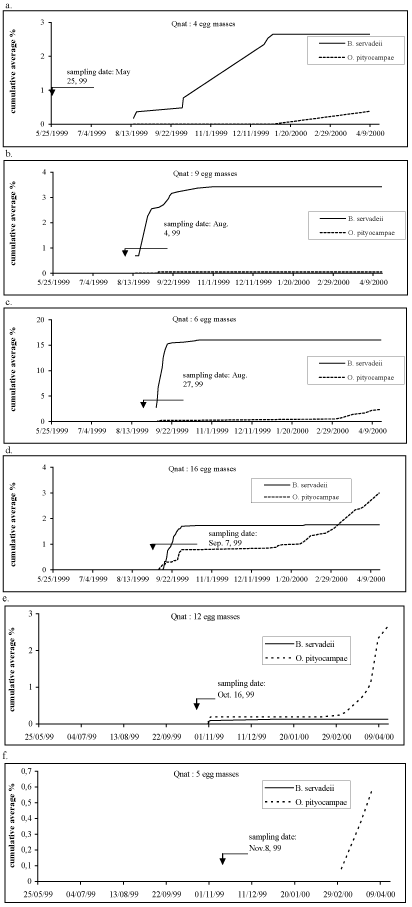
| In Qnat, parasitoid emergence occurred before and after egg hatching of the PPM during August and September, 1999 till April 17, 2000 (Figure 3). Egg masses collected in May 1999 and coming from hatched egg masses of the previous year revealed the presence of overwintering B. servadeii parasitoid (Figure 3a). O. pityocampa showed a consistency in its emergence pattern with the exception of one sampling date (August 4, 1999, Figure 3b) where no emergence was detected. | |
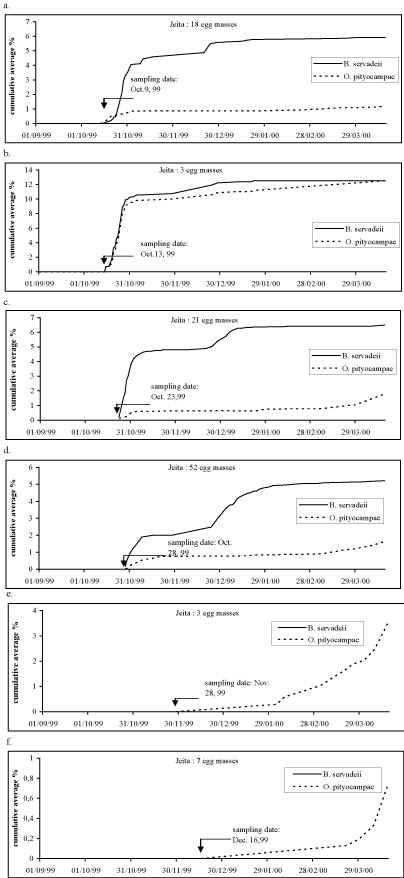
| For samples collected in October from Jeita, the parasitoids readily emerged and they coincided with egg hatching period of Thaumetopoea (Figure 4). However, it is observed that a greater number of B. servadeii emerged than O. pityocampa. | |
| Discussion | |
| The egg batches of the two sampling sites studies were collected at random and they were similarly arranged. The number of eggs was comparable for the two locations as none of the pine in the locations was subject to any treatment and as the weather conditions were mild during the previous years. The mean number of eggs per batch was slightly lower in Jeita than Qnat and more similar to the numbers found for T. pityocampa in SW Turkey [11]. However lower number of eggs per batch were found for the PPM in Israel [14]. The coefficients of correlation between the number of eggs per egg mass and the length of the egg mass were found to be highly significant for a given row (Table 1) which is a typical characteristic of the PPM in other countries [15,16]. Although the parasitoids exploited a large number of egg masses, their impact remain average or below average. Many reasons explain this behavior of the parasitoids. The first reason being linked to the constraints as wind influence and eventual predators that intervene during oviposition [17]. The reproductive success of parasitoids depends on a broad variety of abiotic and biotic mortality risks occurring during their lifetime. Adult parasitoids foraging for suitable hosts are subject to low temperatures, wind and rain [18]. Moreover, adult parasitoids are also attacked by polyphagous predators such as spiders and other generalist predators. This type of interference between species which belong to the same guild is referred to as intraguild predation [19]. | |
| The second reason is represented by the fact that the females may not have enough eggs in their ovarioles for complete exploitation of the eggs. In case of O. pityocampae, oogenesis is not complete at emergence [20]. O. pityocampae is more abundant in the basal and apical parts of the egg mass [21]. This choice could be due to the absence or partial presence of scales on both extremities of the egg mass [22]. Biliotti (1958) observed in the laboratory that O. pityocampae chooses eggs of the PPM that have their scales removed. On the other hand, B. servadeii has a more diffuse distribution but lays its eggs equally on both extremities of the host egg mass [23]. The latter can lay its eggs no matter the position of the host egg in the egg mass because of the adaptive advantage of its elongated abdomen, allowing it to enter easily through the protective barriers of the scales. | |
| In our study unhatched eggs were considered as natural or physiological mortality which is caused by many factors. Temperatures less than 12°C and greater than 28°C are able to induce egg mortality at the embryo stage. Maximal temperatures exceeding 30°C can perturb parasitoid activity [24]. Observations of Tsankov et al. [25] showed that O. pityocampa cannot be active except between 20- 30°C whereas B. servadeii is more tolerant to high temperatures. One of the consequences to the constraints related to the parasitoids’ effectiveness is the desynchronization between them and their host, and the reduction in parasitic efficacy. The latter concerns especially B. servadeii, for which host finding depends on the aggregation intensity of female individuals [20]. The highest percentage of natural egg mortality is recorded reaching peaks of 40% or even 45% [25]. Mortality varies depending on location characteristics from one year to the other. Tiberi (1978) in Italy registered 61.72% mortality [26]. Other results exceed 50% as in Spain [27]. In Morocco, they are comprised between 27.6 and 54.7% [28]. In our study, the ovolarval mortality of the PPM in Lebanon appeared lower compared to that recorded in most countries indicating that the climatic factors in Lebanon are more suitable for the development of the eggs than other countries where extremes in temperature are often found. | |
| The variations in adult emergence of B. servadeii and O. pityocampae from the egg masses collected at the different locations may be due to sampling dates of the egg masses and the conditioning of the egg masses at 22 ± 2.5°C. Conditioning the collected egg masses at 20-22°C nd a natural photoperiod allows suspending the stage of development acquired by the parasitoids in nature [24]. In the present study, during egg sampling just after and before egg lying of the PPM, a high activity of the parasitoids in nature was noticed in the two locations. For the egg masses reared in the laboratory, it appeared that ceasing of diapause was earlier and development was faster than for the egg masses in nature. | |
| Zamoum [24] presented similar results for an Algerian location, Djelfa (alt. 1144 m) that has a similar altitude as Qnat. A very small proportion of individuals at Djelfa adopted a strict diapause and emerged from the egg masses during the presence of the PPM egg masses in August as was the case in Qnat with the sampling date May 25, 1999 (Figure 3a). The emergence of B. servadeii took place in August at the same time of egg mass presence of T. wilkinsoni (Figures 3b and 3c). Thus, the further one moves in time away from Thaumetopoea hatching, the further is the date for parasitoid emergence in the egg mass. This also indicated that parasitoids that do not emerge from the eggs the same year may adopt a longer diapause cycle to coincide with the egg laying period of the prey. However, the parasitoid emergence was found to start in November for egg masses sampled in October and in February for egg masses sampled in November (Figures 3e and 3f). It is noticed that the peak emergence for Baryscapus was reached in December 1999. However, the parasitoids will continue to emerge one year after their sampling date [24]. Thus, one complete year after egg collection is needed to specify the exact peak for their emergence. Sampling dates in September yielded both parasitoids promptly; this activity was comparable to that in nature at that time (Figure 3d). Under the effect of a constant temperature of 20-22°C, the population proportion of B. servadeii that emerged in spring, for lots sampled in August and October, can be explained by the fact that it develops in possibility to alternate from one form to the other under the conjugated effects of the location factors and those in relation to temperatures. In nature, the observed delay in emergence of B. servadeii with respect to the egg mass deposition of Thaumetopoea are due to the presence of daily temperatures that exceed 30°C for many consecutive days from end of June till mid August [24]. | |
| The early emergence of O. pityocampae in spring i.e. in April observed in this study, was also reported in Algeria which may explain the polyphagous character of this parasitoid [25]. However, it was noticed that the emergence of this parasitoid at the end of summer and the beginning of autumn gave very active adults on T. pityocampa eggs in different pine forests in Italy [12]. | |
| Unlike Qnat, egg masses from Jeita sampled in November and December will readily hatch after sampling and released O. pityocampae because it seems they would not have entered diapause in nature due to low altitude and mild temperatures in the beginning of winter (Figures 4e and 4f). The peak emergence of O. pityocampae occurred in October 1999 and is likely to occur in May and June in nature especially that an increase in number was detected in April 2000 under laboratory conditions. The fiure 4b showed an emergence pattern which is not consistent with the others sampling dates and is probably due to the low number of egg masses sampled for this date. | |
| The delay observed in the exit rhythms of B. servadeii with respect to the peak of egg laying for T. pityocampa is perhaps due to the presence of daily temperatures exceeding 30°C for many consecutive days beginning end of June till mid-August. According to [29] temperatures exceeding 28°C reduce the development process of the host and the parasitoids. It is equally probable that the absence of a perfect coincidence between the presence of the egg masses of T. pityocampa and the adults of B. servadeii is due to the fact that the effect of temperatures is different for parasitoids developing on the tree than for pupae developing underground. | |
| In conclusion, egg parasitoids were present in both sites in this study at a rate ranging from 10-16%. The work presented also reports for the first time the presence on the three egg parasitoids: B. servadeii, O. pityocampae and A. bifasciatus in Lebanon. The follow-up on parasitoid emergence showed different peak emergences for the two major egg parasitoids, O. pityocampae and B. servadeii. Thus, it is important to understand the coincidence of egg deposition by the T. wilkinsoni and emergence of the adult parasitoids. Egg parasitoids may undergo a one year diapause and emerge at the time of egg lying by the PPM. The prevalence of one species over another is dependent on many factors such as the age of the pine forest, the location (altitude/latitude) and the development of the host population which need to be studied. | |
| Acknowledgements | |
| This study was supported by a grant from the University Research Board, American University of Beirut. Our special thanks to Hala Dakhil, Houry Zournajian and Michel Bassil for their assistance in field and laboratory work | |
| References | |
References
|
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
|||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 14903
- [From(publication date):
September-2013 - Aug 28, 2025] - Breakdown by view type
- HTML page views : 10238
- PDF downloads : 4665
