Healthcare Resource Utilization and Cost Related to Nosocomial Pneumonia Caused by Staphylococcus aureus and Pseudomonas aeruginosa in France: A 2010-2011 Population-Based Cohort Study Using a National Claims Database
Received: 04-Aug-2017 / Accepted Date: 10-Aug-2017 / Published Date: 20-Aug-2017 DOI: 10.4172/2161-1165.1000318
Abstract
Background: Nosocomial pneumonia is a leading cause of hospital-acquired infection. However, evidence is limited regarding resource utilization and healthcare costs associated with nosocomial pneumonia in France.
Methods: This retrospective case-control study used a nationwide hospital claims database for cases of nosocomial pneumonia caused by P. aeruginosa or Pseudomonas aeruginosa. Hospital stay costs were retrieved during index hospitalization and at 30-days and 90-days post-discharge. Cost was calculated using Diagnosis Related Group (DRG) codes and daily cost estimates.
Results: Of 7,793 patients discharged with S. aureus or P. aeruginosa pneumonia between January 2010 and December 2011, 1,453 and 1,449 cases were included, respectively. Cases were matched with controls in terms of DRG root, age, gender, Charlson comorbidity score, and hospital region. Cases demonstrated significantly higher Charlson comorbidity scores (p<0.01) and almost four times higher mean index hospitalization duration (p<0.001), critical care unit stays (p<0.001), mechanical ventilation procedures (p<0.001), and mortality (p<0.001) compared to matched controls. Univariate analysis indicated significantly higher cost of treating cases compared to controls in terms of DRG (S. aureus cohort: €21,540 vs. €6,426; P. aeruginosa cohort: €20,732 vs. €6,172; p<0.001) and daily valuation costs (S. aureus cohort: €28,063 vs. €5,976; P. aeruginosa cohort: €30,827 vs. €5,819; p<0.001). Multivariate analysis showed that nosocomial pneumonia increased mean DRG costs at 90 days by €13,500 to €16,700 for S. aureus cohort, and by €13,300 to €20,200 for P. aeruginosa cohort.
Conclusions: Nosocomial pneumonia due to S. aureus or P. aeruginosa was associated with considerable patient morbidity and hospital costs in France.
Keywords: Pneumonia; Nosocomial infection; P. aeruginosa; Pseudomonas aeruginos osts; mortality
165844Background
Nosocomial infections are an increasing cause of concern worldwide, responsible for substantial morbidity, mortality, and expense. In 2008, the average healthcare cost for nosocomial infections in Europe was estimated by the European Commission to be €5.48 billion per year [1,2]. A single-center French cohort study in 2008 estimated the additional direct medical costs associated with nosocomial infections to be €3.2 million per year.
Prolonged hospitalization alone accounted for 69% of the total charges [3]. The importance of nosocomial infections to public health and healthcare costs, as well as the increasing concern around the growth of antibiotic resistance, has led to a number of prevention efforts and recommendations [4].
Nosocomial pneumonia is the leading cause of mortality due to hospital-acquired infections, with mortality rates reported to be as high as 30% [5]. The onset of nosocomial pneumonia increases hospital length of stay by an average of 7 day to 10 days, and in the case of ventilator-associated pneumonia, it has been estimated to cost between $10,000 and $40,000 per case [1].
In France, hospital-acquired pneumonia is the second most common nosocomial infection (16.7% of all nosocomial infections in 2012), 44.3% being acquired in critical care unit [2]. The two most frequent causative microorganisms are Pseudomonas aeruginosa (18.1%) and P. aeruginosa (14.7%).
There are limited published data on resource utilization and annual healthcare costs in patients who develop S. aureus or P. aeruginosa nosocomial pneumonia in France. Our objectives were to describe the healthcare resource utilization and costs related to hospital stays and during 90 days of follow-up among patients with or without a record of nosocomial pneumonia caused by S. aureus or P. aeruginosa .
Methods
Data source
This national population-based matched case-control study used data collected from the French hospital claims database–Program of Medicalization of Information Systems (PMSI) [6]. All admissions to French public and private hospitals who are covered by the national insurance are included in the PMSI database, hence the high coverage of the database which is close to 93% of all hospitalizations in France. All hospitals are legally obliged to report activity for collation, otherwise remuneration for the procedures performed is withheld by the local health authority [7]. The PMSI is based on the systematic collection and computerized processing of minimal standardized medical and administrative information, allowing classification of each hospital stay in a homogeneous patient group similar to the Diagnosis Related Group (DRG). Each DRG is associated with a homogeneous hospitalization group and its corresponding cost in Euros, which is used to determine reimbursement by the French Health Insurance [8,9].
The medical information includes the primary diagnosis having led to patient’s admission, related and associated diseases, and medical and surgical procedures performed during hospitalization. The administrative information includes patient’s demographic data (date of birth, gender), medical ward codes representing specific costs, and month of admission and duration of stay. Diagnoses are coded according to International Classification of Diseases, 10th revision (ICD-10), and medical and surgical procedures are coded according to the French Common Classification of Medical/Surgical Procedures (CCAM).
In each French hospital, a physician specialized in coding conducted regular quality control over what was recorded in the database. Data included in the database are for hospitalization only; hence, the 30-day and 90-day post-discharge estimates of care do not include outpatient care costs. Hospitalization data were extracted by Structured Query Language querying from the PMSI database produced by the Technical Information Agency on Hospitalization [6]. The PMSI data are totally anonymized; thus, the study did not require an ethics committee approval.
Cases and controls
We identified all patients with a first hospital stay between 1st January 2010 and 31st December 2011 who had nosocomial pneumonia caused by S. aureus or P. aeruginosa . Patients with multiple hospital stays during the observation period were only included once (i.e. for the first observed stay).
Nosocomial pneumonia was defined using a combination of ICD-10 codes. There is no single ICD-10 diagnosis code that refers to nosocomial infections. We considered any combination of a diagnosis code specifying pneumonia and infection by the bacteria of interest with the ICD-10 supplemental factor nosocomial condition (code Y95) to be a nosocomial pneumonia of interest. The S. aureus and P. aeruginosa cohorts constituted of patients coded with pneumonia due to staphylococcus (code J15.2) and pseudomonas (code J15.1), respectively, as primary, related, or associated diagnosis.
Patients were excluded if they were less than 18 years of age, had a previous nosocomial condition recorded within 90 days prior to index hospitalization, had both S. aureus and P. aeruginosa infection during or within 90 days after discharge from the index hospitalization, or had a DRG code indicative of a recording error (90Z00Z). Additionally, patients with cystic fibrosis (Codes E84.x and P75) were excluded from the P. aeruginosa cohort.
We matched one control for every case from all patients hospitalized during the same period who did not have nosocomial S. aureus or P. aeruginosa infection diagnoses. Controls were individually matched to cases by DRG root [10], age (± 1 year), gender, Charlson comorbidity score (± 1) [11,12], and hospital region. However, only few of the cases had a match in the PMSI national population-based claims database on all of these criteria. Indeed, the cases and controls are fundamentally different patients, especially in terms of their comorbidities. Including only cases for which we would have find perfect matches in the study would have caused a selection bias due to the exclusion of a large number of cases. Hence, the matching process used an optimized step-by-step algorithm to identify candidates by decreasing order of matching criteria. The algorithm first tried to identify controls who met all of the matching criteria.
If no match was found, then it released a criterion (the least relevant criterion) at each subsequent step until a matching control was found with only one criterion remaining (see Supplementary Table 1).
| Eligible | Case subjects with nosocomial pneumonia caused by | |
|---|---|---|
| Staphylococcus aureus N=1799 | Pseudomonas aeruginosa N=1958 | |
| Not included n (%) | 346 (19.2) | 509 (26.0) |
| Reasons for non-inclusion n (%) | ||
| Nosocomial infection within 90 days before index hospitalization | 19 (1) | 24 (1) |
| Age< 18 years | 72 (4.0) | 53 (2.7) |
| Both infections during or within 90 days after index hospitalization | 235 (13.1) | 414 (21.1) |
| Error in DRG code | 48 (2.7) | 37 (1.9) |
| Cystic fibrosis | NA | 8 (0.4) |
| Included n (%) | 1453 (80.8) | 1449 (74.0) |
DRG = Diagnosis Related Group; NA = Not Applicable
Table 1: Reasons for exclusion of cases from study population.
This approach avoided leaving many cases without any matched control, as would have occurred if matching on all criteria were required. Once a control was identified, his/her record was deleted from the list of potential control patients to avoid reuse of the same control and facilitate handling of potential confounding factors such as age, gender, and initial medical condition.
Data collected
Data collected were age, gender, region of hospitalization, type of hospital, month and year of hospital admission, length of stay, entry mode, diagnoses using ICD-10 codes from 90 days before to 90 days after discharge from index hospitalization, type of ward (including critical care unit or intensive care unit [ICU]), medical and surgical procedures according to the French CCAM classification [9], rehospitalizations, drugs administered from the list of expensive drugs, and all-cause in-hospital mortality. In France, drugs used in hospital can be either included in hospital-stay fees based on DRG tariffs or belong to the list of expensive drugs defined as hospital-only costly drugs that are additionally charged to health insurance.
In the French system, the ICU houses patients of intermediate acuity-between the critical care unit and the general ward. Critical care units generally manage and care for patients at high risk of developing, or already suffering from, multiple organ dysfunctions (vs. failure of a single organ in ICU) and/or under mechanical ventilation and/or neuro-sedated (vs. no intubated/ventilated patients in ICU, except in some cases where the hospital has an exemption, but only for managing such patients over a duration shorter than two days).
Data analysis
The outcomes of interest were the total hospital-related healthcare resource utilization and costs per patient for the index hospitalization and post-discharge periods (30 days or 90 days post-discharge), and total all-cause readmission rate and all-cause in-hospital mortality rate among patients with or without record of nosocomial pneumonia caused by S. aureus or P. aeruginosa . Total healthcare resource utilization cost was defined as the cost of hospitalization plus the hospital cost of post-discharge periods (30 days or 90 days postdischarge). These two costs were first assessed separately and then combined.
The occurrence and number of re-hospitalizations were evaluated and described by the cumulative number of hospital stays in days over the follow-up period of 30 days and 90 days post-discharge from index hospitalization. Hospitalization costs were estimated by two methods: DRG costs valuation and daily costs valuation. In the first method, assumption was made that DRG cost reflects the real cost of hospital stay. The French classification of DRG considers four severity levels for each DRG class, permitting cost estimation by DRG severity levels regardless of the length of stay. Costs related to each DRG can be obtained from officially published source for both public and private hospitals [13]. In the PMSI database, the classification algorithm used to obtain the relevant DRG for each stay takes into account the severity score related to a diagnosis of nosocomial infection. Consequently, the algorithm can lead to a more or less severe and costly DRG, depending on the type of infection.
The second method takes into account the impact of the length of stay on the total cost, using the National Mean Length of Stay. For stays where the National Mean Length of Stay was <2 days, the DRG cost was used; in other situations, the cost was split into costs linked to the duration of stay and fixed costs. Costs linked to the duration of stay included clinical expenses such as expenses for continuous monitoring, critical care, intensive care (including medication cost), and logistics and accommodation fees.
Qualitative variables were described by count and percentage in each category. Missing values were not imputed; missing data are inherent in the data source, an administrative database. Quantitative variables were described by their mean, standard deviation, median, 1st and 3rd quartile, and minimum and maximum.
Quantitative variables were compared using ANOVA (comparison of means) under normality assumption; otherwise Wilcoxon/Mann- Whitney or Kruskal-Wallis test (non-parametric approach) was used. Qualitative variables were also compared using chi-square test or Fisher exact test. All statistical tests were two-sided with a significance level of 5%, and p-values were not corrected for multiplicity.
Multivariate analyses were performed to identify cost drivers in terms of DRG costs and daily valuation costs, cumulatively at 90 days after discharge from index hospitalization. The costs were logtransformed before analysis, after checking the shape of distribution of the log-transformed variable and excluding strong violations of normality. All statistical tests and confidence interval computing were made on the log-transformed variables.
Results were then retransformed to the original unit for interpretation. Potential cost drivers, identified among patients’ demographics, and clinical characteristics and factors present at admission were tested one by one using a mixed general linear model, with cost as dependent variable and hospital as random variable, as the costs of patients in the same hospital may be correlated.
Data within the same hospital (same “finess” code) were considered to be correlated, while group and other covariates were considered as fixed variables in this multilevel analysis to account for potential hospital effects. All variables significant at the 5% level were then tested in a global model; independent predictive factors were selected using a descending stepwise method at the 5% level and included in the final multivariate analysis, allowing for determination of the least squares means of costs with their 95% confidence interval and adjusted p-value of comparison between groups.
Results
Characteristics of cases and controls
From 1st January 2010 to 31st December 2011, 7,793 patients were discharged with a nosocomial infection from S. aureus or P. aeruginosa ; 1,799 had S. aureus pneumonia, 1,958 had P. aeruginosa pneumonia, and 375 had both S. aureus and P. aeruginosa pneumonia (exclusion criterion). Table 1 summarizes the included and excluded flow of nosocomial pneumonia cases, which led to the inclusion of 1,453 and 1,449 nosocomial cases of S. aureus and P. aeruginosa pneumonia, respectively. During the same observation period, 2,902 patients (1,453 controls for the S. aureus cohort and 1,449 controls for the P. aeruginosa cohort) without a nosocomial S. aureus or P. aeruginosa infection but presenting the same characteristics as the nosocomial infection population regarding age (±1 year), gender, DRG root, Charlson comorbidity score (±1), and region of hospital were included.
As shown in Table 2, the majority (approximately 68%) of patients was male among cases and controls in both the analysis cohorts; the median age was similar in the P. aeruginosa cohorts (65 years) and in the S. aureus cohorts (63 years). The mean Charlson comorbidity scores indicated a significantly higher possibility of pre-existing comorbidities among cases compared to the controls for S. aureus (5.5 vs. 5.2; p=0.007) as well as P. aeruginosa cohort (5.9 vs. 5.4; p<0.001). Also, the mean number of hospitalizations in the year prior to index hospitalization was significantly higher among cases compared to controls for both the cohorts (p<0.001).
| Parameters | Staphylococcus aureus analysis cohort | Pseudomonas aeruginosa analysis cohort | |||||
|---|---|---|---|---|---|---|---|
| Case N=1453 | Control N=1453 | p | Case N=1449 | Control N=1449 | P | ||
| Gender | Male n (%) | 990 (68.1) | 991 (68.2) | 0.968* | 995 (68.7) | 998 (68.9) | 0.904* |
| Female n (%) | 463 (31.9) | 462 (31.8) | 454 (31.3) | 451 (31.1) | |||
| Age (years) | Mean (SD) | 61 (17) | 61 (17) | 0.998** | 64 (15) | 64 (15) | 0.943** |
| Median | 63 | 63 | 65 | 65 | |||
| 25; 75 percentile | 51; 75 | 51; 74 | 55; 76 | 55; 76 | |||
| Charlson comorbidity index | Mean (SD) | 5.5 (3.4) | 5.2 (3.4) | 0.007** | 5.9 (3.3) | 5.4 (3.1) | <0.001** |
| Median | 5 | 5 | 6 | 5 | |||
| 25; 75 percentile | 3.0; 8.0 | 3.0; 7.0 | 4.0; 8.0 | 3.0; 7.0 | |||
| Number of previous hospitalizations in past year | Mean (SD) | 2.71 (7.80) | 1.15 (4.95) | <0.001** | 2.90 (6.86) | 1.51 (7.41) | <0.001** |
| Median | 1 | 0 | 1 | 0 | |||
| 25; 75 percentile | 0.00; 2.00 | 0.00; 1.00 | 0.00; 3.00 | 0.00; 1.00 | |||
| Time (months) from first previous hospitalization within the pre-index period to index hospitalization | Mean (SD) | 3.98 (4.35) | 2.27 (4.02) | <0.001** | 4.40 (4.37) | 2.39 (4.16) | <0.001** |
| Median | 2 | 0 | 3.02 | 0 | |||
| 25; 75 percentile | 0.00; 7.98 | 0.00; 3.02 | 0.00; 8.05 | 0.00; 3.02 | |||
SD = Standard Deviation; No missing data; *=Chi2 Test; **=ANOVA
Table 2: Characteristics of populations.
The most common primary reasons for the index hospitalization were respiratory, circulatory, and injury (see Supplementary Table 2).
Patients with nosocomial infection caused by P. aeruginosa were numerically more likely than those with S. aureus to have been admitted for respiratory system disease (34.0% vs. 27.0%), while the admissions due to circulatory system diseases (18.8% vs. 13.5%) and injuries (17.7% vs. 8.9%) were numerically higher in the S. aureus cases compared to the P. aeruginosa cases.
Regardless of the bacterial species, patients with nosocomial pneumonia were significantly more likely to have been admitted for neoplasm (S. aureus cohort: p<0.001; P. aeruginosa cohort: p=0.004) or abnormal clinical and laboratory findings (S. aureus cohort: p=0.001; P. aeruginosa cohort: p<0.001) as compared to the matched controls.
Individual comorbidities used to determine Charlson comorbidity score are summarized in (Supplementary Table 3).
| Parameters | Staphylococcus aureus analysis cohort | Pseudomonas aeruginosa analysis cohort | |||||
|---|---|---|---|---|---|---|---|
| Case N=1453 | Control N=1453 | p* | Case N=1449 | Control N=1449 | p* | ||
| Type of hospital - n (%) | Public | ||||||
| University hospital | 407 (28.0) | 304 (20.9) | <0.001 | 313 (21.6) | 226 (15.6) | <0.001 | |
| Regional hospital |
204 (14.0) | 207 (14.2) | 285 (19.7) | 223 (15.4) | |||
| Other public hospital | 778 (53.5) | 745 (51.3) | 769 (53.1) | 765 (52.8) | |||
| Private | 64 (4.4) | 197 (13.6) | 82 (5.7) | 235 (16.2) | |||
| Entry mode - n (%) | Hospital transfer | 272 (18.7) | 105 (7.2) | <0.001 | 254 (17.5) | 87 (6.0) | <0.001 |
| Ward transfer | 14 (1.0) | 8 (0.6) | 17 (1.2) | 5 (0.3) | |||
| Home | 1167 (80.3) | 1340 (92.2) | 1178 (81.3) | 1357 (93.7) | |||
| Stay in intensive care unit - n (%) | 215 (14.8) | 161 (11.1) | 0.003 | 154 (10.6) | 127 (8.8) | 0.09 | |
| Stay in critical care unit - n (%) | 1289 (88.7) | 354 (24.4) | <0.001 | 1320 (91.1) | 344 (23.7) | <0.001 | |
| Duration of index hospitalization | Mean (SD) days | 46.79 (37.35) | 12.86 (17.80) | <0.001 | 53.06 (44.18) | 12.85 (16.14) | <0.001 |
| Median | 38 | 8 | 44 | 8 | |||
| 25; 75 percentile | 22.00; 59.00 | 4.00; 15.00 | 25.00; 68.00 | 4.00; 16.00 | |||
| Re-admissions within 30 days after discharge | n (%) | 292 (20.1) | 312 (21.5) | 262 (18.1) | 328 (22.6) | ||
| Cumulative length of stay 30 days after discharge | Mean (SD) days | 49.69 (38.07) | 14.79 (18.75) | <0.001 | 55.61 (44.75) | 14.62 (17.18) | <0.001 |
| Median | 41 | 10 | 46 | 9 | |||
| 25; 75 percentile | 25.00; 63.00 | 4.00; 19.00 | 28.00; 71.00 | 5.00; 18.00 | |||
| Re-admissions within 90 days after discharge | n (%) | 465 (32.2) | 491 (33.8) | 415 (28.6) | 534 (36.9) | ||
| Cumulative length of stay 90 days after discharge | Mean (SD) days | 53.80 (42.01) | 17.68 (22.23) | <0.001 | 59.14 (47.00) | 18.02 (21.32) | <0.001 |
| Median | 42 | 11 | 48 | 11 | |||
| 25; 75 percentile | 26.00; 69.00 | 5.00; 23.00 | 29.00; 76.00 | 5.00; 22.00 | |||
SD=Standard Deviation; No missing data; *=Chi2 or Fisher exact test; **=ANOVA
Table 3: Hospitalization characteristics.
Regardless of the bacterial species, a significantly higher occurrence of congestive heart failure, chronic pulmonary disease, cerebrovascular disease, hemiplegia/paraplegia, renal disease, and peptic ulcer disease was observed in the nosocomial pneumonia cohorts compared to the matched controls (p<0.01).
Furthermore, the occurrence of mild liver disease and moderate-tosevere liver disease was significantly higher in S. aureus cases compared to the matched controls (p<0.05), while a significantly higher occurrence of peripheral vascular disease was reported P. aeruginosa cases compared to the matched controls (p=0.021).
Interestingly, the occurrence of malignancies and metastatic solid tumors was significantly lower among S. aureus cases compared to the matched controls (p<0.05).
Characteristics of hospital stays
The hospital stay characteristics summarized in Table 3 indicate that more than half of the hospitalizations were reported in public hospitals (including university hospitals, regional hospitals, and any other public hospital) for cases and controls in both the analysis cohorts.
A majority of the patients were admitted from home among cases (S. aureus cohort: 80.3%; P. aeruginosa cohort: 81.3%) as well as controls (S. aureus cohort: 92.2%; P. aeruginosa cohort: 93.7%).
The number of critical care unit stays during index hospitalization was significantly higher for cases than controls in the S. aureus cohort (88.7% vs. 24.4%; p<0.001) as well as the P. aeruginosa cohort (91.1% vs. 23.7%; p<0.001).
The number of stays in ICU was also significantly higher among cases than controls in the S. aureus cohort (14.8% vs. 11.1%; p=0.003); however, the P. aeruginosa cohort reported only numerically higher number of ICU stays among cases compared to controls (10.6% vs. 8.8%; p=0.090).
The mean index hospitalization duration was significantly longer for cases than controls in the S. aureus cohort (46.8 days vs. 12.9 days; p<0.001) and P. aeruginosa cohort (53.1 days vs. 12.9 days; p<0.001). Cumulative length of stay was also significantly longer among cases compared to controls at 30 days (p<0.001) and 90 days (p<0.001) postdischarge from index hospitalization.
When admitted for index hospitalization, cases were more likely to undergo surgery than controls (data not shown), with higher rates of tracheotomy (S. aureus : 22.2% vs. 1.5%; P. aeruginosa : 26.5% vs. 1.5%) and gastrostomy (S. aureus : 7.6% vs. 0.8%; P. aeruginosa : 6.7% vs. 0.9%). When surgery was the primary reason for hospitalization, other cases of surgery were similarly distributed between cases and controls; this is expected because they were part of the DRG root used for matching cases and controls.
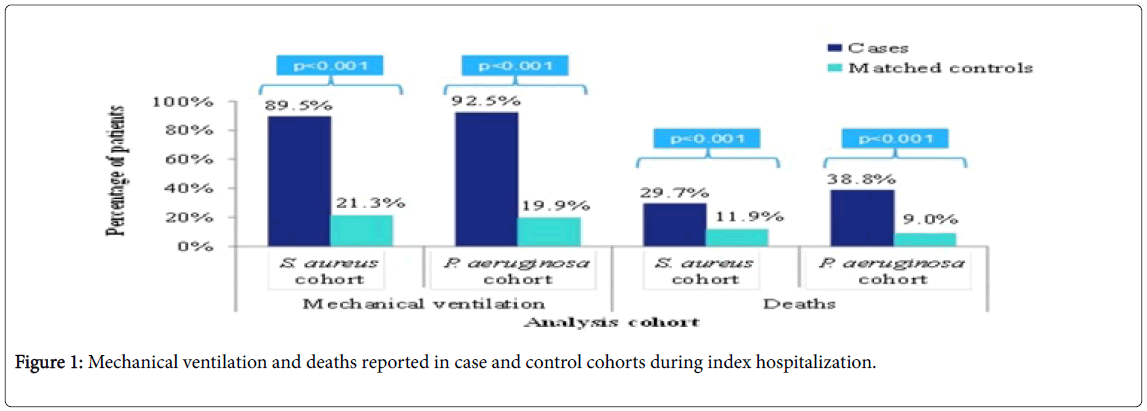
As shown in (Figure 1), mechanical ventilation procedures were significantly more frequent during the index hospitalization for nosocomial pneumonia cases than the matched controls for S. aureus cohort (89.5% vs. 21.3%; p<0.001) as well as P. aeruginosa cohort (92.5% vs. 19.9%; p<0.001). Overall, there were similar number of procedures in cases and controls. However, the type of procedures differed, with up to 99% of cases receiving procedures affecting the respiratory and circulatory systems as compared to 66.6% to 69.7% of controls (Table 4).
| Class of procedure, n (%) | Staphylococcus aureus analysis cohort | Pseudomonas aeruginosa analysis cohort | ||||
|---|---|---|---|---|---|---|
| Cases N=1453 | Controls N=1453 | p* | Cases N=1449 | Controls N=1449 | p* | |
| At least one | 1450 (99.8) | 1378 (94.8) | <0.001 | 1446 (99.8) | 1368 (94.4) | <0.001 |
| Respiratory system | 1440 (99.1) | 996 (68.5) | <0.001 | 1438 (99.2) | 1010 (69.7) | <0.001 |
| Circulatory system | 1407 (96.8) | 967 (66.6) | <0.001 | 1413 (97.5) | 998 (68.9) | <0.001 |
| Adjustments for the transitional French National Classification of surgical procedures | 1215 (83.6) | 902 (62.1) | <0.001 | 1227 (84.7) | 901 (62.2) | <0.001 |
| Digestive system | 1193 (82.1) | 536 (36.9) | <0.001 | 1226 (84.6) | 582 (40.2) | <0.001 |
| Procedures without topographical precision | 979 (67.4) | 363 (25.0) | <0.001 | 961 (66.3) | 343 (23.7) | <0.001 |
| Central, peripheral or autonomic nervous system | 822 (56.6) | 474 (32.6) | <0.001 | 677 (46.7) | 315 (21.7) | <0.001 |
| Additional anesthesia and additional procedures | 728 (50.1) | 354 (24.4) | <0.001 | 745 (51.4) | 333 (23.0) | <0.001 |
| Genitourinary system | 621 (42.7) | 216 (14.9) | <0.001 | 690 (47.6) | 216 (14.9) | <0.001 |
| Immune and hematopoietic systems | 641 (44.1) | 174 (12.0) | <0.001 | 758 (52.3) | 213 (14.7) | <0.001 |
| Lower limb musculoskeletal system | 186 (12.8) | 134 (9.2) | 0.002 | 157 (10.8) | 90 (6.2) | <0.001 |
| Neck and trunk musculoskeletal system | 226 (15.6) | 142 (9.8) | <0.001 | 160 (11.0) | 98 (6.8) | <0.001 |
| Upper limb musculoskeletal system | 156 (10.7) | 80 (5.5) | <0.001 | 102 (7.0) | 43 (3.0) | <0.001 |
| Skin and mammary gland | 93 (6.4) | 66 (4.5) | 0.034 | 91 (6.3) | 54 (3.7) | 0.002 |
| Head musculoskeletal system | 87 (6.0) | 60 (4.1) | 0.027 | 71 (4.9) | 40 (2.8) | 0.004 |
| Musculoskeletal system without topographic details | 41 (2.8) | 22 (1.5) | 0.021 | 34 (2.3) | 17 (1.2) | 0.023 |
| Eye and annexes | 44 (3.0) | 31 (2.1) | 0.16 | 33 (2.3) | 30 (2.1) | 0.799 |
| Metabolism and endocrine system | 10 (0.7) | 12 (0.8) | 0.831 | 13 (0.9) | 15 (1.0) | 0.85 |
| Ear | 8 (0.6) | 5 (0.3) | 0.58 | 10 (0.7) | 5 (0.3) | 0.3 |
| Procedures relative to procreation, pregnancy and the newborn | 4 (0.3) | 1 (<0.1) | 0.375 | 5 (0.3) | 2 (0.1) | 0.453 |
* Chi2 or Fisher exact test
Table 4: Patients with medical acts and procedures during hospitalization.
Approximately one-third of patients in each cohort had a discharge ICD-10 code for bacterial pathogens resistant to antibiotics (S. aureus cohort: 37.2%; P. aeruginosa cohort: 31.5%) (data not shown). The percentage of deaths during index hospitalization was significantly higher in the nosocomial pneumonia cases than in matched controls for S. aureus cohort (29.7% vs. 11.9%; p<0.001) and P. aeruginosa cohort (38.8% vs. 9.0%; p<0.001) (Figure 1). By 90 days of follow-up, an additional 3% of case and control patients had died in both analysis cohorts.
Table 5 summarizes the expensive drugs received by patients during index hospitalization stay; in France, expensive drugs refer to the hospital-only costly drugs that are charged to health insurance in addition to hospital stay fees based on DRG tariffs (“liste T2A”). Significantly more cases received expensive treatments during index hospital stay than controls (S. aureus : 19.1% vs. 5.4%; P. aeruginosa : 25.4% vs. 5.9%; p<0.001 for both comparisons). The use of expensive drugs was significantly higher among cases compared to matched controls for each class of drugs (p<0.01), except for the anti-neoplastic and immunomodulating agents and drugs for musculoskeletal system.
| Class of expensive drug | n (%) | Staphylococcus aureus analysis cohort | Pseudomonas aeruginosa analysis cohort | ||||
|---|---|---|---|---|---|---|---|
| Case N=1453 |
Control N=1453 |
p* | Case N=1449 |
Control N=1449 |
p* | ||
| At least one expensive drug | 277 (19.1) | 79 (5.4) | <0.001 | 368 (25.4) | 86 (5.9) | <0.001 | |
| Anti-infectives for systemic use | All | 132 (9.1) | 22 (1.5) | <0.001 | 242 (16.7) | 25 (1.7) | <0.001 |
| Anti-mycotics for systemic use | 117 (8.1) | 16 (1.1) | <0.001 | 201 (13.9) | 15 (1.0) | <0.001 | |
| Immune sera and immunoglobulins | 23 (1.6) | 7 (0.5) | 0.005 | 49 (3.4) | 14 (1.0) | <0.001 | |
| Blood and blood-forming organs | All | 167 (11.5) | 53 (3.6) | <0.001 | 179 (12.4) | 50 (3.5) | <0.001 |
| Anti-hemorrhagics | 105 (7.2) | 33 (2.3) | <0.001 | 111 (7.7) | 30 (2.1) | <0.001 | |
| Anti-anemic preparations | 48 (3.3) | 21 (1.4) | 0.001 | 65 (4.5) | 17 (1.2) | <0.001 | |
| Antithrombotic agents | 21 (1.4) | 0 (0.0) | <0.001 | 21 (1.4) | 4 (0.3) | 0.001 | |
| Anti-neoplastic and immunomodulating agents | All | 19 (1.3) | 8 (0.6) | 0.051 | 27 (1.9) | 17 (1.2) | 0.171 |
| Musculoskeletal system | All | 3 (0.2) | 6 (0.4) | 0.507 | 8 (0.6) | 3 (0.2) | 0.226 |
No missing data
*Chi2 or Fisher exact test
Table 5: Patients receiving treatments belonging to the list of expensive drugs during index hospital stay.
Costs analysis
Costs associated with nosocomial pneumonia were three times higher than those without a hospital-acquired infection, whether calculated relative to DRGs or to daily costs (Table 6). In the S. aureus cohort, the mean costs of index hospitalization were significantly higher for cases compared to controls in terms of DRG cost (€21,540 vs. €6,426; p<0.001) as well as daily cost (€28,063 vs. €5,976; p<0.001). Similarly, in the P. aeruginosa cohort, significantly higher mean costs were reported for cases compared to matched controls in terms of DRG cost (€20,732 vs. €6,172; p<0.001) and daily cost (€30,827 vs. €5,819; p<0.001). After 30 days and 90 days, the cumulative costs had increased by at least €1,500 for cases and €1,000 for controls; however, the majority of costs in this analysis of hospitalizations were incurred during the index hospitalization.
| Univariate analysis | Staphylococcus aureus analysis cohort | Pseudomonas aeruginosa analysis cohort | |||
|---|---|---|---|---|---|
| Case N=1453 |
Control N=1453 |
Case N=1449 |
Control N=1449 |
||
| DRG cost estimation | |||||
| At hospital discharge | Missing | 3% (n=44) | 1.7% (n=25) | 3.9% (n=56) | 2.9% (n=42) |
| Mean | 21539.5 | 6425.6 | 20731.5 | 6171.7 | |
| 95% CI | 20842.2; 22260.1 | 6117.0; 6749.7 | 20063.0; 21422.3 | 5869.5; 6489.5 | |
| p value | <0.001 | <0.001 | |||
| After 30 days follow-up | Missing | 3.2% (n=47) | 2.1% (n=31) | 4.3% (n=63) | 3.2% (n=47) |
| Mean | 22956.5 | 7320.3 | 22159.1 | 7101.2 | |
| 95% CI | 22212.9; 23724.9 | 6971.8; 7686.3 | 21437.8; 22904.6 | 6752.3; 7468.1 | |
| p value | <0.001 | <0.001 | |||
| After 90 days follow-up | Missing | 3.3% (n=48) | 2.4% (n=35) | 4.4% (n=64) | 3.9% (n=57) |
| Mean | 24417.5 | 8255.9 | 23537.4 | 8190.8 | |
| 95% CI | 23610.8; 25251.7 | 7849.9; 8682.8 | 22756.6; 24345.0 | 7776.8; 8626.8 | |
| p value | <0.001 | <0.001 | |||
| Daily cost estimation | |||||
| At hospital discharge | Missing | 3% (n=44) | 1.7% (n=25) | 3.9% (n=56) | 2.9% (n=42) |
| Mean | 28063.1 | 5975.9 | 30827.2 | 5819.0 | |
| 95% CI | 26962.0; 29209.2 | 5663.8; 6305.2 | 29590.8; 32115.2 | 5508.6; 6146.8 | |
| p value | <0.001 | <0.001 | |||
| After 30 days follow-up | Missing | 3.2% (n=47) | 2.1% (n=31) | 4.3% (n=63) | 3.2% (n=47) |
| Mean | 29746.1 | 6908.5 | 32537.6 | 6760.6 | |
| 95% CI | 28598.8; 30939.3 | 6550.9; 7285.7 | 31251.1; 33877.1 | 6401.4; 7140.0 | |
| p value | <0.001 | <0.001 | |||
| After 90 days follow-up | Missing | 3.3% (n=48) | 2.4% (n=35) | 4.4% (n=64) | 3.9% (n=57) |
| Mean | 31458.2 | 7843.4 | 34275.6 | 7900.8 | |
| 95% CI | 30229.5; 32736.8 | 7422.1; 8288.7 | 32915.0; 35692.5 | 7467.8; 8359.0 | |
| p value | <0.001 | <0.001 | |||
CI = Confidence Interval; DRG = Diagnosis Related Group
Table 6: Univariate analysis for cost estimations (in Euros).
Multivariate analysis of costs
Out of all pre-specified potential cost drivers, nine variables were identified as having an independent influence on DRG costs: gender, cerebrovascular disease, dementia, peptic ulcer disease, diabetes with chronic complication, any malignancy, metastatic solid tumor, age at hospitalization, and Charlson comorbidity index. For daily costs, only six of the variables were identified as having an independent influence, with gender, cerebrovascular disease and malignancy dropped from the model.
Once adjusted with these factors, the costs could be compared between cases and controls to identify the additional costs of nosocomial pneumonia (Table 7). Nosocomial pneumonia increased mean DRG costs at 90 days by €13,500 to €16,700 for S. aureus cohort, and by €13,300 to €20,200 for P. aeruginosa cohort. All these costs were higher when excluding patients who died during the first hospital stay.
| Multivariate analysis | Estimates | P. aeruginosa analysis cohort | Pseudomonas aeruginosa analysis cohort | |||||
|---|---|---|---|---|---|---|---|---|
| Case N=1453 |
Control N=1453 |
Case N=1449 |
Control N=1449 |
|||||
| All patients | ||||||||
| Cumulative DRG cost at 90 days | LS Means | 20813.80 | 7329.14 | 20880.47 | 7593.66 | |||
| 95% CI | 17478.40; 24785.69 | 6196.18; 8669.26 | 17500.10; 24913.80 | 6395.72; 9015.98 | ||||
| Difference | 13484.66 | 13286.81 | ||||||
| Cumulative daily costs at 90 days | LS Means | 22662.67 | 5985.53 | 26766.40 | 6520.08 | |||
| 95% CI | 18612.18; 27594.64 | 4939.59; 7252.95 | 21973.57; 32604.62 | 5374.27; 7910.19 | ||||
| Difference | 16677.14 | 20246.32 | ||||||
| Death during considered hospitalization excluded | ||||||||
| Cumulative DRG costs at 90 days | LS Means | 24391.46 | 7724.32 | 22401.98 | 7108.22 | |||
| 95% CI | 19779.52; 30078.76 | 6317.76; 9444.04 | 17911.74; 28017.86 | 5732.43; 8814.20 | ||||
| Difference | 16667.14 | 15293.76 | ||||||
| Cumulative daily costs at 90 days | LS Means | 27023.29 | 6201.67 | 26756.56 | 5501.58 | |||
| 95% CI | 21435.47; 34067.73 | 4950.60; 7768.89 | 21057.57; 33997.93 | 4362.82; 6937.55 | ||||
| Difference | 20821.62 | 21254.92 | ||||||
CI = Confidence Interval; DRG = Diagnosis Related Group; LS = Least Squares
Table 7: Multivariate final analysis of costs estimation (in Euros).
Discussion
This population-based study is the first to our knowledge to describe the healthcare utilization of adult subjects with nosocomial pneumonia due to S. aureus or P. aeruginosa in France. The PMSI was ideal as a national hospital claims database for this study, with a very high coverage of the French population. This permitted identification of a large number of subjects with the nosocomial pneumonias of interest in a recent timeframe from both public and private hospitals across 23 cities in France; this also permitted identification of enough controls.
An increase in hospital cost and lengths of stay have been highlighted in cases of nosocomial infection, including nosocomial pneumonia, by the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) [14,15-17]. Our study confirms that nosocomial pneumonia due to S. aureus or P. aeruginosa is associated with considerable patient morbidity and substantial hospital costs in France, with significantly longer lengths of hospital stay observed among patients with nosocomial pneumonia due to S. aureus or P. aeruginosa as compared to matched controls without nosocomial infection (p<0.001). In addition to the cost incurred due to prolonged hospital stay, nosocomial pneumonia cases were associated with a significantly increased use of mechanical ventilation (p<0.001) and critical care unit stays compared to the matched controls (p<0.001). Notably, the total cost of treatment is also increased due to significantly higher administration of at least one expensive (liste T2A) drug among cases compared to the matched controls (p<0.001).
These findings are consistent with those previously reported in other countries. In a matched-cohort analysis of patients with ventilatorassociated pneumonia (VAP) in the United States (US), Kollef and colleagues observed that VAP patients were in hospital for an average of 32.6 days vs. 19.5 for matched controls (p<0.0001) [18]. In a retrospective study using data from US Nationwide Inpatient Sample database, patients with nosocomial pneumonia following intraabdominal surgery were hospitalized a mean of 17.1 days compared to 6.1 days for those who did not acquire pneumonia (p<0.001) [16]. This compares to a mean index hospitalization duration of approximately 50 days compared to 13 days for cases and controls, respectively, in our study.
Prompt diagnosis can reduce the mortality and morbidity of patients with nosocomial infection [19]. Care should be taken to detect and prevent nosocomial infection early because despite the high treatment cost, it was observed in our study that death occurred during the index hospitalization in a significantly higher proportion of cases compared to the matched controls (p<0.001) [16]. Increased mortality has been previously associated with nosocomial pneumonia: a study by Guzman-Herrador and colleagues demonstrated that nosocomial pneumonia is independently associated with mortality in hospitalized patients [20,21], and a systematic review of VAP studies found the odds of mortality to be 2.03 higher in patients with VAP compared to those without VAP [22]. The ATS/ IDSA 2005 guideline reported an estimated mortality rate of 33% to 50% for patients with VAP, which was consistent with our observed mortality of 29.7% in the S. aureus cohort and 38.8% in the P. aeruginosa cohort [14].
By DRG code, using univariate analysis, the cost per index hospitalization for cases was approximately €21,000 for cases compared to less than €6,500 for the controls (p<0.001). Similar significant high cost was associated with nosocomial infections compared to matched controls in terms of daily costs. Costs for cases were in fact higher when assessed using the daily cost estimation method compared to the DRG cost. The trend continued through 90 days post-discharge from index hospitalization. By multivariate analysis, at 90 days after discharge from the index hospitalization, costs per case of S. aureus pneumonia were €20,814 and €22,662 using the DRG and daily cost methods, respectively; for P. aeruginosa , comparable figures were €20,880 and €26,766. By either method, control case costs were less than €8,000. Our observation of higher costs for patients with nosocomial pneumonia is also consistent with the published literature in a French population of nosocomial pneumonia following head and neck cancer surgery [23] and a US population of nosocomial pneumonia following intra-abdominal surgery [16], as compared to matched populations without nosocomial infections. In our analysis, P. aeruginosa pneumonia had slightly higher costs than S. aureus pneumonia patients. This may be due to the longer length of stay observed and higher use of expensive antiinfectives in the P. aeruginosa pneumonia cohort.
It is noteworthy that one-third of patients in both pneumonia cohorts of our study had antibiotic-resistant infections, potentially contributing to higher costs. The presence of antimicrobial resistance increases the risk of inappropriate therapy [24,25]. Resistant infections may lead to treatment failures and the need to use second or third-line antibiotics, increased length of stay, and/or mortality. Studies have also demonstrated that healthcare resource utilization typically rises with failure of initial empiric therapy [24-27].
A study on referral patterns between hospitals for nosocomial infections in the Netherlands highlighted that patients referred through the Dutch National Health Care Networks form a bridge between hospitals and provide a path that can facilitate the spread of hospital-acquired infections, such as methicillin-resistant S. aureus , between hospitals [28]. Our study also demonstrated a higher proportion of hospital-transferred patients among cases compared to the controls (S. aureus cohort: 18.7% vs. 7.2%; P. aeruginosa cohort: 17.5% vs. 6.0%). This suggests a plausible route of spreading infection between hospitals through transferred patients, thereby necessitating proper hospital infection control measures for such referred patients.
Strengths of this study include the data source, which represents all hospitalizations in French public and private hospitals and is tied to reimbursement, thereby ensuring completeness of data. Thus, this retrospective study took into account the countrywide French adult population as a representation of nosocomial pneumonia due to S. aureus or P. aeruginosa ; no selection bias was possible, as there was no sampling (all identified cases were included). The study’s sample size was also very large, contributing to the ability to find controls. An additional strength is the evaluation of costs by two different approaches, wherein one approach was independent of the length of stay, while the other approach was based on the total length of stay and on the use of specific medical resources (i.e. costly drugs, expensive devices, emergency room referrals), which are not specifically timedependent. It was assumed that if the actual length of stay is longer than the national mean length of stay associated to a DRG, the daily cost estimation is closer to actual expenses for a given disease. Our study demonstrated that this was the case for nosocomial pneumonia, thereby warranting accuracy of the cost estimates.
There are also limitations to our analysis. First, the lack of a specific ICD-10 code for nosocomial pseudomonas or staphylococcal pneumonia may have affected correct identification of all cases. In addition, subjects and cases could not be fully matched, with differences in specific comorbidities evident at index hospitalization; there was a significantly increased likelihood that cases suffered from congestive heart failure, chronic pulmonary disease, cerebrovascular disease, hemiplegia/paraplegia, renal disease, or peptic ulcer disease as compared to controls at the time of index hospitalization. Finally, only hospitalization costs were included in the analysis, with outpatient costs not represented. This may lead to an under-estimation of the total cost for nosocomial infections through 90 days post-discharge. However, the objective of this study was not to calculate the overall cost of nosocomial infections, but to evaluate the additional hospitalization related cost represented by these infections when compared to matched controls. In addition, the analysis through 90 days post-discharge represents costs for a greater number of days for cases than for controls, given the longer hospital stay for cases; however, since the majority of cost was incurred during index hospitalization, these factors would not alter the conclusion considerably.
It should also be noted that the PMSI database does not provide the date of admission. In this database, hospitalizations are presented by the month of discharge and the duration of stay in days. As a result, the exact dates of admission and discharge are unknown. Although this limitation is pertinent for both sub-groups of patients and its effect on the comparison tables is limited, the number of follow-up days was calculated after the month of discharge and, therefore, is not precise. Finally, the PMSI database is an administrative claims database that is designed for payment and not for analytical purposes. Thus, it may be subject to potential coding bias, inconsistencies, and missing data, thereby leading to under-coded and under-estimated nosocomial infections.
Our study highlighted the considerable hospitalization cost and morbidity burdens associated with cases of nosocomial pneumonia caused by S. aureus and P. aeruginosa , the two most common bacterial causes of nosocomial pneumonia. Although it cannot be concluded with certainty that the significantly higher costs among cases (p<0.001) are due to the nosocomial pneumonia alone, we tried to match controls with cases based on demographic and baseline disease characteristics, making it likely that the increased cost was attributable to nosocomial infection. Cases had longer initial stays with greater complexity of care; greater use of medical procedures (notably, mechanical ventilation); greater use of treatments included in the list of expensive drugs; and longer hospital stays upon readmission after discharge from the index hospitalization compared to the controls.
In our analysis, all cases of pneumonia were nosocomial infections and, therefore, were potentially preventable. By virtue of multivariate analysis, the study was able to identify potential factors driving the occurrence of nosocomial pneumonia in France. Hospitals should, therefore, consider focusing on the factors associated with development of nosocomial pneumonia, including those identified through our analysis, to target infection prevention efforts and new therapies on patients at highest risk of developing nosocomial pneumonia. The increasing growth of resistance and relative dearth of new effective antibiotics underlines the urgent need of measures and recommendations to better control nosocomial infection occurrences. In line with the snapshot of the French situation presented in this study, evidence across other geographies can help consolidate the cost burden of nosocomial pneumonia and identify potential preventable factors on a global scale.
Acknowledgements
This study was funded by MedImmune. All authors were involved in developing the study concept and design. MT designed the study, performed the research, interpreted the data, and edited the paper. IB extracted, analyzed, and interpreted the data, and edited the paper.
HSJ, JF, and KR analyzed and interpreted the data, and edited the paper; and all authors reviewed and approved the final version of the manuscript for submission.
MT and IB are employees of IMS Health, an independent company providing data and research services to pharmaceutical companies and governments. HSJ and JF are employees of MedImmune; KR is an employee of AstraZeneca; all three may own AstraZeneca stock.
We would also like to thank Moe Kyaw, PhD for helpful discussions and input during the early stage of study concept and design, as well as Paranjoy Saharia for his review and improvement of the manuscript.
References
- Commission (2008) Questions and Answers on patient safety, including the prevention and control of healthcare associated infections
- D’alerte R (2012) National Survey of Prevalence of Nosocomial Infections and Infectious Therapy in Health Care Facilities. Results. Saint-Maurice: Institute for Public Health Surveillance.
- Defez C, Fabbro-Peray P, Cazaban M, Boudemaghe T, Sotto A, et al. (2008) Additional direct medical costs of nosocomial infections: an estimation from a cohort of patients in a French university hospital. J Hosp Inf 68: 130-136.
- Kaier K, Lambert ML, Frank UK, Vach W, Wolkewitz M, et al. (2014) Impact of availability of guidelines and active surveillance in reducing the incidence of ventilator-associated pneumonia in Europe and worldwide. BMC Infec Dis 14: 199.
- Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, et al. (2003) Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med 31: 1312-1317
- ATIH (2013) Programme de médicalisation des systèmes d’information en médecine, chirurgie, obstétrique et odontologie (PMSI MCO).
- Code de la santé publique (2013) publique, C.d.l.s. Legifrance L6113-6117.
- Tarifs MCO applicables (2013) Agence technique de l'information sur l'hospitalisation.
- Bousquet C, Trombert B, Souvignet J, Sadou E, Rodrigues JM (2010) Evaluation of the CCAM Hierarchy and Semi Structured Code for Retrieving Relevant Procedures in a Hospital Case Mix Database. AMIA Annu Symp Proc 2010: 61-65.
- Fetter RB, Brand DA (1991) DRGs: Their design and development. Health Administration Press.
- Romano PS, Roos LL, Jollis JG (1993) Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 46: 1075-1079.
- Schneeweiss S, Maclure M (2000) Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol 29: 891-898.
- ATS/IDSA (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med  171: 388-416.
- Glied S, Cohen B, Liu J, Neidell M, Larson E (2016) Trends in mortality, length of stay, and hospital charges associated with health care-associated infections, 2006-2012. American J Inf Cont 44: 983-989.
- Thompson DA, Makary MA, Dorman T, Pronovost PJ (2006) Clinical and economic outcomes of hospital acquired pneumonia in intra-abdominal surgery patients. Ann Surg 243: 547-552.
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, et al. (2013) Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Int Med 173: 2039-2046.
- Kollef MH, Hamilton CW, Ernst FR (2012) Economic impact of ventilator-associated pneumonia in a large matched cohort. Inf Cont Hosp Epidemiol 33: 250-256
- Schellack N (2015) Hospital-acquired pneumonia and its management: Review. SA Pharma J 82: 26-32.
- Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, et al. (1993) Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. American J Med 94: 281-288.
- Melsen WG, Rovers MM, Bonten MJ (2009) Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med 37: 2709-2718.
- Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33: 2184-2193.
- Penel N, Lefebvre JL, Cazin JL, Clisant S, Neu JC, et al. (2008) Additional direct medical costs associated with nosocomial infections after head and neck cancer surgery: a hospital-perspective analysis. Int J oral maxillofacial Surg 37: 135-139.
- Leone M, Garcin F, Bouvenot J, Boyadjev I, Visintini P, et al. (2007) Ventilator-associated pneumonia: Breaking the vicious circle of antibiotic overuse. Crit Care Med 35: 379-385
- Zilberberg MD, Shorr AF, Micek ST, Mody SH, Kollef MH (2008) Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest 134: 963-968.
- Rello J, Ulldemolins M, Lisboa T, Koulenti D, Manez R, et al. (2011) Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Euro Res J 37: 1332-1339.
- Wilke M, Grube RF, Bodmann KF (2011) Guideline-adherent initial intravenous antibiotic therapy for hospital-acquired/ventilator-associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Euro J Med Res 16: 315-323.
- Donker T, Wallinga J, Grundmann, H (2010) Patient Referral Patterns and the Spread of Hospital-Acquired Infections through National Health Care Networks. PLOS Comp Biol 6: e1000715.
Citation: Toussi M, Bardoulat I, Jafri HS, Falloon J, Ryan K (2017) Healthcare Resource Utilization and Cost Related to Nosocomial Pneumonia Caused by P. aeruginosa and Pseudomonas aeruginosa in France: A 2010-2011 Population-Based Cohort Study Using a National Claims Database. Epidemiology (Sunnyvale) 7:318. DOI: 10.4172/2161-1165.1000318
Copyright: © 2017 Toussi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3659
- [From(publication date): 0-2017 - Aug 25, 2025]
- Breakdown by view type
- HTML page views: 2765
- PDF downloads: 894