HER2-Neu Gene Testing in Gastric Cancer by Immunohistochemistry in Tunisian Patient��?s Samples
Received: 07-Nov-2016 / Accepted Date: 21-Feb-2017 / Published Date: 27-Feb-2017 DOI: 10.4172/2476-2253.1000110
Abstract
Immunohistochemical (IHC) testing for HER2/neu is becoming the standard of care for guiding the adjuvant treatment of gastric carcinoma with trastuzumab. Up to now, gastric cancer has been considered the most commonly diagnosed tumors leading to death. In this study, we evaluate the detection of HER2 expression by IHC in Tunisian patients with gastric cancer according to the international consensus. A total of 84 tumor specimens was assessed for HER2 expression by immunohistochemistry (IHC) using the antibodies HercepTest™. Doubtful IHC results (IHC 2+) were resolved by HER2 Chromogenic in situ hybridization (CISH). Thus, 10.5% of samples were HER2-positive (3+), 8.3% having a negative score IHC, Her2-Neu (+1) and 3.6% to be a dubious immunostaining Her2-Neu (2+).
Keywords: HER2-Neu Gene; Gastric cancer; Trastazumab; Immunohistochemistry; In Situ Hybridization
7805Introduction
Gastric cancer (GC) is the fourth most commonly diagnosed tumor and the second leading cause of cancer-related death in the world [1-4]. Conventional cancer therapies are surgery, radiation, and chemotherapy [1]. Surgery is the main treatment for early stage GC, while patients with advanced GC require chemotherapy to improve their chances of survival [2]. Nevertheless, chemotherapy can cause damage or toxicity to both the adjacent and distant normal tissues, which in turn limits the effectiveness of these therapy approaches [1,2].
The ToGA study showed that trastuzumab plus chemotherapy prolonged median survival in patients with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer [5-7].
Trastuzumab, is a monoclonal antibody used to target Human Epidermal Growth Factor receptor 2 protein and is used worldwide with standard chemotherapy [4,6].
The HER2 protein is a member of the epidermal growth factor receptor family and is coded by HER2/neu gene located on the long arm of chromosome 17 [8]. The product is a 185 kD transmembrane glycoprotein [9]. It is a transmembrane tyrosine kinase receptor [3], involved in tumor cell proliferation, apoptosis, adhesion, migration, and differentiation [8].
It was confirmed that tumor cells release circulating HER2 peptides originated from the plasmic membrane, and thus it could be used as a diagnostic marker for tissue HER2 status [10]. Meanwhile tissue HER2 assessment by immunohistochemistry (IHC) and in situ hybridization has become a routine practice in the analysis of advanced gastric cancer [4,10].
In this study of 84 samples, we carried out our assessment by using the approach of immunohistochemistry and we confirmed doubtful samples by in Situ hybridization.
Material and Methods
Our study is retrospective, it was performed on 84 cases of gastric adenocarcinoma involving 42 gastric biopsies, 37 operating samples of gastrectomy and 5 biopsies of metastasis.
All tissue samples were processed according to standard protocols, with formalin fixation. Clinicopathological parameters, including age, gender, histological classification, and pathological TNM stage, were retrieved from the medical folder. Histological classification was determined according to the Lauren’s classification:
- All tissues were fixed with 10% buffered formalin and then paraffinembedded.
- Sections (4 μm thick) were de-paraffinized in xylene and hydrated through a graded ethanol series.
- IHC staining of HER2 was manually performed with the HercepTest II™ (DAKO, Glostrup, Denmark).
- Out of the 84 specimens, 3 expressing HER2 were defined as either or IHC 2+ and subsequently were evaluated by CISH.
- CISH analysis was carried-out using the ZytoDot SPEC Her2-Neu.
Statistical analysis
Statistical analysis was performed using the chi-square test to analyze associations between HER2 status and clinicopathological parameters.
A value less than 0.05 was considered significant. Data were analyzed using the SPSS statistical software program for Microsoft Windows.
Results
Clinical investigation
Out of 84 patients, 67% were male. The mean age of our patients at diagnosis was 59 years with extremes ranging from 27 to 86 years. The maximum frequency is between 40 and 79 years. Patients’ clinicopathological data are summarized in Tables 1-3.
| Her2-Neu 0 | Her2-Neu 1 | Her2-Neu 2 | Her2-Neu 3 | Total | ||
|---|---|---|---|---|---|---|
| Age | ≤60 | 36 | 3 | 2 | 2 | 43 |
| >60 | 30 | 4 | 1 | 6 | 41 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
| Gender | Male | 44 | 4 | 2 | 6 | 56 |
| Female | 22 | 3 | 1 | 2 | 28 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
| Digestive history | Cardia | 8 | 0 | 1 | 1 | 10 |
| Fundus | 8 | 0 | 1 | 0 | 9 | |
| Body | 5 | 0 | 0 | 2 | 6 | |
| Den | 20 | 6 | 1 | 3 | 30 | |
| Pylorus | 1 | 0 | 0 | 0 | 1 | |
| Location broadcasts | 23 | 1 | 0 | 2 | 26 | |
| Total | 65 | 7 | 3 | 8 | 83 | |
| Macroscopic | Budding | 8 | 0 | 0 | 2 | 10 |
| Infiltrating | 7 | 0 | 0 | 1 | 8 | |
| Rankled | 9 | 0 | 1 | 0 | 10 | |
| CD-budding | 24 | 4 | 2 | 5 | 35 | |
| CDinfiltrating | 18 | 3 | 0 | 0 | 21 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
Table 1: Correlations between epidemiological, paraclinical data and the score of the Her2-Neu status.
| Her2-Neu 0 | Her2-Neu 1 | Her2-Neu 2 | Her2-Neu 3 | Total | ||
|---|---|---|---|---|---|---|
| Size of the tumor | ≤5cm | 26 | 4 | 0 | 3 | 33 |
| >5cm | 40 | 3 | 3 | 5 | 51 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
| Histological type | Intestinal type | 40 | 7 | 2 | 6 | 55 |
| Diffuse type | 26 | 0 | 1 | 2 | 29 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
| Histological differentiation | Well-differentiated | 3 | 0 | 0 | 0 | 3 |
| Medium Differentiated | 22 | 5 | 1 | 4 | 32 | |
| Undifferentiated | 15 | 2 | 1 | 2 | 20 | |
| Isolated cells in ring kitten | 26 | 0 | 1 | 2 | 29 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
| Mucosecretion | Tumor mucosecreteur | 32 | 1 | 1 | 2 | 36 |
| Tumornot mucosecreteur | 34 | 6 | 2 | 6 | 48 | |
| Total | 66 | 7 | 3 | 8 | 84 | |
| Associated precanceros lesions | Chronic gastritis | 36 | 3 | 1 | 5 | 45 |
| Intestinal metaplasia | 18 | 4 | 0 | 2 | 24 | |
| Helicobacter pylori | 13 | 2 | 1 | 2 | 18 | |
| Operating parts | 30 | 3 | 0 | 4 | 37 | |
| Histopathological sampling type | Gastric biopsy | 32 | 4 | 3 | 3 | 42 |
| Biopsy of metastasis | 4 | 0 | 0 | 1 | 5 | |
Table 2: Correlations between histopathological data and the score of the Her2-Neu status.
| Her2-Neu 0 | Her2-Neu 1 | Her2-Neu 2 | Her2-Neu 3 | Total | ||
|---|---|---|---|---|---|---|
| T-stage | T1+T2 | 16 | 3 | 0 | 2 | 21 |
| T3+T4 | 34 | 4 | 3 | 6 | 47 | |
| Total | 50 | 7 | 3 | 8 | 68 | |
| N-stage | N (+) | 42 | 6 | 3 | 7 | 51 |
| N (-) | 14 | 1 | 0 | 1 | 16 | |
| Total | 56 | 7 | 3 | 8 | 74 | |
| M-Stage | M (+) | 28 | 3 | 2 | 5 | 37 |
| M (-) | 10 | 4 | 1 | 3 | 14 | |
| Total | 38 | 7 | 3 | 8 | 56 | |
Table 3: Correlations between TNM Stage and the score of the Her2-Neu status.
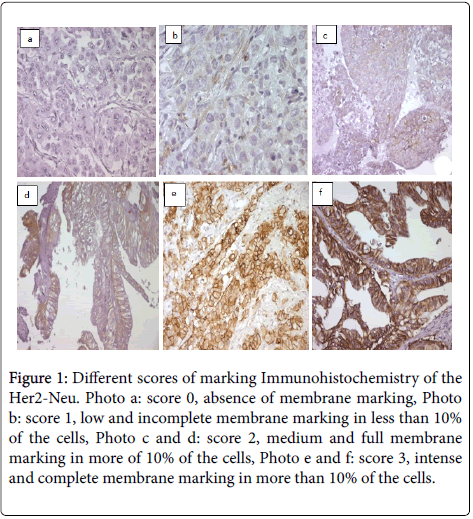
An immunostaining was revealed in 18 samples, 21.4% of cases. Eight patients, (10.5%) of cases have an intense and full stained membrane in 'U', covering 40% to 95% of the cells, and therefore having a score IHC 3+ (Figures 1e and 1f).
Figure 1: Different scores of marking Immunohistochemistry of the Her2-Neu. Photo a: score 0, absence of membrane marking, Photo b: score 1, low and incomplete membrane marking in less than 10% of the cells, Photo c and d: score 2, medium and full membrane marking in more of 10% of the cells, Photo e and f: score 3, intense and complete membrane marking in more than 10% of the cells.
Three patients, (3.6%) of cases have a moderate stained membrane, full for two of them and incomplete 'L' for the third, covering respectively 20%, 30% and 70% of the cells, and therefore a score IHC 2+ (Figures 1c and 1d).
Seven patients, (8.3%) of cases have an incomplete low stained membrane, detected in 20% to 60% of the cells, and thus having a score IHC 1+ (Figure 1b).
The rest of cases (77.6%) showed no marking and therefore an IHC score 0 (Figure 1a).
Chromogenic in Situ Hybridization CISH
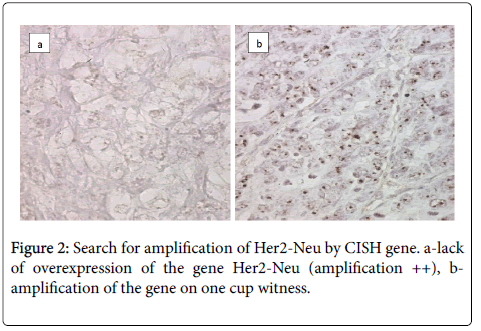
The CISH technique on three ambiguous cases Her2-Neu (2+) looking for a possible amplification of Her2-Neu gene was used. The result was negative and there was no CISH signal (Figure 2).
Discussion
In our study a total of 84 tumor samples (42 gastric biopsies, 37 gastrectomy operating parts and 5 biopsies of metastasis) were tested. 10.5% of samples were Her2Neu (3+), 3.6% were 2+ and 8.3% were 1+. The proportion of stained cells was more than 10% except for cells with score 1+, it was less than 10%.
Recent studies have reported that overexpressing Her2 samples in gastric and gastroesophageal junction carcinomas is ranging between 10% and 20% [7]. But Saito et al. [10], in a series of 224 patients, they found 21% of tissues were HER2-positive. Furthermore, Huang et al. determined only a proportion of 7.8% of HER2-positive in fourteen biopsy and surgical specimens [8]. However, in the study of Rajagopal et al., in 60 cases, positive HER2 expression was observed only in 26.7% of tumors, predominantly in males and intestinal type [3]. Besides, in the study of Koopman et al. [11], HER2 positivity was found in 50 samples out of 323 (15.5%). Whereas, Van et al. determined the status of HER2 in 3803 tumor samples, they found 22.1% of positivity rate. Rates were similar between European and Asian patients (23.6% vs. 23.9%), but higher in intestinal- vs . diffusetype (31.8% vs. 6.1%), and gastroesophageal junction cancer versus gastric tumors (32.2% vs. 21.4%) [12,13]. Moreover, Yoshida et al. evaluated the expression of Her2 Neu gene by fluorescence in situ hybridization and HER2 overexpression was observed in 17% of both surgically resected tumors and biopsy specimens [14].
Rakhshani et al. examined the overexpression of HER2/neu in 101 of gastric tissue samples by IHC. In their study, they demonstrated a significant difference in the overexpression of HER2/neu in gastric tumors. They found that the overexpression of HER2/neu was significantly higher in intestinal type, poorly differentiated grade, large size (≤ 5 cm) and positive nodal involvement tumors [15]. In our study, we found an increase Intestinal type expression of Her2-Neu comparing with diffuse type. Additionally, we found that Her2 was overexpressed in medium differentiated type.
Rabahao et al. used several antibodies to study the expression of Her2. HER2-positive expression (3+) in the whole-tissue sections was observed in 23 cases (11.6%) using the 4B5 antibody, in 18 cases (9.1%) using the SP3 antibody and in 10 cases (5.1%) using the HercepTest antibody [16].
In a different study, HER2/neu overexpression (IHC 3+) was detected in 19% (10 of 54) of gastric tumors. HER2-positive expression (3+) in the whole-tissue sections was observed in 23 cases (11.6%) using the 4B5 antibody, in 18 cases (9.1%) using the SP3 antibody and in 10 cases (5.1%) using the Hercep Test antibody [17].
HER2 overexpression (2+ or 3+) was observed in 40 (13.8%) cases by HercepTest, 46 (15.9%) by A0485, 40 (13.8%) by 4B5 and 27 (9.3%) by CB11 [18].
In our study, we used HercepTest antibody and we found Her2 expressed (3+) in 8 cases (10.5%).
In another study Hunag et al. reported that HER2-positive tumors were identified in 12.0% (88/734) of the GC and GJC cases. No significant difference in HER2 positivity was identified between resection and biopsy samples, or between early and advanced disease stages [19].
In the study of Gasljevic et al. HER2 over-expression was found in 25.2%; HER2 3+6.6%, HER2 2+ 18.7% of tumors [20].
In the study of Shan et al., HER2 overexpression (3+) was detected in 9.8% of carcinomas and more frequently observed in GEJ cancer cases, in the intestinal type, and in the well or moderately differentiated type [21].
In a series of the 775 gastric cancer samples examined by IHC, a total of 88 (11%) cases were positive for HER-2/neu overexpression at a score of 3+; 44 (6%) cases were equivocal with a score of 2+; and the rest 643 (83%) cases were negative scored as 0/1+. Intestinal-type and early-stage cancers exhibited higher rates of HER-2/neu overexpression than those of diffuse/mixed-type and advanced cancers [22]. Similarly, Sekaran et al. confirmed the overexpression of HER2 in 23 of 52 (44.2%) patients. Two patients had equivocal result by IHC (2+), one of whom was positive on analysis by FISH [23]. Conversely, in another study, HER2 amplification was found prevailed in intestinal-type and low grade tumors, showing no correlation with patients’ age/sex, tumor location, stage, and Ming histotype.
The expression of the Her2-Neu gene increases with age (16.2% for patients older than 60 years compared to 26.8% for very elderly patients (over 60 years)), but there was no significant correlation between the overexpression of Her2-Neu and age (P=0, 23). Similarly, very elderly patients overexpress more this bothers Her2-Neu (3+) (14.6% vs. 4.6%).
Yan et al. have reported that HER2-neu over-expression significantly predicts poor outcome in pN0 EGC, and exact HER2-neu testing would be done before endoscopic therapy. For HER2/neupositive patients, radical surgery should be performed [24].
In the study of Madani SH et al., patients with advanced cancer of GC and GEJ, HER2-neu overexpression was more associated with the intestinal cancer subtype. This could be a guide to new complementary therapy for affected patients [25].
In his report, Liu X argued that the value of HER2/neu for a potential role as a negative prognostic factor in the equivocal gastric cancer cases is limited. Indeed, FISH (Fluorescent In Situ Hybridization is necessary for further classification when IHC (Immunohistochemistry) gives a score of 2+ [9].
Beside FISH assessment, HER2 DISH (Dual In Situ Hybridization) assay, utilizing 10% buffered formalin-fixed CB, would be a reliable and the ideal method to assess the HER2 gene status of breast cancer cytological specimens [26].
More new techniques as next generation sequencing enable Reliable Detection of HER2 (ERBB2) Status in Breast Cancer and Provides Ancillary Information of Clinical Relevance [27].
With the help of NGS, we have now been able to identify actionable mutations such as in the isocitrate dehydrogenase 1 (IDH1), FGFR2, BRAF and HER2/neu genes for targeted therapeutics and correlate the genetic variations with distinct clinical prognoses. This recent genetic information has the potential to make precision medicine a part of routine clinical practice for the management of BTC patients [28].
In our study the expression of Her2 (2+, 3+) was 14.1%. Three cases (2+) were negative on CISH.
There was no difference in HER2 overexpression (positivity) or negativity in relation to age, gender, tumor site, histological subtype, tumor differentiation, serosal involvement or lymph nodal status. HER2 overexpression rates were similar to intestinal type as compared to diffuse histologically [23].
In the study of Sin et al., HER2 gene amplification was detected in 10/85 (11.8%) cases of gastric carcinoma. In the 10 cases with HER2 amplification, HER2 immunoreaction scorings of 3+, 2+ and 0/1+ were present in 7, 2 and 1 cases, respectively [29].
An accurate assessment of HER2 expression in gastric cancer patients is of importance and utility in the optimal selection of patients for Trastusumab (Herceptin) therapy. Our study found an HER2 overexpression of 10.5% in gastric cancers similar to many studies in the world. Additional studies are needed to explore the role of HER2 as an independent prognostic factor. Though Herceptin is approved for advanced gastric and GEJ cancers, role of herceptin in adjuvant/neoadjuvant setting in early stages needs to be evaluated with newer agents like Pertuzumab, Bevacizumab, especially in young patients.
References
- Ito K, Mitsunaga M, Arihiro S, Saruta M, Matsuoka M, et al. (2016) Molecular targeted photoimmunotherapy for HER2-positive human gastric cancer in combination with chemotherapy results in improved treatment outcomes through different cytotoxic mechanisms. BMC Cancer 16: 37.
- Cui Y, Li SB, Peng XC, Wu J, Fu GH (2015) Trastuzumab Inhibits Growth of HER2-Negative Gastric Cancer Cells Through Gastrin-Initialized CCKBR Signaling. Dig Dis Sci 60: 3631-3641.
- Rajagopal I, Niveditha SR, Sahadev R, Nagappa PK, Rajendra SG (2015) HER 2 Expression in Gastric and Gastro-esophageal Junction (GEJ) Adenocarcinomas. J Clin Diagn Res 9: EC06-10.
- Cappellesso R, Fassan M, Hanspeter E, Bornschein J, d'Amore ES, et al. (2015) HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum Pathol 46: 665-72.
- Gong J, Liu T, Fan Q, Bai L, Bi F, et al. (2016) Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer 16: 68.
- Yamada T, Yamamoto Y, Moriwaki T, Hyodo I (2016) Is serum HER2 ECD a predictive biomarker for response to trastuzumab in advanced gastric cancer? J Gastroenterol 51: 506-507.
- Grillo F, Fassan M, Sarocchi F, Fiocca R, Mastracci L (2016) HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J Gastroenterol 22: 5879-87.
- Huang SC, Ng KF, Lee SE, Chen KH, Yeh TS, et al. (2016) HER2 testing in paired biopsy and excision specimens of gastric cancer: the reliability of the scoring system and the clinicopathological factors relevant to discordance. Gastric Cancer 19: 176-82.
- Liu X, Wang X, Wang B, Ren G, Ding W (2016) HER2 Gene Amplification by Fluorescence In Situ Hybridization (FISH) Compared With Immunohistochemistry (IHC) in 122 Equivocal Gastric Cancer Cases. Appl Immunohistochem Mol Morphol 24: 459-64.
- Saito M, Yamashita K, Arimura Y, Kaneto H, Okuda H, et al. (2016) Serum HER2 as an adjunct to assess HER2 status for advanced gastric cancer: A prospective multicenter trial (SHERLOCK). Acta Oncol 55: 309-17.
- Koopman T, Louwen M, Hage M, Smits MM, Imholz ALT (2015) Pathologic diagnostics of HER2 positivity in gastroesophageal adenocarcinoma. Am J Clin Pathol 143: 257-64.
- Van Cutsem E, Bang YJ, Feng-YiF, Xu JM, Lee KW, et al. (2015) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18: 476-84.
- Kimura Y, Oki E, Yoshida A, Aishima S, Zaitsu Y, et al. (2014) Significance of accurate human epidermal growth factor receptor-2 (HER2) evaluation as a new biomarker in gastric cancer. Anticancer Res 34: 4207-12.
- Yoshida H, Yamamoto N, Taniguchi H, Oda I, Katai H, et al. (2014) Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch 465: 145-54.
- Rakhshani N, Kalantari E, Bakhti H, Sohrabi MR, Mehrazma M (2014) Evaluation of HER-2/neu overexpression in gastric carcinoma using a tissue microarray. Asian Pac J Cancer Prev 15: 7597-7602.
- Abrahao-Machado LF, Jacome AA, Wohnrath DR, dos Santos JS, Carneseca EC, et al. (2013) HER2 in gastric cancer: comparative analysis of three different antibodies using whole-tissue sections and tissue microarrays. World J Gastroenterol 19: 6438-46.
- Jeung J, Patel R, Vila L, Wakefield D, Liu C (2012) Quantitation of HER2/neu expression in primary gastroesophageal adenocarcinomas using conventional light microscopy and quantitative image analysis. Arch Pathol Lab Med 136: 610-7.
- Cho EY, Srivastava A, Park K, Kim J, Lee MH, et al. (2012) Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology 44: 216-20.
- Huang D, Lu N, Fan Q, Sheng W, Bu H, et al. (2013) HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One 8: e80290.
- Gasljevic G, Lamovec J, Contreras JA, Zadnik V, Blas M, et al. (2013) HER2 in gastric cancer: an immunohistochemical study on tissue microarrays and the corresponding whole-tissue sections with a supplemental fish study. Pathol Oncol Res 19: 855-65.
- Shan L, Ying J, Lu N (2013) HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population. Diagn Pathol 8: 76.
- Liu W, Zhong S, Chen J, Yu Y (2012) HER-2/neu overexpression is an independent prognostic factor for intestinal-type and early-stage gastric cancer patients. J Clin Gastroenterol 46: e31-37.
- Sekaran A, Kandagaddala RS, Darisetty S, Lakhtakia S, Ayyagari S, et al. (2012) HER2 expression in gastric cancer in Indian population--an immunohistochemistry and fluorescence in situ hybridization study. Indian J Gastroenterol 31: 106-110.
- Yan Y, Lu L, Liu C, Li W, Liu T, et al. (2015) HER2/neu over-expression predicts poor outcome in early gastric cancer without lymph node metastasis. Clin Res Hepatol Gastroenterol 39: 121-126.
- Madani SH, Rahmati A, Sadeghi E, Khazaei S, Sadeghi M, et al. (2015) Survey of Her2-neu Expression and its Correlation with Histology of Gastric Carcinoma and Gastroesophageal Junction Adenocarcinoma. APJCP 16: 7755-7758.
- Nishimura R, Okamoto N, Satou M, Kojima K, Tanaka S, et al. (2016) Bright-field HER2 dual in situ hybridization (DISH) assay on breast cancer cell blocks: a comparative study with histological sections. Breast cancer 23: 917-21.
- Pfarr N, Penzel R, Endris V, Lier C, Flechtenmacher C, et al. (2016) Targeted Next Generation Sequencing Enables Reliable Detection of HER2 (ERBB2) Status in Breast Cancer and Provides Ancillary Information of Clinical Relevance. Genes Chromosomes Cancer 56: 255-265.
- Jain A, Javle M (2016) Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol 7: 797-803.
- Sin LF, Vong HT, Wen JM, Yip YC (2012) Amplification of HER2 gene in gastric carcinoma detected by dual in-situ hybridization. Zhonghua Bing Li Xue Za Zhi 41: 168-71.
Citation: Gharsalli T, Bouazzi H, Aiwasiyah B, Sassi M, Sriha B (2017) HER2-Neu Gene Testing in Gastric Cancer by Immunohistochemistry in Tunisian Patient’s Samples. J Cancer Diagn 2: 110. DOI: 10.4172/2476-2253.1000110
Copyright: © 2017 Gharsalli T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6926
- [From(publication date): 0-2017 - Aug 17, 2025]
- Breakdown by view type
- HTML page views: 5919
- PDF downloads: 1007