Research Article Open Access
Hybrid Amniotic Membrane Dressing with Green Silver Nanoparticles as Bioengineered Skin for Wounds and Burns: A Pilot Studies
Balasundari Ramesh1*, Jaikanth Chandrasekaran2, Sangeetha Jeevankumar2, George Jacob1and Kotturathu Mammen Cherian21Apollo Cancer Speciality Hospital, Teynampet, Chennai, 600035, India
2Frontier Lifeline Pvt. Ltd., R80C, Ambattur Industrial Estate Road, Mugappair, Chennai 600101, India
- *Corresponding Author:
- Ramesh B
Department of Regenerative Medicine
Apollo Cancer Speciality Hospital, Teynampet
Chennai, India 600035
Tel: 09036464769
E-mail: drbalasundari_r@apollohospitals.com
Received date: July 06, 2017; Accepted date: September 21, 2017; Published date: September 28, 2017
Citation: Ramesh B, Chandrasekaran J, Jeevankumar S, Jacob G, Cherian KM (2017) Hybrid Amniotic Membrane Dressing with Green Silver Nanoparticles as Bioengineered Skin for Wounds and Burns: A Pilot Studies. J Biotechnol Biomater 7:272. doi:10.4172/2155-952X.1000272
Copyright: © 2017 Ramesh B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Fabrication of 3D novel scaffolds is very challenging and critical to achieve the appropriate function for tissue regeneration. This study aims to develop a hybrid biological nano-scaffold using Liquid nitrogen (LN2) decellularised human amniotic membrane (hAM), human umbilical cord derived collagen nano-fibres and green silver nanoparticles obtained by green synthesis of curcumin. A novel attempt was made to develop in vitro bioengineered dermal layer by seeding the scaffold with cord blood derived Mesenchymal stem cells (CBMSCs) and trans-differentiating them to keratinocytes and dermal fibroblast like cells using keloid foreskin conditioned media. Furthermore, the small animal experiments in Albino Wistar rats prove it to be a safe healing product without scar formation. This novel scaffold can be a promising dressing material for none healing ulcers and burns. This scaffold has long shelf life, easy to apply, have sustained/controlled release of silver, the minimum frequency of dressing changes, manage excessive exudates, moisture retainment, reduce inflammation and facilitate autolytic debridement.
Keywords
Human amniotic membrane; Collagen nanofibers; Keloid foreskin conditioned media; Umbilical cord; Hybrid bandages
Introduction
The application of biological wound dressings to heal the skin goes back to centuries to the first written report on the skin xenograft in the 15th century BC as mentioned in the Papyrus of Ebers. Various xenogenic dressings from different animal sources ranging from frogs to porcine skins were in the application. The clinical use of human skin allograft was first described in the manuscript of Branca of Sicily in 1503 [1]. A variety of techniques have been attempted to reduce wound sepsis, and variable results have been reported. Human Amniotic membrane (hAM) is a versatile biological dressing with least ethical/religious problems and elicits neither immunological problems nor allergic responses [2]. hAM has been used with variable success for superficial burns, deep burns, after necrosectomy, on large granulating wound surfaces, on autografts, in donor regions, and after dermabrasion [3]. Though the risk of the transmission of certain viral infections is possible, it can be minimized through proper blood diagnostics and maintenance of aseptic conditions during retrieval of amniotic membrane. Bacterial examinations performed over burn wounds covered with amniotic membrane showed little or no bacterial colonization over open injury [4].
Hybrid skin grafts derived from electrospun nano fibre have become a new horizon in wound healing. The hybrid electrospun fibres show excellent support for normal human dermal fibroblast (NHDF) cells adhesion and proliferation [5]. Silver nanoparticles act as an antibacterial and also dampen the process of inflammation, thus promoting scarless pro-healing activity. According to Tian et al. silver nanoparticles can modulate local and systemic inflammatory response following burn injury by cytokine modulation [6]. Moreover, Curcumin is a well-known topical wound healing agent for both regular and diabetic-impaired wounds. Hence we ventured to develop a novel hybrid bandage with the dried amniotic membrane, cord collagen nanofibers and green silver nanoparticles synthesized by curcumin reduction.
Keloid scars are one of the rapid healing processes of skin with high levels of all collagen, proteoglycan and water. Keloid fibroblasts are highly active compared to normal dermal fibroblast [7]. Hence an attempt was made to use the 3D scaffold as in vitro bio engineered skin differentiating CBMSCs to skin cells using keloid scar conditioned media.
This is an in vitro and in vivo small animal study to evaluate the efficacy of hAM hybrid scaffold derived from amniotic membrane and cord collagen impregnated with green silver nanoparticles as a wound healing product.
Methodology
The study was conducted after a proper approval from various committees, which includes Animal ethical committee (FLL/ IAEC/04/2015) and stem cell ethics committee of Frontier lifeline Hospital (IC-SCR), Chennai (Approval No.s: FLL/IC-SCR/02/2015). All the animals involved in this research were treated with humane. Informed consent was taken from the patients or their parents for collecting Amniotic membrane and cord blood. The patients were prescreened for HIV I and II, HBsAg, HCV, CMV and venereal diseases.
Preparation of 3D scaffold
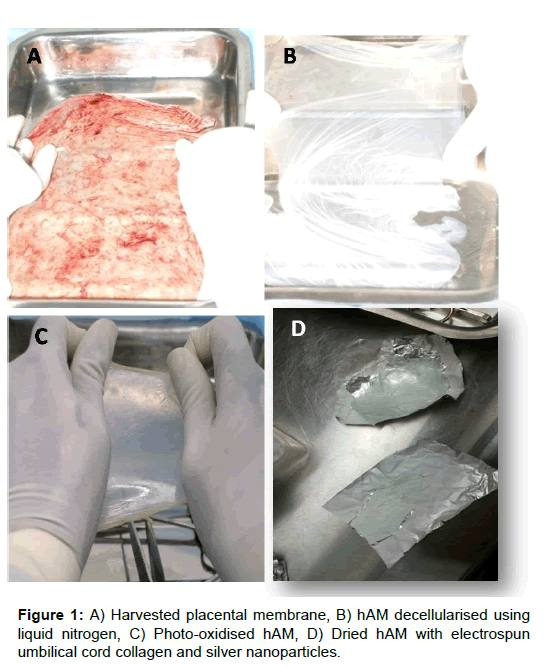
The placental membranes were collected in Hanks balanced salt solution (HBSS-Himedia TS1003) with Antibiotic solution (Himedia A002A) from C-Section delivery and transported to the laboratory with chilled packs within 6 h. The amniotic membrane was separated from chorionic membrane manually. The amniotic membrane was washed well with normal saline (0.9% W/V) and Antibiotics to remove the residual blood stains. Further decellularisation was done by snap freezing with liquid nitrogen for a short duration of 1 min to remove the single layer of epithelial cells. The decellularised amniotic membrane was cross-linked using the photo-oxidizing method using Methylene blue solution 100 μl (Sigma Aldrich 1808) in 500 ml of Dulbecco's Phosphate buffered saline (DPBS, Himedia-TS1006) and at UV wavelength of 480 nm for 2 h. The cross-linked membrane was electro spun with cord derived collagen nanofibers and green silver nano particles obtained by green synthesis from curcumin.
Green silver nanoparticles
The green silver nanoparticle was prepared by green synthesis method of reducing silver nitrate by curcumin. 3.68 mg curcumin (Sigma Aldrich 08511) was dissolved in 2 mL of 10 mM aqueous NaOH solution (Merck 106462) and the volume was made up to 10 mL by water. To synthesize green silver nanoparticles, 1 mL of 1 mM AgNO3 (Merck 101510) was added to 8 mL water. To this 1 mL of freshly prepared curcumin solution was added drop wise and was allowed it to stir for 2 h. The solution turned from yellow to red in a water bath at 95°C. The silver nanoparticle solution thus obtained was purified by repeated centrifugation at 12,000 rpm for 20 min. The supernatant was discarded, and the pellet was lyophilized and stored as powder form. The physical and biological parameters of the green synthesized nanoparticles were characterized using UV-Vis spectroscopy, Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), FTIR, X-ray crystallography, Cytotoxicity studies using 3T3 balb/c cell line, Minimal inhibitory concentration for microorganisms.
Collagen extraction from human umbilical cord
The human umbilical cord was chopped into pieces and washed well with Double Distilled water to remove the blood stains. The tissues were homogenized with 0.5 M acetic acid (Merck 137000) using a blender until a uniform homogenate was obtained. Pepsin (Sigma 96887, 10 mg/100 ml of tissue homogenate) was added to initiate the digestion of protein and placed in the cold room (4-8°C) by constant stirring using magnetic stirrer. After 48 h of digestion, the homogenates were centrifuged at 8000 rpm for 1 h at 4°C. The sediments were discarded, and the supernatant was precipitated by adding 0.7 M concentration of Sodium Chloride (NaCl, Merck 7710) until a thick precipitate was obtained. The precipitated solution was centrifuged at 8000 rpm for 30 min. The supernatant was discarded and the pellet was dialysed using sterile double distilled water for 48 h in cold room. After dialyzation the collagen was lyophilized and stored in -20°C.
Electro spinning of umbilical cord collagen on dried amniotic membrane
480 mg of umbilical cord collagen and 50 μg of green silver nanoparticles were weighed using sterile protocol and dissolved in 5 ml of 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP, Aldrich 105228). The mixture was thoroughly dissolved well in cold room using a magnetic stirrer for 3 days. Finally, it was electro spun on the dried amniotic membrane using syringe pump (Fisher scientific) with 18 blunt gauge needles at the rate of 1 ml/h, air gap distance of 15 cm and 15 kV.
Finally, the 3D scaffold was air dried, gamma sterilized and used for burn and wound dressing. Physical and biocompatibility validation of the dressing material was done by tensile strength, Stability, Surface tension, Fluid uptake, Differential scanning calorimetry (DSC), Fourier transform infra red spectrophotometry (FTIR), Thrombogenicity assay and Toxicity assay (in vitro cytotoxicity, skin irritation and percutaneous toxicity studies) assay following the ISO 10993 updated version.
Protocol for In Vitro Bioengineered Dermal Layer
Isolation of mononuclear cells
Cord blood from the mother undergoing Cesarean section delivery was collected with pre-informed consent. The samples were collected in heparin sodium (15 IU/ml) as an anticoagulant and processed within 6-12 h of collection. The mono nuclear cells were isolated by centrifuging at 1500 rpm for 20 min with Ficoll-Paque density 1.073 ± 0.0001 g/ ml (GE Health care 17-5446-52). The isolated mononuclear cells were washed twice with sterile DPBS and plated in the T25 flask (Nunc) along with complete Dulbecco's Modified Eagles media (DMEM, HyClone- Thermo SH30021.01) and 10% fetal bovine serum (FBS Gold, PAA A15-151). The plastic adherent Mesenchymal stem cells (MSCs) were passaged using 0.5 g Trypsin and 0.2 g Ethylene diamine tetraacetic acid EDTA (Sigma T3924). Passage 2 (P2) cells were used for differentiation assays after characterization of stem cells. The stem cell characterization was done by Reverse transcriptase polymerase chain reaction (RT-PCR), Flow cytometry (FACS) and Immunocytochemistry (ICC).
Preparation of conditioned media
The Keloid foreskin from a burn patient (45 years/F) was surface disinfected using 70% alcohol and bacillocid, alternatively. The scar tissue was excised during plastic surgery and collected in HBSS with antibiotics from with informed consent. Approximately 100 mg of keloid tissue was homogenized by sterile methods and incubated for 48 h with DMEM (Serum free). The media was centrifuged and filtered 0.2 μm Sartorius filter and stored in -80°C freezer as conditioned media.
Differentiation of cord blood derived stem cells to skin cells
The isolated passage number two, CBMSCs were seeded on the 3D hAM hybrid scaffold and was differentiated to skin cells (Keratinocytes, skin fibroblast) using keloid tissue conditioned media. After 3 weeks of incubation, the in vitro developed biological skin was histopathologically compared with native skin. The differentiated skin like cells was tested for the presence of skin fibroblast marker and keratinocyte markers.
Protocol for RT-PCR
Total RNA from the differentiated cells was extracted using TRI Reagent (Sigma 93289). cDNA was synthesized from 1 μg total RNA using Super Script II reverse transcriptase (New England Bio labs) following the manufacturer's protocol. The concentration and the purity of RNA were determined spectrophotometrically. After the reverse transcription step, cDNA samples were subjected to PCR amplification with primers selective for human Mesenchymal stem cells, dermal fibroblast and keratinocytes (Table 1). After an initial denaturation step for 5 min at 95°C, 35 cycles of amplification was performed as follows: Denaturation at 94°C for 30 s, annealing at 57°C for 30 s and DNA extension at 72°C for 60 s, followed by an additional cycle of 5 min at 72°C to complete partial polymerizations. Amplified products will analyze using horizontal 1.5% Agarose (Sigma A9539) gel electrophoresis.
| Conc of AgNo3� � (�µg/ml) | N | %Viability | P value |
|---|---|---|---|
| Mean �± SD | (Treatment Vs Control) | ||
| 0 | 3 | 99.90 �± 0.10 | |
| 1 | 3 | 99.50 �± 0.26 | >0.05 |
| 2 | 3 | 99.40 �± 0.17 | >0.05 |
| 4 | 3 | 99.49 �± 0.35 | >0.05 |
| 6 | 3 | 99.10 �± 0.17 | >0.05 |
| 8 | 3 | 98.13 �± 0.23 | <0.05 |
| 10 | 3 | 96.26 �± 0.35 | <0.05 |
| Total | 21 |
Table 1: Cytotoxicity study using 3T3 balb/c cell line: 3T3 balb/c cells were seeded in 96-well plates and treated with different concentrations of green synthesised� AgNo3 for 24 h. the percentage viability was analyzed by measuring fluorescence at� 560 nm after the addition MTT� reagent. Data were represented as mean�± standard deviation (n=3) P<0.05 were statistically significant.
In Vivo Small Animal Experiment
The small animal experiments were done after the regulatory clearance of Institutional Animal Ethics committee. Albino Wistar rats (24 nos, Male, weighing 200 ± 20 g) purchased from Tamil Nadu Veterinary and Animal Sciences University were used for in vivo studies. They were housed individually in the standard laboratory environment (CPCSEA approved animal facility 871/10/ReReBt/SL/05) for one week to acclimatize and fed ad libitum with commercial pellets and water. All the animals were anaesthetized intraperitoneally by using Ketamine (75 mg/kg) and Xylazine (20 mg/kg) before experiments.
Excised wounds
Two wound sites were chosen in each Albino Wistar rat model (6 Nos), with one on either side of the thoracolumbar spine. Each site was shaved, and a 2 cm full-thickness laceration was made at each site. Bleeding was controlled with sterile gauze pressure. 24 h after excision hybrid bandage was applied on one site and another site was used as the control.
Infected wounds
After Excision of skin, the wounds were immediately injected intradermally with 10-106 colony forming units (CFU)/ml of Pseudomonas aeruginosa and Methicillin resistant Staphylococcus aureus (MRSA). After 24 h of contamination, one side of the infected wound was treated with the hybrid bandage and the undressed other side was left as a control.
Incision wound
The pre auricular area of each animal (6 Nos) was shaved. A vertical incision through the skin was made of the masseter muscle, 1.5 cm long and extending to the lateral surface of the mandibular ramus. After the skin was reflected, an identical second incision and the third incision were made in the muscle at 3 mm interval and parallel to the first one. One side of the incision area was dressed with the hybrid bandage and the other undressed considered as control.
Infected Burn wounds
Albino Wistar rat model (6 Nos) of the full-thickness burn was used as a burn model by applying two preheated brass blocks (92-95°C) to the opposing sides of an elevated skin folder on the backs of shaved rats for 5 s. The combined brass block area was corresponding to a 5% of total body surface area (TBSA). After the infliction of burns, the scars were immediately injected intradermally with the aforementioned dilution of P. aeruginosa and MRSA. 24 h after infection the hybrid bandage was applied on one side of the burnt wound. Another side was used as a control. At the interval of every week the bandage was removed from mouse and checked for the healing process.
At the range of every week, the bandage was removed from the rats and tested for the healing process. Every week one animal from each group was euthanized using Barbiturate (100 mg/kg) and sacrificed. At 4th week all the remaining animals were sacrificed, and the healed portion of skin was explanted, fixed using 10% buffered formalin and analyzed histopathologically by Hematoxylin & Eosin stain.
Wound Healing Evaluation Parameters
Measurement of wound contraction
An excision wound margin was traced after wound creation by using transparent paper and area measured by graph paper. Wound contraction was measured in each 7 days interval, until complete wound healing and expressed in percentage of the healed wound area. The evaluated surface area was then employed to calculate the percentage of wound contraction, taking the initial size of wound, 2 cm diameter, like 100%, by using the following formula as:
![]()
Epithelialization period
It was evaluated by noting the number of days required for the Escher to fall off from the wound surface exclusive of leaving a raw wound behind.
Statistical Analysis
All treated groups were compared with the control groups. The results were analyzed statistically using one-way analysis of variance (ANOVA). The results were found to be significantly at P<0.05 All tests were conducted using SPSS Software, (version 19.0).
Results
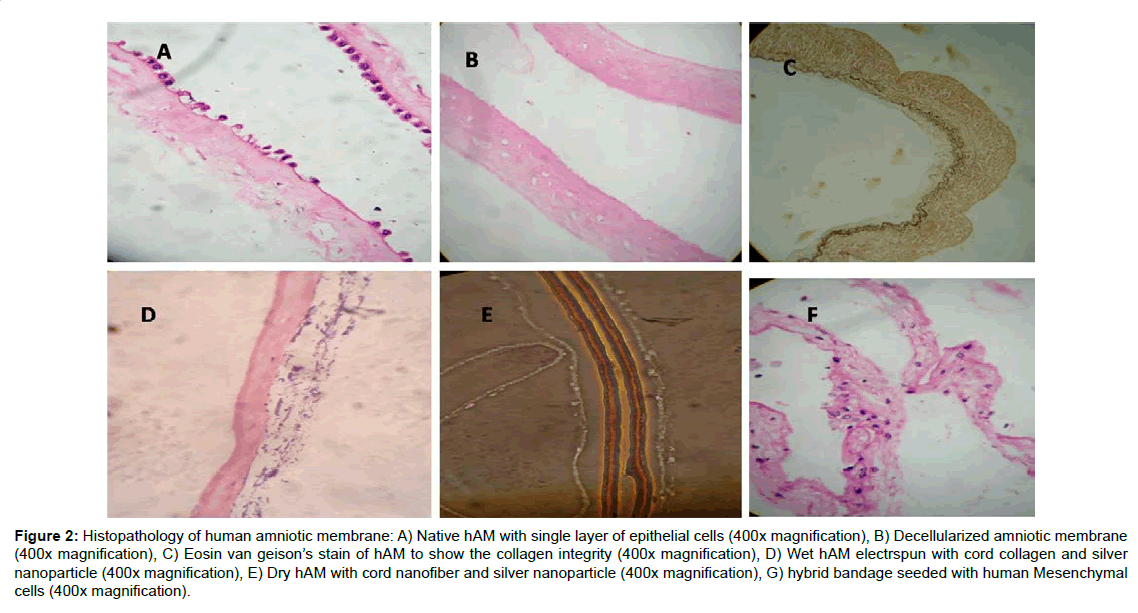
The amniotic membrane (hAM) being the innermost layer of the foetal membranes of the placenta, is avascular and consists of a cuboidal epithelial layer with a thin basement membrane followed by a sub-adjacent avascular stromal layer. The collagen rich basement layer is composed of reticular fibres that support the growth and maintenance of epithelial cells. The decellularized and electrospun hybrid hAM bandage was fixed and subjected to H&E staining (Figure 1). The Liquid nitrogen snap freezing method is the one of the efficient recellularization protocol for the removal of the single layer of epithelial cells. The collagen architecture remains unchanged even after decellularization (Figure 2). The hAM hybrid bandages have good tensile strength compared to native hAM. The tensile strength of native hAM is 5.47 ± 3.06 MPa, whereas the dried hybrid bandages have 7.96 ± 4.12 MPa. The thermal stability of hybrid bandages was analysed by Differential scanning calorimetry. The heat of denaturation has been over 120°C. Fourier transform infrared spectroscopy to assess resident collagen shows the absence of carboxyl peak in the processed amniotic membrane confirms that chemicals used during processing do not cause the significant effect on calcification or immunological effects. There was no evidence of residual HFIP. In vitro cytotoxicity experiment using 3T3 balb cell lines shows that the hybrid bandages do not posses any cytotoxicity (Supplementary Table 1).
Figure 2:Histopathology of human amniotic membrane: A) Native hAM with single layer of epithelial cells (400x magnification), B) Decellularized amniotic membrane (400x magnification), C) Eosin van geison�s stain of hAM to show the collagen integrity (400x magnification), D) Wet hAM electrspun with cord collagen and silver nanoparticle (400x magnification), E) Dry hAM with cord nanofiber and silver nanoparticle (400x magnification), G) hybrid bandage seeded with human Mesenchymal cells (400x magnification).
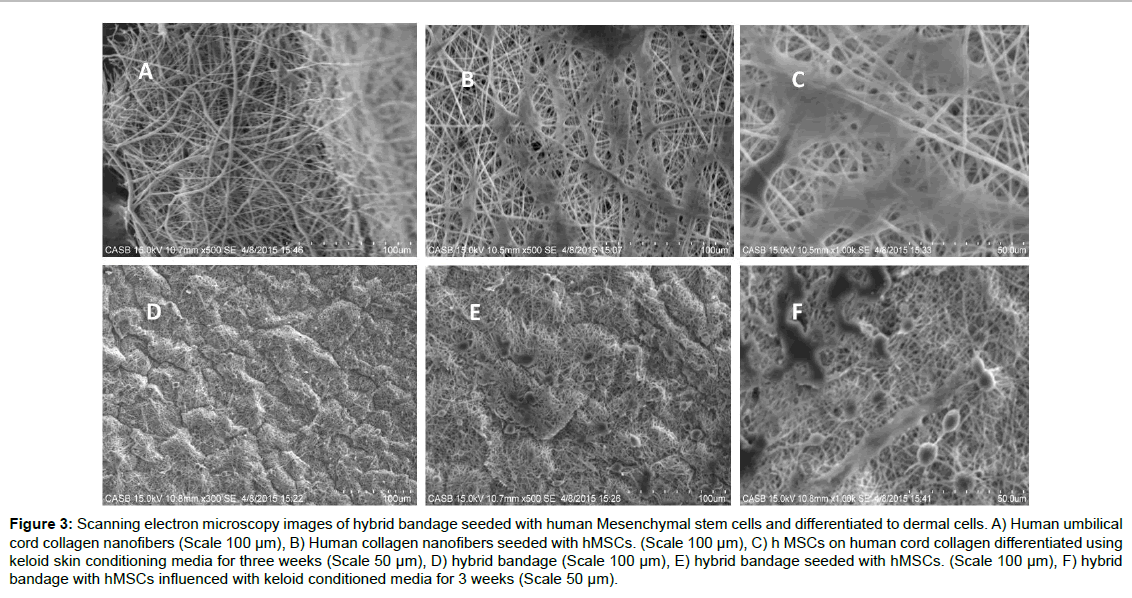
The efficacy of the hybrid bandage to differentiate Mesenchymal stem cells to skin cells (Keratinocytes or melanocytes or skin fibroblasts) was experimented by seeding cord blood derived human MSCs on the hybrid bandages and differentiating them to skin cells using novel keloid foreskin conditioned media. Electrospun nanofibers were used as the control. After 3 weeks of differentiation, the MSCs on the hybrid bandage and the MSCs seeded on nanofiber collagen were fixed and analysed using SEM (Figure 3). The cells on the scaffold were subjected to RT PCR analyses for keratocytes marker and fibroblast marker (Supplementary Table 2 and Supplementary Figure 1). This in vitro study reveals that the MSC's can readily differentiate to skin cells in the presence of keloid foreskin conditioned media.
Figure 3:Scanning electron microscopy images of hybrid bandage seeded with human Mesenchymal stem cells and differentiated to dermal cells. A) Human umbilical cord collagen nanofibers (Scale 100 �µm), B) Human collagen nanofibers seeded with hMSCs. (Scale 100 �µm), C) h MSCs on human cord collagen differentiated using keloid skin conditioning media for three weeks (Scale 50 �µm), D) hybrid bandage (Scale 100 �µm), E) hybrid bandage seeded with hMSCs. (Scale 100 �µm), F) hybrid bandage with hMSCs influenced with keloid conditioned media for 3 weeks (Scale 50 �µm).
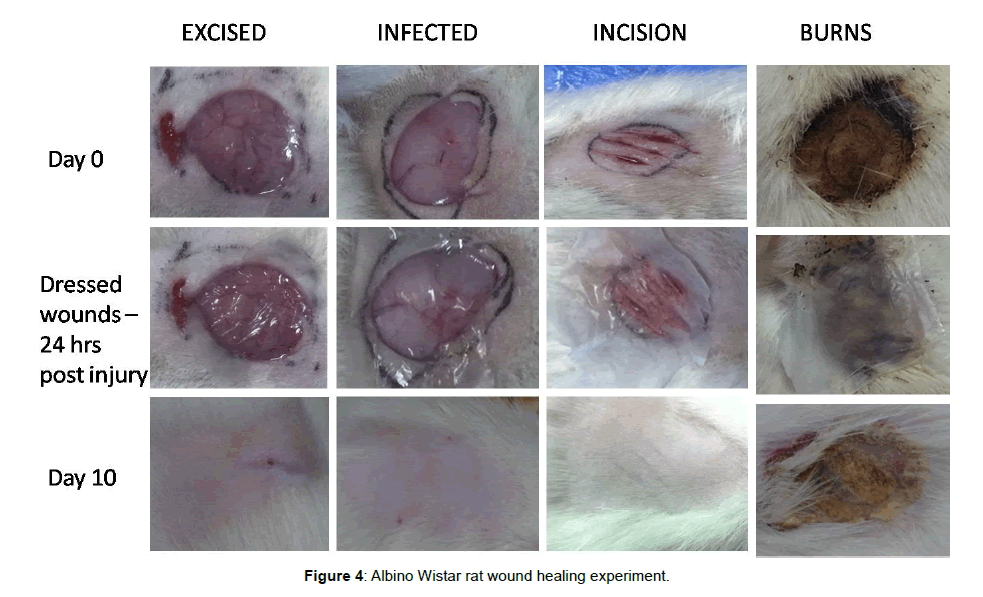
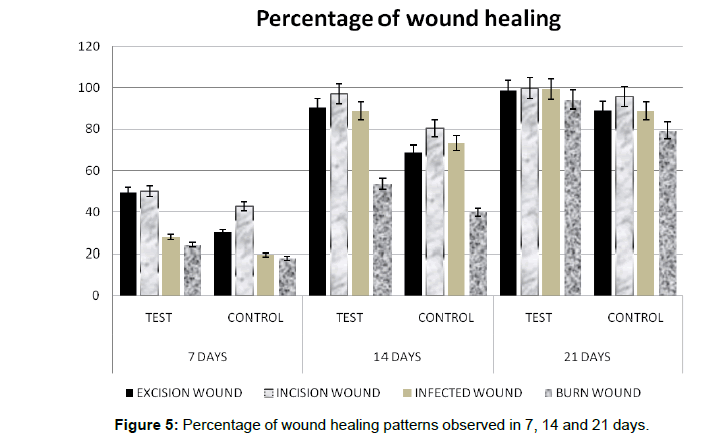
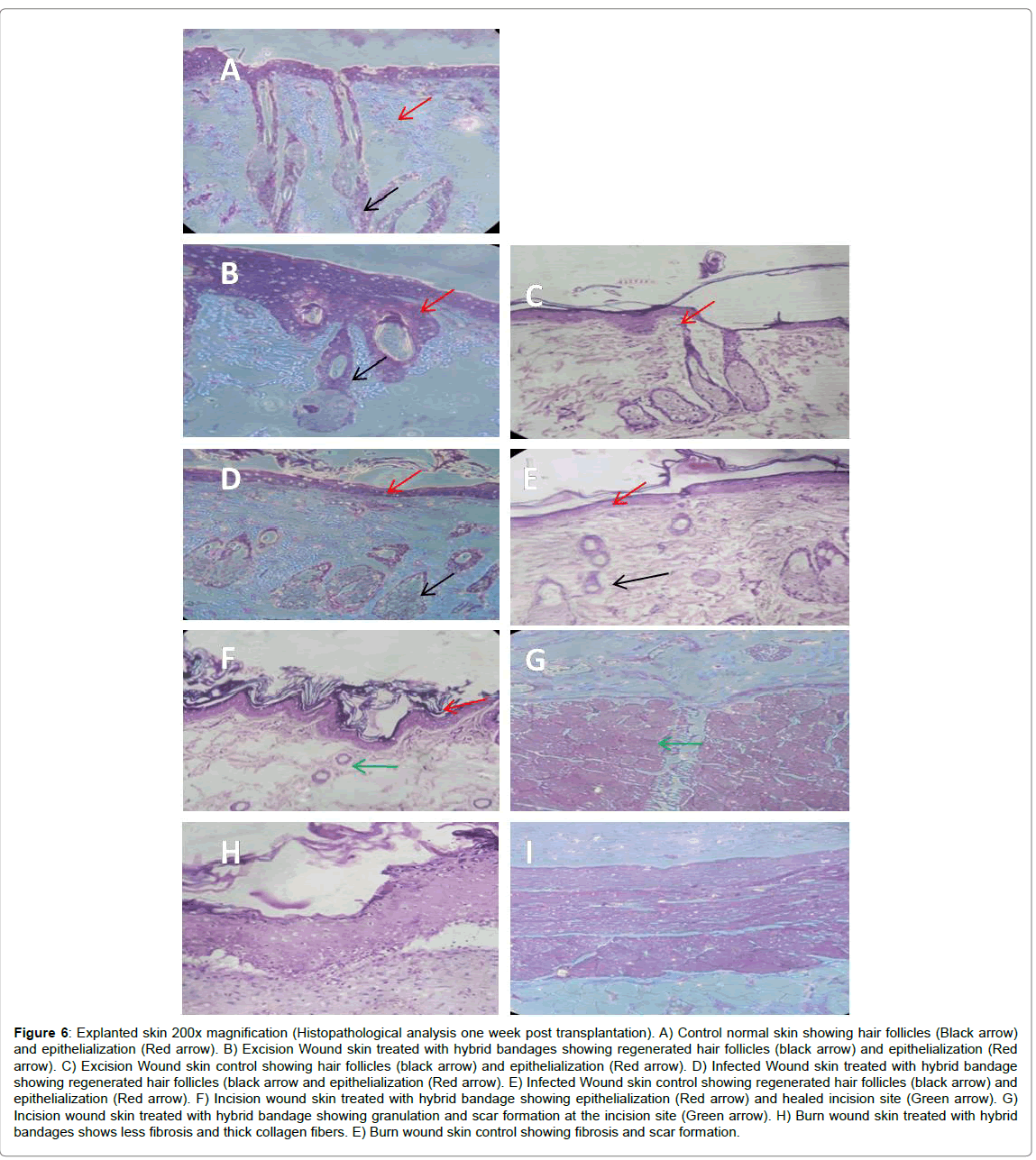
The in vivo small animal experiments in Albino Wistar rats indicates that the excision wound, infected wound and the incision wounds could be healed without any scar formation and the Escher had fallen by 10-12 days of treatment with the hybrid bandage. However, the treated burn wound Escher had fallen only at the end of the 3rd week for all the animals (Figure 4). The percentage of wound healing patterns observed in 7, 14 and 21 days are shown in Figure 5. Although the hAM is highly hydrophobic, the layer of electrospun umbilical cord collagen with porosity remains intact with wound surface and maintains the moisture. The H&E stain of the explanted skin (wounds and burns) treated with hybrid bandage reveals that the skins regenerated with newly formed hair follicles, sweat glands and epithelization in excision, infected and incision wound models. A prolonged healing process of 2-3 weeks was observed in burn wounds (Figure 6).
Figure 6:Explanted skin 200x magnification (Histopathological analysis one week post transplantation). A) Control normal skin showing hair follicles (Black arrow) and epithelialization (Red arrow). B) Excision Wound skin treated with hybrid bandages showing regenerated hair follicles (black arrow) and epithelialization (Red arrow). C) Excision Wound skin control showing hair follicles (black arrow) and epithelialization (Red arrow). D) Infected Wound skin treated with hybrid bandage showing regenerated hair follicles (black arrow and epithelialization (Red arrow). E) Infected Wound skin control showing regenerated hair follicles (black arrow) and epithelialization (Red arrow). F) Incision wound skin treated with hybrid bandage showing epithelialization (Red arrow) and healed incision site (Green arrow). G) Incision wound skin treated with hybrid bandage showing granulation and scar formation at the incision site (Green arrow). H) Burn wound skin treated with hybrid bandages shows less fibrosis and thick collagen fibers. E) Burn wound skin control showing fibrosis and scar formation.
Discussion
Wound healing is a natural process of regenerating the damaged or lost tissue. However, certain complications such as sepsis, multi resistant bioburden, biofilm, maggot formation and extension of infection to adjacent and interior organs, hamper the healing process [8]. Recently there have been great developments in wound care, with allografts, amniotic membranes, artificial dermis, and synthetic and semi-synthetic dressing materials. However, bacterial infection competes with host cells for oxygen and nutrients. Henceforth, infection raises blood cytokines and matrix metalloproteinase, reduces growth factors, causes wound hypoxia and vessel occlusions, thus delaying wound healing, while their metabolites are toxic to host cells [9].
To accelerate the healing process, a combination of growth factors has to act synergistically [10]. For wound healing treatments different combinations of growth factor supplements can be administered, however, it tends to be very expensive. Under such circumstances hAM is one of the most efficient biological dressings with myriad of growth factors, cytokines and extracellular matrix such as Collagen I, III, IV, V, VI, laminins, fibronectin, proteoglycans, etc. hAMs are used to treat burns and wounds for almost a century [11].
Thomas Koob et al. performed an Elisa assay on hAM samples and found quantifiable levels of growth factors such as platelet-derived growth factor-AA (PDGF-AA), PDGF-BB, transforming growth factor α (TGFα), TGFβ1, basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), placental growth factor (PLGF), granulocyte colony-stimulating factor (GCSF), Interleukins-4, 6, 8 and 10 and TIMP 1, 2 and 4 [12]. Anti Inflammatory effect of hAM is due to the presence of cytokines and natural metalloprotease (MMP’s) inhibitors. The Anti-scarring property of hAM is achieved with the help of tissue inhibitor of metalloproteases and TGFβ that reduce protease activity and activate fibroblasts. hAM has been reported to exert antimicrobial activity through direct contact with bacteria [13] with the presence of factors such as defensins [14] to ensure septic conditions. Our decellularization protocol with LN2 may preserve the growth factors in hAM and incorporating silver nitrate gives a better therapeutic effect than plain amniotic membranes.
In recent years with increased multi resistance in bacteria and need for low-cost products for wound management, there is a renewed interest in the usage of silver in nano dimensions in the medical field. Microbial resistance to elemental silver is exceedingly rare [15], emphasizing the presence of multiple bactericidal mechanisms acting in synergy. For active bactericidal effect, nano silver acts on 3 main bacterial components.
1. Silver ions interact with the peptidoglycan layer of the plasma membrane, altering the membrane permeability, enhancing membrane degradation and cell death and also inhibit the respiratory chain thereby limiting the formation of energy molecules such as Adenosine triphosphate [16].
2. The nano silver ion interact with sulphur-containing bacterial proteins and phosphorous containing bacterial components such as DNA, RNA, to inhibit replication [17].
3. The bacterial enzymes involved in cellular processes such as electron transport chain, glycolysis, etc., are inactivated [18].
According to Park et al. [18] the antimicrobial mechanism of silver nanoparticles is specific, favouring interaction with the peptidoglycan layer in bacterial species rather than the mammalian cells. The potency of silver as an antimicrobial was found to be related to the amount and rate of free silver released onto the wound bed [19]. The greater the ratio of surface area to volume, higher is the number of silver particles coming in contact with the open wounds and ulcers, thereby accelerating the bioactivity and silver solubility.
Silver treatment not only acts as antibacterial but also directly works on dampening the process of inflammation, thus promoting scarless wound healing. According to Tian et al. [6], silver nanoparticles can modulate local and systemic inflammatory response following burn injury by cytokine modulation. In vitro and in vivo experimental findings have shown that silver nanoparticles are effective at decreasing inflammation and initiating pro healing activity [20,21].
The eco-friendly biosynthesis of silver nanoparticles using curcumin makes them superior wound healing product with enhanced biofilm activity [22-24]. Nano silver nitrate incorporated hybrid hAM bandage increases their manageability, provides the easier application to the burned area and creates a bactericidal effect, therefore reducing the risk of contamination and infection. One of the main advantages of wound coverage with hAM hybrid bandages is that it enhances reepithelization, reduces fluid, protein, heat and energy loss.
Conclusion
The hybrid amniotic membrane with electrospun umbilical cord collagen nanofibers and eco-friendly green silver nanoparticles derived from curcumin reduction have excellent adherence property to wound, maintains a moist environment and augments epithelialization. It is known to reduce inflammation, pain and scarring while providing a natural biological barrier with a matrix for cell colonisation and regeneration. In vitro culture system using the hybrid bandage as a 3D scaffold induces the differentiation of human CBMSCs towards keratinocytes and skin fibroblast cells. This in vitro developed culture system can be utilised for validating various cosmetic products as the scaffold mimics 3D skin layers. The in vivo small animal experiments applying the dried hybrid dressing augments the wound healing process and prevents scar formation. These grafts are viable, bioabsorbable, off the shelf available and easy logistics. Therefore, hAM hybrid dressings are useful in preventing secondary infections on the wound and promoting rapid wound healing in burn and infected wound models. Cheap manufacturing and storage costs make it an ideal dressing for application, especially in countries where economic factors prevent the purchase of commercially available costly dressings.
Acknowledgement
We acknowledge the PG diploma in stem cells and tissue engineering students of Madras University, 2015 batch (Mahalekshmi, Alsa and Usha) for their help with stem cell experiments and SEM and Rochana Sashmita of Prathusa Engineering College for her help in animal experiments. I thank our lab assistant Mrs. L. Lavanya and Pathologist Dr. Sarasabarati Arumugam for their help in histopatology analysis.
Conflict of Interest
The authors declare no conflict of interest.
References
- Halim AS, Khoo TL, Mohd Yussof SJ (2010) Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg 43: S23-28.
- Bujang-Safawi E, Halim AS, Khoo TL, Dorai AA (2010) Dried irradiated human amniotic membrane as a biological dressing for facial burns: A 7 year case series. Burns 36: 876-882.
- Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G (2007) Bioengineered skin substitutes for the management of burns: A systematic review. Burns 33: 946-957.
- Hadjiiski O, Anatassov N (1996) Amniotic membranes for temporary burn coverage. Ann Burns Fire Disasters 9: 2.
- Jesada C, Uracha R, Pitt S (2015) Hybrid biomimetic electrospun fibrous mats derived from poly(e-caprolactone) and silk fibroin protein for wound dressing application. J Appl Polym Sci 132: 41653.
- Tian J, Wong KK, Ho CM, Lok CN, Yu WY, et al. (2007) Topical delivery of silver nanoparticles promotes wound healing. Chem Med Chem 2: 129-136.
- Ghahary A, Shen YJ, Scott PG, Gong Y, Tredget EE (1993) Enhanced expression of mRNA for transforming growth factor-beta, type I and type III procollagen in human post-burn hypertrophic scar tissues. J Lab Clin Med 122: 465-473.
- Templeton S (2005) Management of chronic wounds: the role of silver-containing dressings.
- Fu YC, Jin XP, Wei SM (2000) The effects on cell growth of tea polyphenols acting as a strong anti-peroxidatant and an inhibitor of apoptosis in primary cultured rat skin cells. Biomed Environ Sci 13: 170-179.
- Woodward M (2005) Silver dressings in wound healing: What is the evidence? Primary Intention 13: PP153-609
- Broeckx SY, Borena BM, Van Hecke L, Chiers K, Maes S, et al. (2015) Comparison of autologous versus allogeneic epithelial-like stem cell treatment in an in vivo equine skin wound model. Cytotherapy 17: 1434-1446.
- Borena BM, Martens A, Broeckx SY, Meyer E, Chiers K, et al. (2015) Regenerative skin wound healing in mammals: State-of-the-art on growth factor and stem cell based treatments. Cell Physiol Biochem 36: 1-23.
- Donald EF, Robert J S (2012) Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds 24: 299-307.
- Thomas JK , Robert R, Nicole Z, Michelle M, Jeremy JL, Johnna ST, William WL, Geoffrey G (2013) Biological properties of dehydrated human amnion/chorion composite graft: Implications for chronic wound healing. Int Wound J 10: 493-500.
- Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y (1991) Antibacterial properties of human amniotic membranes. Placenta 12: 285-288.
- Kjaergaard N, Helmig RB, Sch�¸nheyder HC, Uldbjerg N, Hansen ES, et al. (1999) Chorioamniotic membranes constitute a competent barrier to group b streptococcus in vitro. Eur J Obstet Gynecol Reprod Biol 83: 165-169.
- Siddhartha S, Tanmay B, Arnab R, Gajendra S, Ramachandrarao P, et al. (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnol 18: 225103-225112.
- Park HJ, Kim JY, Kim J, Lee JH, Hahn JS, et al. (2009) Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res 43: 1027-1032.
- Yang W, Shen C, Ji Q, An H, Wang J, et al. (2009) Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnol 20: 085102.
- Wright JB, Lam K, Buret AG, Olson ME, Burrell RE (2002) Early healing events in a porcine model of contaminated wounds: Effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen 10: 141-151.
- Lansdown AB (2002) Silver: Its antibacterial properties and mechanism of action. J Wound Care 11: 125-130.
- Wong KK, Cheung SO, Huang L (2009) Further evidence of the anti-inflammatory effects of silver nanoparticles. Chem Med Chem 4: 1129-1135
- Nadworny PL, Wang JF, Tredget EE, Burrell RE (2010) Anti-inflammatory activity of nanocrystalline silver-derived solutions in porcine contact dermatitis. J Inflam 7: 13.
- Ching YL, Ramin R, Paul MY , Daniela T, Rosalia C, et al. (2016) Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. J Agric Food Chem 64: 2513-2522.
- El Khoury E, Abiad M, Kassaify ZG, Patra D (2015) Green synthesis of curcumin conjugated nanosilver for the applications in nucleic acid sensing and anti-bacterial activity. Biointerfaces 127: 274-280.
- Alves TF, Chaud MV, Grotto D, Jozala AF, Pandit R, et al. (2017) Association of silver nanoparticles and curcumin solid dispersion: Antimicrobial and antioxidant properties. AAPS PharmSciTech, pp: 1-7.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 7263
- [From(publication date):
September-2017 - Aug 17, 2025] - Breakdown by view type
- HTML page views : 6107
- PDF downloads : 1156