Special Issue Article Open Access
Interaction Effect of Indole-3-Butyric Acid and �?±-Naphthalene Acetic Acid on In Vitro Rooting of Two Sugarcane (Saccharum officinarum) Genotypes
| Dereje Shimelis1*, Kassahun Bantte2and Tilaye Feyissa3 | ||
| 1Ethiopian Sugar Corporation, Research and Training Division, Variety Development Directorate, Biotechnology Research Team, Wonji Research Center, P. O. box 15, Wonji, Ethiopia | ||
| 2Jimma University College of Agriculture and Veterinary Medicine, Jimma, Ethiopia | ||
| 3Addis Abeba University, Science and Technology faculty, Addis Abeba, Ethiopia | ||
| Corresponding Author : | Dereje Shimelis Ethiopian Sugar Corporation Research and Training Division Variety Development Directorate Biotechnology Research Team Wonji Research Center P. O. box 15, Wonji, Ethiopia Tel: +25191081644 E-mail: d.shimelis@yahoo.com |
|
| Received March 31, 2015; Accepted May 01, 2015; Published May 03, 2015 | ||
| Citation: Shimelis D, Bantte K, Feyissa T (2015) Interaction Effect of Indole- 3-Butyric Acid and α-Naphthalene Acetic Acid on In Vitro Rooting of Two Sugarcane (Saccharum officinarum) Genotypes. Adv Crop Sci Tech S1:001. doi: 10.4172/2329-8863.1000S1-001 | ||
| Copyright: ©2015 Shimelis D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
In Ethiopia, sugarcane is being grown as an important cash and industrial crop among many crops and plays an important role for the development of the economy of the country. Improvement of this crop through conventional breeding takes 8 to 10 years and its propagation by cutting takes 6 to 7 years for commercial scale. During these periods of years the crop might starts to detiorarte genetically by biotic and abiotic factors. To solve the limitations, tissue culture (micropropagation) was born as best alternative. Once millions of shootlets multiplied in vitro by micropropagation, they should have roots to be transplanted to the field. Hence, this research was launched to optimize appropriate concentration of IBA (0, 1, 2 and 3) mgl-1 and NAA (0, 1, 2, 3, 4 and 5) mgl-1 combinations for root induction of C86-12 and C86-56 genotypes in completely randomized design with 4×6×2 factorial treatment combinations arrangements. The analysis of variance showed that the interaction effects of concentrations of IBA and NAA combinations and genotypes were highly significant (p=0.001) for mean number of roots per shoot and mean root length.1/2 MS medium supplemented with 2 mgl-1 IBA+1 mgl-1 NAA for C86-56 has gave best number of roots (14.88) but best root length (3.14 cm) was obtained on ½ MS+3 mgl-1 IBA+4 mgl-1 NAA. For C86-12 genotype 5 mgl-1 NAA alone has gave best number of roots (17.8) and root length (3.2 cm). Out 100 Plantlets taken for acclimatization from best media of root induction, 87% for C86-12 and 93% for C86-56 were survived. Thus these media combinations are the best media for induction of roots after multiplication stage of micropropagation for these genotypes.
| Keywords | |
| Sugarcane; Indole-3-Butyric Acid (IBA); α-Naphthalene Acetic Acid (NAA); Root induction | |
| Introduction | |
| Sugarcane belongs to the genus Saccharum officinarum L., of the tribe Andropogoneae in the grass family (Poaceae). This tribe includes tropical and subtropical grasses including the cereals like Sorghum and Corn with an octaploid 2n=8x=80 number of chromosome [1]. It is a perennial tropical grass that tillers at the base to produce unbranched stems of 3- 4 m length or more with a thickness of approximately 5 cm in diameter [2]. The plant was domesticated by the Polynesians for its sweet stem [3]. The commercially cultivated sugarcane was originated from New Guinea and Northern India [4]. | |
| Sugarcane is C4 plant of the most efficient converters of solar energy into sugars and other renewable forms of energy and stores in its internode [5]. It accounts for approximately 75% of the world’s sugar and is economically important cash crop in tropical and sub-tropical regions of many countries [5,6]. It is cultivated as a commercial crop in nearly 60 countries spread over 5 continents and Brazil and India are the largest one [7]. Beside to white sugar, it is being used as a source of other products like animal feed, antibiotics, particle board, biofertilizer and raw material for generating electricity and furthermore it is lately emerged as an important base material for bio-ethanol production [5]. | |
| ¨In Ethiopia, sugarcane is being grown as an important cash and industrial crop among many crops and plays an important role for the development of the economy of the country. It is used for the production of white and brown sugar and bi-products like molasses, bagasse and press mud (filter cake) and no bi-product is thrown as non- useful matter. Molasses is a chief bi-product and is the main raw material for the production of drinking alcohol after distillation in Ethiopian Ethanol alcohol and liquor factories. Ethanol is also used as source of energy by mixing with benzene to reduce the cost of increasing oil prices and to make environment friendly fuel. Moreover, molasses can be used for making asphalt roads as a replacement of range. Bagasse is the fiber part of the sugarcane and used for cogeneration of electric power for the factory as well for the communities around the factory, for manufacturing paper and particle board. Filter cake contains considerable amounts of plant nutrients [8], so that it has been used as source of bio-fertilizer which reduces the cost incurred for chemical fertilizers and toxic residues from the soil. Furthermore, in Ethiopia the sugar factories are creating job opportunities, and trying to full fill the domestic demand of sugar and fuel. | |
| Sugarcane is a highly poly-aneuploid crop, is impeded by its narrow gene pool, complex genome, poor fertility and long selection cycle makes the conventional breeding difficult for this crop [9]. Conventional breeding of this crop takes 8 to 10 years to release new improved variety [4,10]. The planting material of the released variety is so limited; hence, conventional vegetative propagation of sugarcane takes 6-7 years to commercialize, by the time it might starts to detoriate by biotic and abiotic factors [4,11]. This long duration causes major bottleneck in conventional breeding programmes [12]. The planting materials (setts)are prepared when the age of sugarcane reaches 7 to 10 months and one hector of nursery can plant 10 to 15 hectors of land if the setts are with two to three buds respectively [5,13]. Which means large area of nursery is mandatory to plant the planned new and the expansion projects of Ethiopia Sugar Corporation. The other disadvantage of vegetative propagation of sugarcane is the accumulation and transmission of diseases and pests over vegetative cycles which leads to further yield and quality reduction [5,13]. | |
| To overcome the problems of conventional breeding and vegetative propagation of sugarcane, biotechnological tool (plant tissue culture) was born as a best alternative to intervene the challenges existing in Ethiopian sugar production. Advantages of this technology lies in the production of high quality, uniform and disease free planting materials that can be multiplied on a year-round basis anywhere irrespective of the season and weather and it also used to rejuvenate old varieties of sugarcane. Furthermore it saves the time needed to release and commercialize newly improved variety and the planting materials needed per hector. This technology is being used by India, Pakistan, Australia and etc [5]. So far in Ethiopia, new released varieties have been imported and propagated for commercial scale by vegetative propagation method. But now the country is already started to propagate enough planting material by in vitro micropropagation technology. Once, the required number of shoots produced, root induction is a must to enable the plantlets to absorb water and nutrients for further growth and development in the field. Usually auxins (IBA and NAA) are the common plant growth regulators used for in vitro root induction of many plants. Hence, this research was done to optimize an appropriate concentration of IBA and NAA combination to induce roots on the produced shoots of sugarcane genotypes. | |
| Materials and Methods | |
| The experiment was conducted at Plant Tissue Culture Laboratory of Jimma University College of Agriculture and Veterinary Medicine (JUCAVM), Ethiopia. Two sugarcane genotypes (C86-12 and C86- 56) were used for the study. They were imported from Cuba in 2006 and passed through agronomic performance evaluation. They were among the selected ones to be commercialized. The setts of these genotypes were prepared and treated with hot water. The setts were taken to JUCAVM green house and planted. After two to three months of growing, shoot tip explants were taken from the sugarcane plants. The explants were prepared by the procedure described by Jalaja [5]. They were washed under running tap water and liquid detergents. They were socked in fungicide solution (0.3% kocid) for 30 minutes under laminar flow cabinet containing three drops of tween-20. After the kocid was properly washed off from the explants, they were rinsed three times with distilled water and disinfected with 70% of ethanol for one minute. The ethanol was poured off and the explants were rinsed again with sterile distilled water. Disinfection of explants was done with 0.1% of HgCl2 for 10 minutes [2] followed by 3-4 washing with sterile distilled water. The required amounts of all stock solutions of ½ MS media (macro and micro nutrients, vitamins, amino acids, agar [14] and 60 gL-1 [11] sucrose and combinations of different concentrations of IBA and NAA were mixed in a beaker and the pH was adjusted to be 5.8. This was followed by addition of 0.8% agar for solidifying the media. Then, it was heated to melt the agar and 30 ml media was dispensed in to culture jars. Finally, it was autoclaved at temperature of 121°C for 20 minutes with 15 psi of pressure. | |
| Initiated explants were multiplied and uniform micro-shoots of about 5-6 cm length of the two genotypes were excised from culture jars. They were washed by warm water and cultured on ½ MS medium under laminar flow hood aseptically. The cultures were transferred to the growth room at which the growth conditions were adjusted to be 16 hours of photoperiod with 25 μmolm−2 s−1 photosynthetic photon flux intensity and 26 ± 2°C of temperature. The experiment was laid down in factorial treatment combination in complete randomized design with factorial treatment combination arrangements. Five micro shoots were cultured in a jar and each of treatment was replicated three times and per a treatment there were 15 micro shoots. Data on number of roots per shoot and root length were collected after 30 days of culturing. Uniform sizes of 100 plantlets of each genotype were acclimatized in greenhouse on 1:1:1 ratio of sand, soil and cow dung and data of survival percentage was recorded. Finally data were subjected to analysis of variance (ANOVA) using SAS statistical software version 9.2 and treatments’ means were separated by using REGWQ (Ryan- Einot-Gabreil-Welsch Multiple range test) mean separation method. | |
| Results | |
| The analysis of variance showed that the interaction effects of concentrations of IBA and NAA combinations and genotypes were highly significant (p=0.001) for mean number of roots per shoot and mean root length. Both genotypes exhibited a significant variation both in the mean number of roots per shoot and mean root length. This because it is a general truth that different genotype of the same species may have different response to a media. No rooting was observed in the absence of plant growth regulators (Table 1). Similar results were reported by Tilahun [15] on half MS medium without PGRs. | |
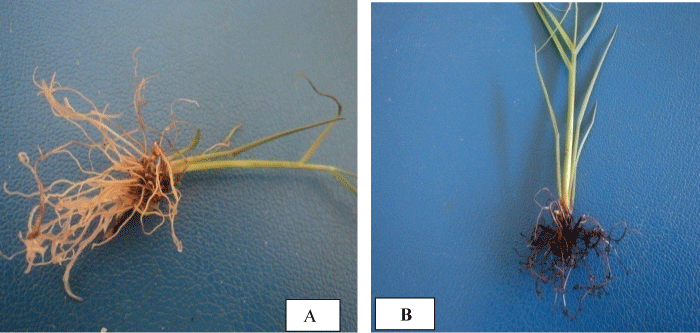
| The highest mean number of roots per shoot was recorded on ½ MS medium supplemented with 0.0 mgl-1 IBA+5 mgl-1 NAA for C86- 12; 2 mgl-1 IBA+1 mgl-1 NAA, 2 mgl-1 IBA+4 mgl-1 NAA and 3 mgl-1 IBA+3 mgl-1 NAA for C86-56. ½ MS medium supplemented with 5 mgl-1 NAA+60 gl-1 sucrose resulted in 17.5 ± 0.00 mean number of roots per shoot and 3.2 ± 0.223 cm mean root length in C86-12 (Table 1 and Figure 1A) but it only gave 7.76 ± 0.750 mean number of roots per shoot with 0.84 ± 0.055 cm mean root length and ½ MS+3 mgl-1 IBA+4 mgl-1 NAA is best media that gave best root length (3.14 ± 0.167 cm ) for C86-56. Though all the three media gave statistically insignificant mean number of roots per shoot in C86-56 genotypes, ½ MS medium supplemented with 2 mgl-1 IBA+1 mgl-1 NAA gave relatively better mean number of roots per shoot (14.88 ± 0.164) and root length (2.80 ± 0.02 cm ) (Table 1 and Figure 1B). However this medium combination resulted in only 11.80 ± 0.570 mean number of roots per shoot and 2.46 ± 0.057 cm mean root length in C86-12. | |
| Increasing the concentration of NAA from 0.0 mgl-1 to 5 mgl-1 in the absence of IBA showed a significant increase in both the mean number of roots per shoot and mean root length from 0.00 ± 0.00 to 17.5 ± 0.00 and 0.00 ± 0.00 cm to 3.2 ± 0.223 cm in C86-12 respectively. For C86-56, the same trend holds true in the root length (0.0 ± 0.0 to 0.84 ± 0.055) but the mean number of roots per shoot increased discontinuously from 0.0 ± 0.0 to 7.76 ± 0.750. Similarly increasing the concentration of NAA from 0.0 mgl-1 to 1 mgl-1 at 2 mgl-1 IBA significantly increased the mean number of roots per shoot and mean root length from 8.22 ± 0.044 to 14.88 ± 0.164 and from 1.36 ± 0.089 cm to 2.80 ± 0.02 cm respectively for C86-56. Increasing the concentration of IBA from 0.0 mgl-1 to 2 mgl-1 with absence of NAA, the mean number of roots per shoot increased from 0.0 ± 0.0 to 11.56 ± 0.966 for C86-12 and from 0.0 ± 0.0 to 8.22 ± 0.044 in C86-56 but increasing the concentration of IBA beyond 2 mgl-1 showed reduction in number of roots per shoot for both genotypes. | |
| Many authors recommend that combination of IBA and NAA are promising for root initiation and elongation of sugarcane. Nadgauda reported 5.0 mgl-1 of NAA or combination of NAA and IBA were good for rooting in sugarcane [16]. Pruski et al. also found that the combinations of IBA and NAA were best for root induction of sugarcane [17]. The current result for the C86-12 genotype is in agreement with result reported by Baksha R who had got 15 ± 0.5 mean number of roots per shoot and mean root length of 4 ± 0.5 cm after seven days of culturing on ½ MS medium supplemented with 5 mgl-1 NAA [18]. A similar result also reported by Singh B profuse rooting of shoots were achieved on ½ MS+5 mgl-1 NAA+60 gl-1 sucrose within 30-40 days of culturing [19]. It also agrees with result reported by Ramanand et al. that showed ½ MS medium supplemented with 5 mgl-1 NAA along with 50 gl-1 sucrose proved to be the best combination for rooting in CoS 96268 and CoS 95255 genotypes [20]. | |
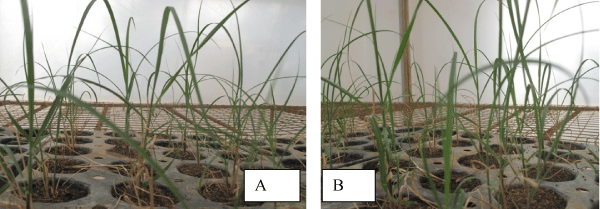
| The best result obtained in C86-56 genotype is contradictory with of Ali [13] who had found a mean root number per shoot of 4.0 ± 0.346 from CP 77,400 after seven days of culturing and 3.4 ± 0.289 from BL-4 genotype after 6 days of culturing on full MS medium supplemented with 2 mgl-1 NAA alone. This might be due to the number of days of culturing, the concentration of medium and genotypes used. From 100 plantlets taken for acclimatization stage, 87% for C86-12 and 93% for C86-56 were survived Figure 2A and Figure 2B respectively. | |
| Conclusion | |
| From the research we can infer that the two genotypes of sugarcane need different concentrations of IBA and NAA combinations for optimum in vitro root induction. Hence, ½ MS medium supplemented with 5 mgl-1 NAA alone for C86 and ½ MS medium with 2 mgl-1 IBA+1 mgl-1 NAA for C86-56 are the best media combination for root induction. | |
| Acknowledgement | |
| First and for most I would like to gratitude Ethiopian Sugar Corporation for funding the research budget and Jimma University College of Agriculture and Veterinary Medicine for facilitating the plant tissue culture laboratory. Lastly I would say thank you my God for all things that you gave me and you are my only trust! | |
| References | |
References
- id="Reference_Titile_Link" value="1">Ather A, Khan S, Rehman A, Nazir M (2009) Optimization of the Protocols for Callus induction, Regeneration and Acclimatization of sugarcane cv thatta-10. Pak J Bot 41: 815-820.
- id="Reference_Titile_Link" value="2">Bisht SS, Routray Ak, Mishra R (2011) Rapid in vitro propagation techniques for sugarcane variety 018. Iipbs 2:0975-6299.
- id="Reference_Titile_Link" value="3">Brandes EW (1958) Origin classification and characteristics. In: Artschwager E, Brandes EW (eds.) Sugarcane (Saccharum officinarum L). US Dept Agric Hand book 122 pp. 1-35.
- id="Reference_Titile_Link" value="4">Sengar RS, Sengar K, Garg SK (2011) Role of tissue culture technique in high sugarcane production. Life Sciences leaflets 21:1008-1017.
- id="Reference_Titile_Link" value="5">Jalaja NC, Neelamathi D, Sreenivasan TV (2008) Micropropagation for quality seed Production in sugarcane in Asia and the Pacific. APCOAB:13-60.
- id="Reference_Titile_Link" value="6">Pandey RN, Rastogi J, Sharma ML, Singh K (2011) Technologies for Cost Reduction in Sugarcane Micropropagation. African Journal of Biotechnology 10:7814-7819.
- id="Reference_Titile_Link" value="7">Viera (2002) Genetic instability of sugarcane plants derived from meristem cultures. Genetic Molecular Biology 25: 91-96.
- id="Reference_Titile_Link" value="8">Sundara B (2000) Sugarcane cultivation. Vikas Publishing house Pvt Ltd, New Delhi.
- id="Reference_Titile_Link" value="9">Manickavasagam M, Ganpati A, Anbazhagan VR, Sudhakar B, Selvaraj N, et al. (2004) Agrobacterium mediated genetic transformation and development herbicide resistant sugarcane (Saccharum species hybrids) using auxiliary buds. Plant Cell Rep 23: 134-43.
- id="Reference_Titile_Link" value="10">Cox M, Hogarth M, Smith G (2000) Cane breeding and improvement,Manual of cane growing, Bureau of sugar Experimental Stations, Indooroopilly, Australia.
- id="Reference_Titile_Link" value="11">Khan SA, Rashid A, Chaudhary MF, Chaudhry Z, Afroz A (2008) Rapid micropropagation of three elite Sugarcane (Saccharum officinarum L) varieties by shoot tip Culture. African Journal of Biotechnology 7:2174-2180.
- id="Reference_Titile_Link" value="12">Siddiqui SH, Khan EA, Kahtri A, Nizamani GS (1994) Rapid multiplication of sugarcane through micropropagation. Pakistan J Agric Res 15: 134-136.
- id="Reference_Titile_Link" value="13">Ali A, Naz S, Siddiqui FA, Iqbal J (2008) An Efficient Protocol for Large Scale Production of Sugarcane through Micropropagation. Pak J Bot 40: 139-149.
- id="Reference_Titile_Link" value="14">Murashige T, Skoog F (1962) A Revised Medium for Rapid Growth and Bioassay with Tobacco Cultures. Physiol Plant 15: 473-479.
- id="Reference_Titile_Link" value="15">Tilahun M (2011) Protocol Optimization for In Vitro Mass Propagation of Two Sugarcane (Saccharum officinarum L) Clones Grown in Ethiopia. An MSc Thesis Presented to School of Graduates Studies of Jimma University College of Agriculture and Veterinary Medicine.
- id="Reference_Titile_Link" value="16">Nadgauda RS (2002) Need for tissue culture, Tissue culture pilot plant, National Chemical Laboratory.Pure pp: 1-4.
- id="Reference_Titile_Link" value="17">Pruski KT, Astatkie, Nowak J (2005) Tissue culture propagation of Mngolian cherry (Prunus fruticosa) and Nanking cherry (Prunus tomentosa). Plant Cell Tiss and Org Cul 82: 207-211.
- id="Reference_Titile_Link" value="18">Baksha R, Alam R, Karim MZ, Paul SK, Hossain MA (2002) In vitro Shoot Tip Culture of Sugar-cane (Saccharium officinarum) Variety Isd. Biotechnology 1: 67-72.
- id="Reference_Titile_Link" value="19">Singh B, Yadar GC, Lal M (2001) An Efficient Protocol for Micropropagation of Sugarcane Using Shoot Tip. Sugar Tech 3:113-116.
- id="Reference_Titile_Link" value="20">Ramanand LM, Singh SB, Singh VP (2007) Optimization of rooting in micropropagated shoots of sugarcane. Sugar Tech 9: 95-97.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 14383
- [From(publication date):
specialissue-2015 - Jul 30, 2025] - Breakdown by view type
- HTML page views : 9710
- PDF downloads : 4673
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
