MUC1 and C-Met Affect Proliferation, Intercellular Junctions and Invasion in Two Head and Neck Carcinoma Cell Lines
Received: 10-Nov-2017 / Accepted Date: 23-Nov-2017 / Published Date: 27-Nov-2017 DOI: 10.4172/2161-0681.1000326
Abstract
Role of metalloproteases and adhesion molecules has been studied in cancer and metastases; tyrosine kinase receptors (TKRs) and mucins are related to their expression.
Objective: To investigate the effects of MUC1/c-Met, and their participation in metastatic mechanism in head and neck carcinoma cell lines.
Materials and methods: Lines Cal27 and A253 from squamous cell carcinoma and submaxillary gland carcinoma were treated with SU11274 (c-Met inhibitor) and GO-201 (MUC1 inhibitor) and evaluated by western blot and immunocytochemistry with anti-claudin 1, 3, and 9, integrin-αVβ1, E-cadherin, MMP2, MMP9, TIMP1 and TIMP2 after inhibition. MMPs’ activity was assessed by zymography.
Results: Claudins were atypically located in the cytoplasm and nucleus and their expression is differentially modified. Migration and invasion rate were affected by inhibition. MMP9 activity was affected.
Conclusion: Our results suggest that the role of regulating MUC1 and c-Met is related to invasion mechanisms by dysregulation of claudins and MMPs activity.
Keywords: Head and neck carcinoma; Invasion; c-Met; MUC1; Claudins
Introduction
Head and neck cancers are a group of neoplasms that start within the mouth, nose, throat, larynx, sinuses, or salivary glands. Of them, oral squamous cell carcinoma (OSCC) is the most frequent accounting for approximately 95% [1]. OSCC is usually diagnosed at a late stage of the disease with locally invasive tumor and regional lymph node metastasis [2]. Salivary gland malignancies comprise 3 to 6% of all head and neck cancers [3]. These neoplasms have varying histology and diverse biologic behaviors that impose a significant challenge on the management of malignancies overall [2].
There are many molecules implicated in cancer development, such as tyrosine kinase receptors (TKRs), adhesion molecules, growth factors, and mucins. c-Met, a transmembrane TKR, is activated upon the binding of the hepatocyte growth factor via phosphorylation of its tyrosine kinase domain [4], resulting in cell motility and proliferation. This activation also promotes tumor progression, invasion, and metastasis in cancer patients [5,6]; its correlation with poor prognosis has been reported in lung, breast, and head and neck cancers [7-14]. Likewise, mucins (high molecular weight glycoproteins) are implicated in cancer progression. MUC1 for example is a prognostic indicator in gastric and colorectal cancer and a marker of progression and metastasis [6]. Inhibition of MUC1 affects its oligomerization domain and signaling in prostate cancer, lung adenocarcinoma, and breast cancer [15-17]. Interaction of MUC1/c-Met has been associated with high motility and invasion in pancreatic adenocarcinoma [18]. Although, this relation has been studied in several carcinomas, the effect on adhesion molecules and metalloproteinases is yet poorly understood.
Loss of cell–cell adhesion is one of the steps in the progression to metastasis. At least three main families of tight junction proteins have been associated with this process: occludin, claudin, and junction adhesion (TJ) molecules [19]. Some TJ proteins are involved in the regulation of cell proliferation and several claudins exhibit abnormal expression in human cancers [20]. Understanding the processes by which tumor cells invade and metastasize to distant sites is one of the great challenges in cancer research, as metastatic spread is responsible for 90% of cancer-related mortality [21]. One mechanism occurs through their ability to recruit signaling proteins; it has been shown that stimulation by growth factors (epidermal growth factor and transforming growth factor-β) and β-catenin/Tcf signaling regulate the expression of various claudins [22]. Alteration of tight junctions and, therefore, the expression and localization of claudins are related to the malignant cell transformation. Loss of claudins and other TJs has been interpreted as a depleting mechanism for cell adhesion and plays a significant role in metastasis progression [22]. E-cadherin expression or its cell surface localization is often lost in advanced tumors and to a higher incidence of metastasis and tumor recurrence. The loss of Ecadherin and the resulting suppression or weakening of cell–cell adhesion has been regarded as a crucial step in the epithelial– mesenchymal transition (EMT) process [23]. EMT is the coordinated destabilization of cell–cell contacts, and the acquisition of a more migratory and invasive mesenchymal phenotype, with the corresponding changes in gene expression patterns, are some relevant events in the metastatic spread of tumor cells [21]. Moreover, the spread of malignant tumors requires degradation or breakdown of the extracellular matrix and connective tissue surrounding tumor cells [24]. For this step, matrix metalloproteinases (MMPs) involved in degradation of various components of the extracellular matrix are required. Some studies have suggested a major role for MMP2 and 9 in the digestion of basement membrane, as an important mechanism for invasion and metastasis in some cancers [25].
We used HNSCC A253 and Cal27 cell lines in this study to understand changes during pharmacologic treatment with inhibitors of the functional activity of MUC1 and c-Met. A253 has been used in xerographs as a well-differentiated squamous cell carcinoma line and with characteristics of therapy resistance [26] and Cal27, which has a G3 differentiation grade. These cell lines differ in their morphological, immunogenicity, growth, and resistance to cytotoxic drugs characteristics. In addition, because of their proven invasive capacity [27,28], they were used in our study to assess the invasion and migration, as well as the expression of MUC1 and c-Met-mediated adhesion molecules. According to our knowledge this is the first report using these inhibitors on Cal27 and A253, both sourced from head and neck neoplasms.
Methods
Chemicals and antibodies
Trypsin-EDTA, DMEM, McCoy medium, SU11274 (c-Met inhibitor), and GO-201 (MUC1 inhibitor) were purchased from Sigma-Aldrich (St Louis, MO, USA); fetal bovine serum (FBS) was purchased from Byproductos (Guadalajara, Jalisco, MEX); 3-(4, 5- dimethyl-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) from Roche (Roche Diagnostics Inc., USA). Antibodies: IgG rabbit polyclonal anti- MMP2, MMP9, TIMP1, TIMP2, Cldn1, Cldn3, E-cadherin, integrin- αVβ1, and IgG mouse monoclonal anti-Cldn9 were purchased from Genetex (Concord, CA, USA); IgG rabbit polyclonal anti-p-Met Y1234 and anti-MUC1 from Abcam (Cambridge, MA. USA), and IgG mouse monoclonal anti-βactin antibody from Santa Cruz Biotechnology (Dallas, Texas, USA).
Cell culture and treatment
Human head and neck cancer cell lines: CAL 27 cells (squamous cell carcinoma of tongue, differentiation grade-G3, ATTC CRL-2095) were established from a lesion of the middle of the tongue [25] and A253 cells were from a submaxillary salivary gland carcinoma (ATCC HTB-41). The cells were grown in DMEM and McCoy medium, respectively, and supplemented with 10% fetal bovine serum, penicillin G (60 mg/L), and streptomycin (100 mg/L) at 37°C in a humidified atmosphere of 5% CO2.
Cell viability assay (MTT)
An amount of 2 × 103 cells was seeded in 96-well chambers until a confluence of 80%. The cells were treated with SU11274 (c-Met inhibitor) and GO-201 (MUC1 inhibitor) at concentrations of 1-100 μM for 24 h. After that time, 10 μL of MTT was added to each well and incubated for 4 h at 37°C in a humid atmosphere. Subsequently, 100 μL of solubilizing solution was added and, after 24 h, cell viability was evaluated in an ELISA microplate reader with a reference of 550-600 nm.
Immunocytochemistry
In a Nunc™ Lab-Tek™ II Chamber Slide™ System, cells were seeded and treated with pharmacological inhibitors at a concentration of 10 μM. Cells were fixed with formalin. After blocking with 5% bovine serum albumin for 1 h, cells were incubated with anti claudins-1, 3, and 9, metalloproteinases 2 and 9, and E-cadherin (Genetex Concord, CA, USA) (1:100) overnight at 4°C. They were then incubated with the Multilink secondary antibodies system (Dako, Denmark A/S, Glostrup Denmark) for 1 h at room temperature, mounted, and visualized with an Olympus microscope DX40.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
The preparation of cell lysates was performed with total extracts prepared using CelLytic MT obtained from Sigma-Aldrich (St Louis, MO, USA) according to manufacturer's instructions. Samples were applied to SDS-PAGE and blotted onto a PVDF membrane. Following the transfer, the membranes were blocked with 5% bovine serum albumin for 1 h at room temperature, then incubated with each primary antibody (1:500 dilution) at 4°C overnight, followed by incubation with an HRP-conjugated IgG secondary antibody (1:1000 dilution) at room temperature for 1 h. Membranes were visualized using the enhanced chemiluminescent (ECL) detection method (ECL Plus Western Blotting Detection System; Amersham Biosciences, Foster City, CA, USA) according to manufacturer’s instructions and finally, exposed to film. The film was scanned for densitometric analysis using my Image Analysis Software (Thermo Fisher Scientific Inc. Wyman Street, Waltham, MA USA) and values were normalized to the densitometric values of actin in each sample. Fold change in protein amount was calculated for the experimental sets and compared to the control.
Gelatin zymography
MMP2 and MMP9 gelatinase activities were determined by gelatin zymography. Briefly, Cal27 and A253 cells were treated with concentrations of 10 μM of SU11274 and GO-201 for 24 h.
Then, the medium was collected and mixed with nonreducing sample buffer, and resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) in the presence of 1 mg/mL gelatin.
The resulting gel was washed in 10 mM Tris (pH 8.0) containing 2.5% Triton X-100, and incubated overnight in a reaction buffer at 37°C; staining with Coomassie brilliant blue and destaining until gelatinases were identifiable as clear bands.
Cell invasion assay
The cell invasion capabilities of cell lines were determined using an EMC invasion assay (Merck Millipore, Darmstadt, Germany). In total, 3 × 105 cells were seeded onto the top of each of the inserts in serumfree medium with and without inhibitors as control. An equal volume of the same medium containing 10% FBS was placed in the lower chamber (the well beneath the filter) to act as a chemoattractant. The assay plate was incubated at 37˚C for 24 h. Cells/media were removed from the top side of the insert, and the invasion chamber was placed into a clean well containing cell detachment solution. After 30 min, cells were dislodged from the underside, tilting the invasion chamber and incubating with CyQuant GR dye with lysis buffer. Finally, we read with a fluorescent plate reader using a 480/520 nm filter.
Cell migration assay
Cal27 and A253 cells were seeded into 6-well plates and incubated until confluence. To perform wound-healing assays, cell monolayers were manually wounded by scraping with pipette tips. After different treatments, the cells were permitted to migrate into the denuded areas and photographed immediately after wounding (0 h) and at 6, 12, or 24 h after wounding with and without treatment. The distance between the two edges of a denuded area was quantified in triplicate and repeated independently thrice. Migration is represented as the percentage of cell migration and was plotted on a graph.
Statistics
Results are presented as means ± SD. Differences between groups were analyzed with a one-way analysis of variance, and corrections for multiple comparisons were made using Tukey's multiple comparison test. Comparisons between two groups were made using the Student's t test. Significant differences were assumed at P<0.05.
Results
Inhibition of MUC1 and c-Met
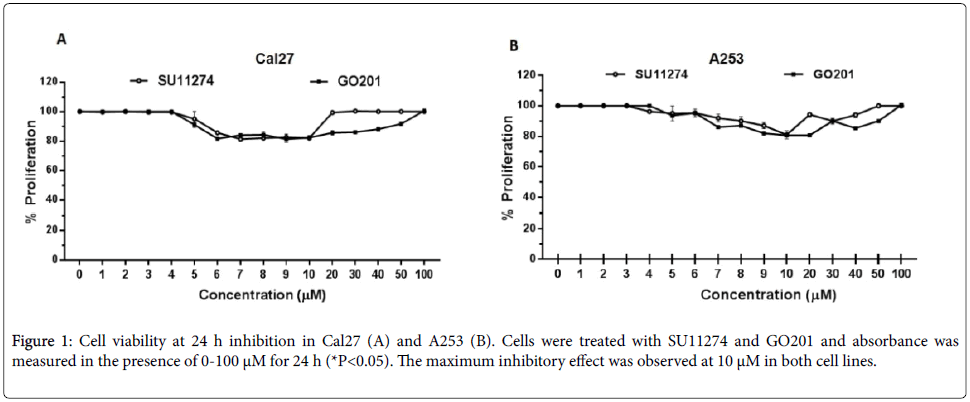
Cal27 and A253 cells were treated with 0-100 μM of SU11274 and GO201 for 24 h and the growth-inhibitory effects were measured. As shown in Figure 1, the maximum inhibitory effect was observed at 10 μM. Then, cells were treated with SU11274 and GO201 for 24 h and the inhibition of MUC1 and c-Met was observed by western blot. Cells treated with SU11274 reduced the phosphorylation status of c-Met in P-Y1234 as observed in Figure 2A. As shown in Figure 2B, cells treated with GO201 reduced the expression of MUC1CT after treatment with 10 μM.
Figure 2: c-Met and MUC1 expression after 24 h inhibition. Cells were treated with 10 μM SU11274 and GO201 and incubated with anti-Met, p-Met Y1349, and anti-MUC1-CT. As shown in (A), after treatment with SU11274, reduction of phosphorylation was observed. (B) MUC1 expression was reduced after GO201 treatment.
Effect of c-Met and MUC1 on junction molecules
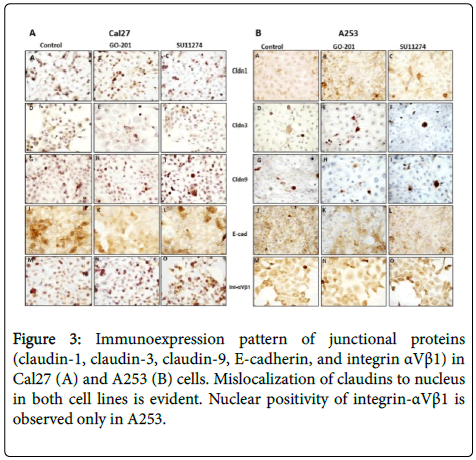
After treatment with 10 μM of SU11274 and GO201 for 24 h, we performed immunocytochemistry and western blot to investigate localization pattern and expression of claudin-1, claudin-3, claudin-9, E-cadherin, and integrin αVβ1. As shown in Figure 3, we observed in control, mislocation of claudin-1, claudin-3, claudin-9 in both cell lines and mislocation of integrin-αVβ1 only in A253 to the cytoplasm and nucleus.
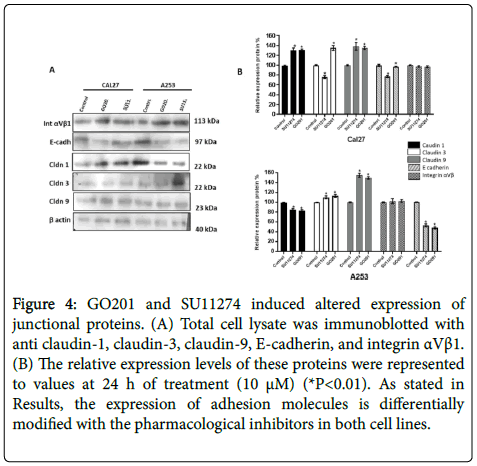
However, no changes of localization were observed after treatment with MUC1 and c-Met inhibitors (Figure 3). As shown by western blot in Figure 4, altered expression of adhesion molecules was observed for claudin-1, 3, 9 and E-cadherin after treatment.
Figure 4: GO201 and SU11274 induced altered expression of junctional proteins. (A) Total cell lysate was immunoblotted with anti claudin-1, claudin-3, claudin-9, E-cadherin, and integrin αVβ1. (B) The relative expression levels of these proteins were represented to values at 24 h of treatment (10 μM) (*P<0.01). As stated in Results, the expression of adhesion molecules is differentially modified with the pharmacological inhibitors in both cell lines.
Metalloproteases expression and activity
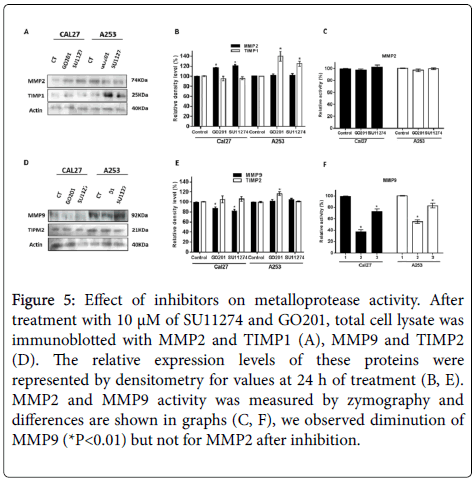
We found that expression of MMP-2 was not affected by the pharmacological treatment and TIMP1 increased after GO201 and SU11243 (Figures 5A and 5B). Expression of MMP-9 was lower after treatment in Cal27 and no change occurred in A253 cells (Figures 5D and 5E). To investigate the mechanisms of MUC1 and c-Met regulating metalloproteases activity in oral cancer cells, we performed gelatin zymography. Our result showed that activity of MMP-2 was not affected after treatment of cells (Figure 5C). MMP-9 activity was lower after treatment of cell lines with both inhibitors (Figure 5F).
Figure 5: Effect of inhibitors on metalloprotease activity. After treatment with 10 μM of SU11274 and GO201, total cell lysate was immunoblotted with MMP2 and TIMP1 (A), MMP9 and TIMP2 (D). The relative expression levels of these proteins were represented by densitometry for values at 24 h of treatment (B, E). MMP2 and MMP9 activity was measured by zymography and differences are shown in graphs (C, F), we observed diminution of MMP9 (*P<0.01) but not for MMP2 after inhibition.
Effects of migration and invasion on Cal27 and A253 cells
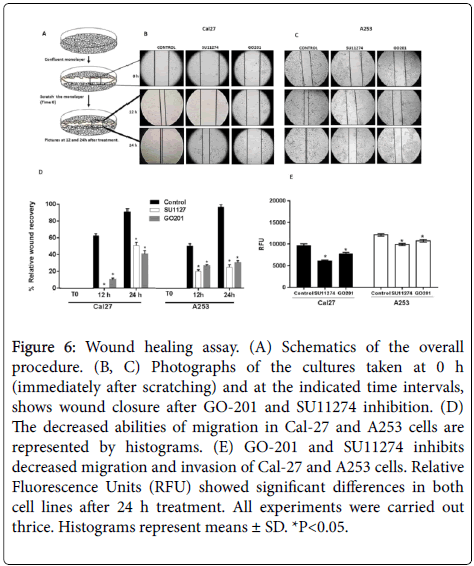
A wound healing assay was used to investigate the effects on cell migration. After treatment with 10 μM SU11274 and GO201 for 24 h, compared to the control group, migration rate was lower in treated cells. Our results suggest that MUC1 and c-Met play a role in the inhibition of cell migration in vitro (Figure 6).
Figure 6: Wound healing assay. (A) Schematics of the overall procedure. (B, C) Photographs of the cultures taken at 0 h (immediately after scratching) and at the indicated time intervals, shows wound closure after GO-201 and SU11274 inhibition. (D) The decreased abilities of migration in Cal-27 and A253 cells are represented by histograms. (E) GO-201 and SU11274 inhibits decreased migration and invasion of Cal-27 and A253 cells. Relative Fluorescence Units (RFU) showed significant differences in both cell lines after 24 h treatment. All experiments were carried out thrice. Histograms represent means ± SD. *P<0.05.
The EMC assay was performed to determine the invasion rate in head and neck carcinoma cells. Our findings show that migration rate was inhibited significantly as well as the invasion of Cal27 and A253 cells (Figure 6).
Discussion
This study was undertaken to elucidate the biological significance of TJs and MMPs expression and activity in head and neck carcinoma cells through MUC1 and c-Met inhibition. A253 and Cal27 cell lines were selected for their different anatomical localization, differentiation degree of the primary tumor, and their proven invasive capacity [26-29].
The pharmacological inhibitor, SU11274 and GO201, has been used to assess the functional activity of c-Met and MUC-1 respectively, in different types of cell lines [30-32]. In our cell lines we observed inhibition in the proliferation rate of Cal27 and A253 cells. Early research identified predominantly claudins as suppressors in human malignancies and it is known that their increased expression leads to drug resistance in colon cancer [33]. Some studies have determined that distribution of claudins, such as claudin-1, in the nucleus and cytosol is related to the up-regulation of transformation and cell proliferation in several cancers [34-36].
The western blot results revealed a differential expression of TJs, which was modified by the use of pharmacological inhibitors. The expression pattern of claudins is highly tissue-specific, and most tissues express multiple claudins. Differential expression of various claudin family members in cancer can potentially be used to confirm the histologic identity of certain types of cancer and exclude others [22]; furthermore, recent studies have identified several growth factors, cytokines, and transcription factors that affect expression of claudins.
Tumor promoting factors, such as hepatocyte growth factor and epidermal growth factor, have been shown to decrease the expression of claudin-7, and to increase the expression of claudin-1, 3, and 4 in lung cancer [22]. In turn, claudin-1 is down-regulated in basal breast cancer [33], whereas it is up-regulated in colon carcinoma [34] and melanoma [37]. Otherwise, nuclear localization of several junction proteins (β-catenin, ZOs) is known to be correlated with oncogenic transformation and cell proliferation [33,34]. Our data demonstrated that claudin-1, 3, and 9, in human cancer cell lines, were frequently mislocalized from membrane to the cytoplasm and nucleus. A proposed mechanism of claudin mislocalization is β-catenin, a component of TJs with well-characterized dual role in cell adhesion (membrane) and in signal transduction (cytoplasmic and nuclear) leading to epithelial cell transformation [38]. Moreover, it seems that this altered location has antiapoptotic effects. Nuclear/cytoplasmic shuttling of signaling proteins, including extracellular signal-regulated kinases, mitogen-activated protein kinase and SMAD3, has been characterized as nuclear translocation signal [34]. In the same way, we found that integrin αVβ1 was mislocated to the nucleus in A253 but not in Cal27 cell line. There are few reports on the altered location of integrin αvβ1 to the nucleus associated with cancer. It has been proposed that this location could promote expression of the c-myc protein, ERK1/2 MAPK, and Ras/Akt [39].
The aggressiveness of tumor cells is dependent on their capability to degrade and remodel the extracellular matrix by activating certain proteases, including plasminogen activator and MMPs [40,41]. Among extracellular remodeling enzymes, tumor cell gelatinase activities of MMP2 and MMP9 are especially relevant [42]. In our experimental setting, gelatinase MMP-9 activity was affected in carcinoma cell lines after 24 h of treatment. The endogenous inhibitor of MMP9, TIMP2 [43], binds it to MMP9 and keeps it inactive [43]. We found that TIMP2 expression increases after treatment with SU11274 and GO201, suggesting a crosstalk regulation between c-Met and MUC1. MMPs are tightly regulated at transcriptional and post-transcriptional levels by several cytokines and growth factors and are controlled at the protein level via their activators, inhibitors, and cell-surface proteins [44]. It has been demonstrated that MUC1-CT physically occupies the MMP promoter. The presence of MUC1-CT in the transcriptional complex AP1 regulates the expression of metalloproteases and their tissular inhibitors [18].
Zimography results revealed decreased activity of MMP-9, which could increase the invasive and metastatic potential in cell carcinoma via extracellular matrix degradation. In many tumor types, MUC1 expression correlates with aggressive, metastatic disease, poor response to therapy, and poor survival [18]. Our results indicated that MUC1 expression in cancer cells might be associated with an invasive phenotype and cancer growth as has been reported in in vivo studies evaluating MUC1 and MUC4 [45]. In our study, proliferation rate was affected by GO201 and c-Met inhibition. By EMC invasion assay and wound healing assay, we demonstrated that, after MUC1 and c-Met inhibition, invasive and motility activities of Cal27 and A253 decreased markedly, suggesting that they might have potential to accelerate progression. It has been reported that MUC1 expression correlates with the adhesion and invasion of MDA-MB-231 cells and suppressing MMP2 and MMP9 [39]. In contrast, our study shows low activity of MMP9 only in cells treated with GO201 but not with SU11274. High MUC1 expression is associated with vascular invasion in oral squamous cell carcinoma and MUC1 represents a useful biomarker to identify occult lymph node metastases in oral squamous cell carcinoma [46,47]. Our study proved a critical role of MUC1 and c- Met in induced invasion of Cal27 and A253 cells, suggesting that MUC1 and c-Met could become a potential target for studying tumor metastases in two types of head and neck carcinomas.
Conclusion
We have shown that MUC1 and c-Met activate proteolytic networks in carcinoma cells and contribute, thereby, to their invasive behavior. We also found mislocation of claudins and integrin αVβ1. However, further studies are needed to elucidate whether MUC1 and c-Met also contribute to the metastasis-promoting activity through other regulator models.
Acknowledgments
We want to thank for the technical support in cell culture to Miss Diana Carolina Martínez Rosas (Laboratory of Immunology, School of Odontology, UNAM). This work was supported by grants UNAM DGAPA-PAPIIT IN217912, DGAPA-PAPIIT-IN221616, and National Council of Science and Technology 167464 (CONACyT, Consejo Nacional de Ciencia y Tecnología).
References
- Vig N, Mackenzie I, Biddle A (2015) Phenotypic plasticity and epithelialâ€toâ€mesenchymal transition in the behaviour and therapeutic response of oral squamous cell carcinoma. J Oral Pathol Med 44: 649-655.
- Paliga A, Mai K (2014) Squamous cell carcinomas of the anterior oral cavity are commonly associated with simplex (or differentiated) oral intraepithelial neoplasia clinical and pathologic significance. Int J Sur Pathol 22: 231-240.
- Pinkston J, Cole P (1999) Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg 120: 834-40.
- Molina J, Adjei A (2006) The Ras/Raf/MAPK pathway. J Thorac Oncol 1: 7-9.
- Birchmeier C, Birchmeier W, Gherardi E, Woude G (2003) Met, metastasis, motility and more. Nat Rev Mol Cel Biol 4: 915-925.
- Uen Y, Lin S, Wu C, Hsieh J, Lu C, et al. (2006) Clinical significance of MUC1 and c-Met RT-PCR detection of circulating tumor cells in patients with gastric carcinoma. Clin Chim Acta 367: 55-61.
- Masuya D, Huang C, Liu D, Nakashima T, Kameyama K, et al. (2004) The tumour–stromal interaction between intratumoral c-Met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non-small-cell lung cancer patients. Br J Cancer 90: 1555-1562.
- Tsao M, Liu N, Chen J, Pappas J, Ho J, et al. (1998) Differential expression of Met/hepatocyte growth factor receptor in subtypes of non-small cell lung cancers. Lung Cancer 20: 1-16.
- Maulik G, Madhiwala P, Brooks S, Ma PC, Kijima T, et al. (2002) Activated câ€Met signals through PI3K with dramatic effects on cytoskeletal functions in small cell lung cancer. J Cell Mol Med 6: 539-553.
- Ghoussoub R, Dillon D, D'Aquila T, Rimm E, Fearon E, et al. (1998) Expression of câ€met is a strong independent prognostic factor in breast carcinoma. Cancer 82: 1513-1520.
- Camp R, Rimm E, Rimm D (1999) Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer 86: 2259-2265.
- Qian C, Guo X, Cao B, Kort E, Lee C, et al. (2002) Met protein expression level correlates with survival in patients with late-stage nasopharyngeal carcinoma. Cancer Res 62: 589-596.
- Lo Muzio L, Farina A, Rubini C, Coccia E, Capogreco M, et al. (2006) Effect of c-Met expression on survival in head and neck squamous cell carcinoma. Tumour Biol 27: 115-121.
- Zhao D, Wang S, Feng Y, Hua C, Zhao J, et al. (2011) Intratumoral c-Met expression is associated with vascular endothelial growth factor C expression, lymphangiogenesis, and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Hum Pathol 42: 1514-1523.
- Joshi M, Ahmad R, Yin L, Raina D, Rajabi H, et al. (2009) MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Can Ther 8: 3056-3065.
- Klinge C, Radde B, Imbert-Fernandez Y, Teng Y, Ivanova M, et al. (2011) Targeting the intracellular MUC1 C-terminal domain inhibits proliferation and estrogen receptor transcriptional activity in lung adenocarcinoma cells. Mol Can Ther 10: 2062-2071.
- Liu X, Ban L, Luo G, Li Z, Li Y, et al. (2016) Acquired resistance to HSP90 inhibitor 17-AAG and increased metastatic potential are associated with MUC1 expression in colon carcinoma cells. Anticancer Drugs 27: 417-426.
- Singh P, Behrens M, Eggers J, Cerny R, Bailey J, et al. (2008) Phosphorylation of MUC1 by Met modulates interaction with p53 and MMP1 expression. J Biol Chem 283: 26985-26995.
- Hart IR, Saini A (1992) Biology of tumour metastasis. Lancet 339: 1453-1457.
- Ikari A, Watanabe R, Sato T, Taga S, Shimobaba S, et al. (2014) Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim Biophys Acta 1843: 2079-2088.
- Canel M, Serrels A, Frame M, Brunton V (2013) E-cadherin–integrin crosstalk in cancer invasion and metastasis. J Cell Sci 126: 393-401.
- Ding L, Lu Z, Lu Q, Chen Y (2013) The claudin family of proteins in human malignancy: a clinical perspective. Cancer Manag Res 5: 367-375.
- Kalluri R, Weinberg R (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420-1428.
- Kohn E, Liotta L (1995) Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res 55: 1856-1862.
- Hwang T, Changchien T, Wang C, Wu C (2014) Claudin‑4 expression in gastric cancer cells enhances the invasion and is associated with the increased level of matrix metalloproteinase-2 and 9 expression. Oncol Lett 8: 1367-1371.
- Bhattacharya A, Tóth K, Mazurchuk R, Spernyak J, Slocum H, et al. (2004) Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clinical Cancer Research 10: 8005-8017.
- Bago R, Pavelić J, VlahoviÄek G, Bosnar M (2009) Nm23â€H1 promotes adhesion of CAL 27 cells in vitro. Mol Carcinog 48: 779-789.
- Kibeey M, Royce L, Dym M, Baum B, Kleinman H (1992) Glandular-like morphogenesis of the human submandibular tumor cell line A253 on basement membrane components. Exp Cell Res 198: 343-351.
- Fujishiro Y, Tonogi M, Ochiai H, Matsuzaka K, Yamane G, et al. (2012) The receptor tyrosine kinase inhibitor vandetanib activates Akt and increases side population in a salivary gland tumor cell line (A253). Int J Oncol 41: 362-368.
- Horm T, Schroeder J (2013) MUC1 and metastatic cancer: expression, function and therapeutic targeting. Cell Adh Migr 7: 187-198.
- Kenessey I, Keszthelyi M, Kramer Z, Berta J, Adam A, et al. (2010) Inhibition of c-Met with the specific small molecule tyrosine kinase inhibitor SU11274 decreases growth and metastasis formation of experimental human melanoma. Curr Cancer Drug Targets 10: 332-342.
- Christensen J, Schreck R, Burrows J, Kuruganti P, Chan E, et al. (2003) Cherrington JM and Mendel DB: A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res 63: 7345-7355.
- Stebbing J, Filipović A, Giamas G (2013) Claudin-1 as a promoter of EMT in hepatocellular carcinoma. Oncogene 32: 4871-4872.
- Dhawan P, Singh A, Deane N, No Y, Shiou S, et al. (2005) Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest 115: 1765-1776.
- Lee J, Hsiao W, Chen H, Hsu L, Chen P, et al. (2010) Upregulated claudinâ€1 expression confers resistance to cell death of nasopharyngeal carcinoma cells. Int J Cancer 126: 1353-1366.
- Ogawa M, Kojima T, Someya M, Nomura K, Takasawa A, et al. (2012) Epidermal growth factor modulates claudins and tight junctional functions in ovarian cancer cell lines. Histochem Cell Biol 138: 323-338.
- Leotlela P, Wade M, Duray P, Rhode M, Brown H, et al. (2007) Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene 26: 3846-3856.
- Reichert M, Müller T, Hunziker W (2000) The PDZ domains of zonula occludens1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta catenin/Tcf/Lef signaling. J Biol Chem 275: 9492-9500.
- Benaud C, Dickson R (2001) Regulation of the expression of c-Myc by b1 integrins in epithelial cells. Oncogene 20: 759-768.
- Hecht M, Papoutsi M, Tran H, Wilting J, Schweigerer L (2004) Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res 64: 6109-6118.
- Tsou H, Chen H, Hung Y, Chang C, Li T, et al. (2013) HGF and c-Met interaction promotes migration in human chondrosarcoma cells. PLoS One 8: e53974.
- Deshmukh H, Case L, Wesselkamper S, Borchers M, Martin L, et al. (2005) Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 171: 305-314.
- Yamazoe S, Tanaka H, Sawada T, Amano R, Yamada N, et al. (2010) RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res 29:53.
- Sternlicht M, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol 17: 463-516.
- Lu L, Deng H, Fan W (2004) Correlation of MUC1 expression to adhesion of breast cancer cell line MDA-MB-231. Ai Zheng 23: 1294-1296.
- Liu X, Chen G, Wang Z, Liu F (2007) Clinical significance of detecting mucin 1 mRNA in diagnosing occult lymph node micrometastasis in esophageal cancer patients. Ai Zheng 26: 194-199.
- Hamada T, Nomura M, Kamikawa Y, Yamada N, Batra S, et al. (2012) DF3 epitope expression on MUC1 mucin is associated with tumor aggressiveness, subsequent lymph node metastasis, and poor prognosis in patients with oral squamous cell carcinoma. Cancer 118: 5251-5264.
Citation: Amador CV, Rodríguez AC, Zenteno E, Guerrero JCH, Farfán MDJ (2017) MUC1 and C-Met Affects Proliferation, Intercellular Junctions and Invasion in Two Head and Neck Carcinoma Cell Lines. J Clin Exp Pathol 7: 326. DOI: 10.4172/2161-0681.1000326
Copyright: ©2017 Amador CV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5362
- [From(publication date): 0-2017 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 4400
- PDF downloads: 962