Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Multimodality Imaging for Malignant Transformation Assessment in Neurofibromatosis Type 1
| Faiq Shaikh1* and Omer Awan2 | |
| 1Molecular Imaging and Informatics, University of Pittsburgh Medical Center, Pittsburgh, USA | |
| 2Department of Radiology, Dartmouth Geisel School of Medicine, Lebanon, USA | |
| Corresponding Author : | Faiq Shaikh Molecular Imaging and Informatics University of Pittsburgh Medical Center Pittsburgh, USA E-mail: faiqahmedshaikh@gmail.com |
| Received: January 09, 2016; Accepted: January 11, 2016; Published: January 14, 2016 | |
| Citation: Shaikh F, Awan O (2016) Multimodality Imaging for Malignant Transformation Assessment in Neurofibromatosis Type 1. OMICS J Radiol 5:e135. doi:10.4172/2167-7964.1000e135 | |
| Copyright: © 2016 Shaikh F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
| Editorial |
| Neurofibromatosis is a common autosomal dominant neurocutaneous disorder. It is also defined as a RASopathy (developmental syndromes caused by germ line mutationsin genes that alter the Ras subfamily and Mitogen activated protein kinases that control signal transduction). It has an incidence of 1:3000 live births and has variable expressivity [1]. Half of the cases of NF demonstrate de novo mutations and it has equal male to female preponderance. It has two types: NF1, which is characterized by neurofibromas, which may induce compressive symptoms and have the tendency to undergo malignant transformation into malignant peripheral nerve sheath tumors (MPNST). NF2 manifests with bilateral acoustic neuromas, which may lead to hearing loss [1]. Patients typically have multiple inherited schwannomas, meningiomas, and ependymomas. |
| MPNST aka Neurofibrosarcoma has an incidence of 1.6/1000 (813% lifetime incidence) [2]. 25-70% of MPNST cases are associated with NF1 [3]. However, only about 812% of patients with NF1 develop a MPNST. It is often detected late and is associated with high mortality. MPNSTs most commonly occur in the deep soft tissues, usually close to a nerve trunk, the mediastinum, and retro peritoneum. The most common sites are the sciatic nerve, brachial plexus, and sacral plexus. MPNST may be distinguished from neurofibroma if there is rapid or infiltrative growth pattern [4]. The superficial lesions are amenable to biopsy while the deep neural lesions are associated with high morbidity (nerve paralysis) and sampling error given the heterogeneous histopathologic appearance. |
| Clinically, malignant transformation in NF may be suspected in the presence of a rapidly growing and/or cystic mass (neurofibroma or plexiform NF), which may or may not be associated with pain, and occurrence or aggravation of neurological symptoms (pain, sensory/ motor deficit, dysphonia, dysphagia). Additional features that may help in differentiating the two entities include large areas of heterogeneity with haemorrhage, necrosis and infiltration of surrounding soft tissue structures for MPNST. Imaging plays a vital role in assessment of malignant transformation of these lesions. MRI can be useful in detecting and differentiating MPNSTs and neurofibroma, as it provides excellent soft tissue contrast and ability to identify a mass arising from a specific nerve and invasion of specific structures such as vessels, muscles, fascia, and subcutaneous fat [5]. Wasa et al. found significant differences between MPNST and neurofibromas for largest dimension of the mass, peripheral enhancement pattern, perilesional edemalike zone, and intratumoral cystic lesion [4]. The presence of two or more of the four features was suggestive of malignancy (MPNST) with a sensitivity of 61% and specificity of 90%. Among cases in NF1 patients, heterogeneity on T1weighted images (T1WI) was also significant in distinguishing MPNST from NF [5]. |
| Another group found that intratumoral lobulation (sensitivity 63%, specificity 83%) and the presence of a high signal intensity area on T1WI (sensitivity 63%, specificity 88%) were considered to be diagnostic indicators of MPNST [6], although there remains debate regarding these findings in the literature. Scheppers et al. suggested that the tumor diameter of greater than 66 mm associated with neural deficits and MRI with no abnormal signal on T2WI, heterogeneous signal on T1WI, and >50% necrosis, was high risk for malignant transformation [7]. Van Herendael et al. suggested if the lesion was intramuscular, perineural, nodular, and MRI showed heterogeneous signal on T1/2WI along with gadolinium enhancement, it was high risk for malignant transformation [8]. |
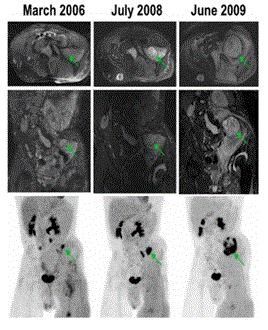
| FDGPET/CT is making strides as a viable adjunct to MRI in detection of malignant transformation in NF1. There are three approaches to interpreting these studies: Qualitative approach is based on visual assessment and is fast but highly operator dependent and with low reproducibility. The second approach is to use standardized uptake value (SUV cut off), whereby PET/CT was shown to have a sensitivity, specificity, positive predictive value, and negative predictive value for separating MPNSTs from BNFs of 91%, 84%, 67%, and 96% versus 91%, 81%, 63%, and 96%, respectively, on 4hour delayed imaging, and showed that the mean SULmax was significantly higher for MPNSTs than BNFs on both early scans (6.5 vs. 2.0, P<0.01) and delayed imaging (8.3 vs. 2.3, P<0.02) [9]. The third approach is to use the ratio SUVmax of tumor to SUV mean of background (liver). This method was found to be reproducible, and less subject to SUVmax variability. The reported NPV and PPV were 98.8% and 65.1% respectively. The recommended T/L ratio is <1.5 for monitoring and >1.5 to intervene (biopsy, surgery) [10]. Serial PET and MRI imaging can be performed for the evaluation of abnormal growth pattern, interval increase in FDG uptake and MRI features suggestive of malignant transformation, as demonstrated in Figure 1. |
| Clinical presentation and multimodality imaging features can be used to detect early stages of malignant transformation of neurofibromas, which is directly related to reducing mortality and improving outcomes. Clinical suspicion is raised when a patient with NF1 presents with a growing mass that is usually painful and associated with a neurologic deficit. MRI and/or PET/CT imaging is then indicated to assess for features as mentioned above to detect early signs of malignant transformation. This information can be then judiciously interpreted for further management. There is a need for methods to create preintervention likelihood scores that are generated through concatenated indices based on the clinical and imaging features in order to minimize unnecessary iatrogenic morbidities and detect cancer early enough to increases chances of survival. |
References
- Rauen KA, Hus on SM, BurkittWright E, Evans DG, Farschtschi S, et al. (2015) Recent developments in neurofibromatoses and RASopathies: management, diagnosis and current and future therapeutic avenues. Am J Med Genet Part A 167A: 1-10.
- Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, et al. (2002) Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 39: 311-314.
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM (1986) Malignant peripheral nerve sheath tumors: a clinicopathologic study ofv120 cases. Cancer 57: 2006-202.
- Hrehorovich PA, Franke HR, Maximin S, Caracta P (2003) Malignant peripheral nerve sheath tumor. Radiographics 23:790794.
- Wasa J, Nishida Y, Tsukushi S, Shido Y, Sugiura H, et al. (2010) Peroneal nerve: Normal anatomy and pathologic findings on routine MRI of the knee. AJR Am J Roentgenol 194: 15681574.
- Matsumine A, Kusuzaki K, Nakamura T, Nakazora S, Niimi R, et al. (2009) Differentiation between neurofibromas and malignant peripheral nerve sheath tumors in neurofibromatosis 1 evaluated by MRI. J Cancer Res Clin Oncol 135:891900.
- Verstraete KL, Achten E, De Schepper A, Ramon F, Parizel P, et al. (1992) Nerve sheath tumors: evaluation with CT and MR imaging. J Belge Radiol 75:311320.
- Van Herendael BH, Heyman SR, Vanhoenacker FM, De Temmerman G, Bloem JL, et al. (2006) The value of magnetic resonance imaging in the differentiation between malignant peripheral nervesheathtumors and nonneurogenic malignant softtissuetumors. Skeletal Radiol 35: 745-753.
- Chirindel A, Chaudhry M, Blakeley JO, Wahl R (2015) 18FFDG PET/CT Qualitative and Quantitative Evaluation in Neurofibromatosis Type 1 Patients for Detection of Malignant Transformation: Comparison of Early to Delayed Imaging With and Without Liver Activity Normalization. J Nucl Med 56: 379385.
- Combemale P, ValeyrieAllanore L, Giammarile F, Pinson S, Guillot B, et al. (2014) Utility of 18FFDG PET with a SemiQuantitative Index in the Detection of Sarcomatous Transformation in Patients with Neurofibromatosis Type 1. PLoS ONE 9: e85954.
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 12538
- [From(publication date):
February-2016 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 11578
- PDF downloads : 960
