Oral Cancers: A New Perspective on Anticancer/Antioxidants
Received: 01-Mar-2023 / Manuscript No. jcd-23-89914 / Editor assigned: 04-Mar-2023 / PreQC No. jcd-23-89914 / Reviewed: 18-Mar-2023 / QC No. jcd-23-89914 / Revised: 22-Mar-2023 / Manuscript No. jcd-23-89914 / Published Date: 29-Mar-2023
Abstract
Globally, oral cancer (OC) is one of the most prevalent malignancies with a negligible prognosis, a high recurrence rate, and high therapeutic costs. Despite many efforts to resolve these issues, no alternative definitively effective solution has yet been presented. From this point of view, it seems necessary to research other new therapeutic methods. Antioxidants are oxidation inhibitors demonstrating diverse physiological functions in the body. In this review, we focused on some compounds that, despite their antioxidant properties, have evidenced anti-cancerous effects on OCs.
Oral cancers and its prevalence
Oral cancers are the sixth most common cancer types worldwide that is more prevalent in men than women in the ethnic categories, based on a report by the World Health Organization (WHO) (Kharbanda et al. 2019). OCs is neoplasia that is perilous, and they occur around the oral cavity and lip. They are usually denominated squamous cell carcinoma (OSCC) because 90% of OCs are histologically associated with the squamous cells (Lingen et al. 2008) [1]. Moreover, OCs has various levels, can progress to node metastasis of the lymph node. The International Agency for Research on Cancer (IARC) has stated that OC, indicated by ICD-10 code C00-08: Lip, Oral Cavity, encompasses various cancers such as saliva glands, parotid, tongue, gingiva, lips and mouth floor. Globally, annual diagnosed cases are more than 300,000 patients, and its fatality rate is about 145,000 (Rivera. 2015). Some regions of the world have a much higher prevalence, such as the Pacific regions, Eastern Europe, Latin America, and South and Southeast Asia (Warnakulasuriya. 2009) [2].
Risk factors
Risk factors involved in oral cancer are chemical factors such as alcohol and tobacco, biological factors such as syphilis and human papillomavirus (HPV), dental trauma, age and family history, and genetic diseases and disorders related to oral cancer (Rivera. 2015 ;Azhar el al. 2018) [3].
Types of oral cancer
The most common type of OC is squamous cell carcinoma, whereas others are less common such as Salivary gland cancer (Lin et al. 2018), Adenoid cystic tumor (Pushpanjali et al. 2014), Basal cell carcinoma (Woods et al. 2014), Oral cavity lymphomas (Silva et al. 2016), Oral malignant melanomas (Padhye and D’souza. 2011) [4].
History of treatments
For more than one hundred years, surgery has been one of the best-established methods to treat the majority of OCs. However, with the discovery of radium, ionizing radiation has become a milestone in oral carcinoma treatment. Nonetheless, in most patients with advanced cancer, a combination of surgery and radiotherapy are usually recommended [5]. During the three decades of 1950, 60, and 70, chemotherapy was considered the palliative method to treat OCs. With the appearance of the anti-cancer drug Cis-platinum, significant positive effects on patients were obtained via induction chemotherapy. However, despite remarkable effectiveness in the head and neck area,induction chemotherapy is not enough to eradicate oral squamous cell carcinomas in all cases (Shah and Gil. 2009) [6].
Surgery
Resection and reconstruction surgery is an indispensable method to treat OC. Resection surgery involves the elimination of the initial tumor ± management of the cervical nodes ± tracheostomy if needed. The resection surgeon attempts to removal the oral cancer region along with the elimination of a margin of normal tissue surrounding the cancer in all three dimensions (Friedland et al. 2011; Wong and Wiesenfeld. 2018). However, novel researchers suggest the removal of a 5 mm microscopic margin of normal tissue all around the tumor [7]. The tumor and the margin of normal tissue are pinpointed by preoperative scans and intraoperative palpation and marked by a special marker. The shrinkage of the tumor after resection and in the period of pathological preparation is changeable, and this is associated with the location, which can be up to 50% (Wong and Wiesenfeld. 2018). On the other hand, reconstructive surgery involves reducing drastically the morbidity of the resection, e.g., substitution of tissue, causing the least impact on speech, swallowing, and mastication [8].
Role of antioxidants in oral cancers
Some antioxidants like provitamin A, vitamin E, vitamin C, β carotene, spirulina, zinc, and selenium have inhibitive functions against OC. Succinctly, antioxidants prevent oral cavity carcinogenesis, diminish the expansion of OC, and leads to recurring premalignant lesions such as oral leukoplakia (Chandra Mouli et al. 2012). Antioxidants diminish the occurrence of various disorders such as diabetes, inflammation, cardiovascular disease, cataracts, liver disease, nephrotoxicity, neurodegenerative disorders, aging, and cancer (Neha et al. 2019). They have considerable functions not only during the free radical injurious process but also when the malignancy spreads (Madhulatha et al.2017). According to some data, antioxidants do not interfere with chemotherapy and can enhance one’s quality of living (Singh et al. 2018) [9].
Anti-cancerous properties
OC therapy utilizes drug alternatives with anti-cancer properties to act with more precision to diminish cancer recurrence with minimum side effects. Most anti-cancer therapies are able to interfere with the molecular agents or signaling pathways activating tumorigenesis, tumor growth and its development (Seebacher et al. 2019). Nonetheless, high tumor recurrence rates and harsh side-effects due to chemotherapeutic components weakens clinical efficacy of a wide range of anti-cancer therapies. Therefore, there is a dire need to introduce more alternative or synergistic anti-cancer therapies with the least side-effects (Ali et al. 2012) [10].
The Function of antioxidants in oral cancers
Studied antioxidants act on OCs via different mechanisms described below:
Flavonoid group
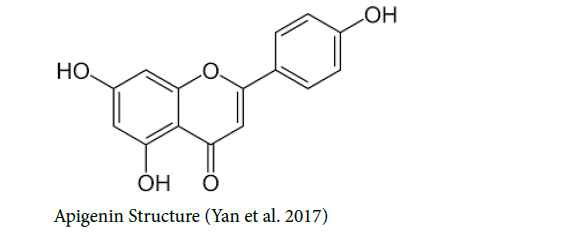
Apigenin: Apigenin or 4′, 5, 7-trihydroxy flavone with the IUPAC name of 5, 7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran- 4-one, belongs to the flavone subclass. Fruits such as grapes, apples, vegetables such as parsley, and chamomile tea and red wine contain large quantities of apigenin (Yan et al. 2017). Apigenin taken orally is systemically absorbed and recirculated by and local intestinal and enterohepatic pathways. Its bioavailability is approximately 30% and once orally absorbed, maximal circulating concentration (Cmax) was realized after a time (Tmax) of 0.5–2.5h with a half-life of 2.52 ± 0.56h (DeRango-Adem and Blay, 2021). Various properties of apigenin such as anti-inflammatory, antioxidant, antiviral activities and antibacterial and anti-cancer capacity have been proven. Apigenin demonstrates remarkable cell cytotoxicity selectively against different types of cancer cells, while not harming the normal cells (Yan et al. 2017). A significant decline of apigenin-induced cell growth has been found in the tongue oral cancer-derived cell line. Apigenin treatment leads to cell-cycle arrest at both G0/G1 and G2/M checkpoints (Maggioni et al. 2013) and it shows antitumor activities through regulating multiple signaling pathways containing PI3K/AKT, NF-kB, Wnt/β-catenin, JAK/STATs, MAPK/ERK, AMPK and JNK (Arango et al. 2013) [11].
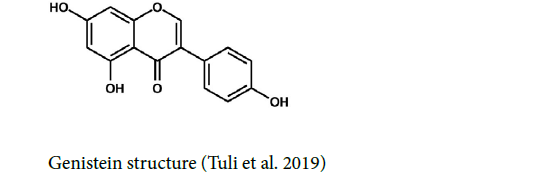
Genistein: Genistein or 4′, 5, 7-trihydroxyisoflavone is a flavone component that is found in soybeans and foods such as cheese, milk, oil, sources, and flour. Also, other legumes such as chickpeas, beans, peas, lentils, and all grains like oats, rye, rice, barley, and wheat possess the antioxidant genistein (Ardito et al. 2017). Genistein has low oral bioavailability and its plasma or tissue concentrations were much lower than its in vitro IC50 (Yang et al. 2012). It can be found as β-glucoside,which is hydrolyzed, in the small intestine, in the form of aglycones, an active pharmacological part (Ardito et al. 2017). Genistein has a short Tmax (<2 hr) indicating that genistein is absorbed fast after oral administration (Yang et al. 2012) [12].
Clinical researches emphasize the chemo preventive abilities of genistein on a wide range of cancers (Ardito et al. 2017). Genistein has anticancer abilities to prevent tyrosine kinase proteins and the cessation of the cell cycle during the G2/M phase. Moreover, the apoptosis process is started by genistein through the activation of caspase-3 and -9 (Banerjee et al. 2008; Yamasaki et al. 2013). A Nano co-adhesive system with the help of lipid-based nanocarriers possessing genistein, which is called Nano emulsions, leads to effective improvement in patients with oral cancer (Gavin et al. 2015; Ardito et al. 2017).
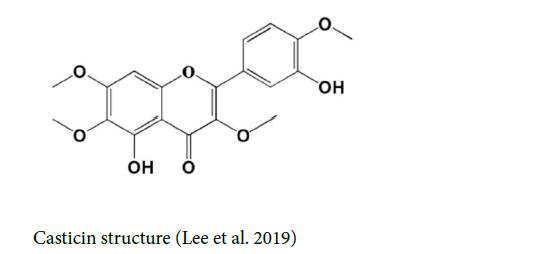
Casticin: Casticin (CTC), also known as vitexicarpin or 3′, 5-dihydroxy-3, 4′, 6, 7-tetramethoxy flavone, belongs to the class of 7-O-methylated flavonoids. It is insoluble in water and weak acids (Lee et al. 2019). The bioavailability of casticin was reported to be 45.5% ± 11.0%, through both intravenous and oral methods of administration (Ramchandani et al. 2020) [13].
Casticin is able to induce cell morphological alterations, DNA condensation and subsequently destruction, attenuating the live cells, and compelling G2/M phase arrest in SCC-4 cells. Casticin boosts the productions of ROS and Ca²⁺, elevates the levels of the mitochondrial membrane potential (ΔΨm), and amplifies caspase-3,-8, and -9 functions in SCC-4 cells (Chou et al. 2018). Casticin can act as a potential antineoplastic agent and prevent the activities of cell viability, invasion, and migration on OSCC cell lines. It is able to reverse the epithelial-Mesenchymal transition (EMT) process and hamper the β‐ catenin expression in OSCC (Xie et al. 2019) [14-19].
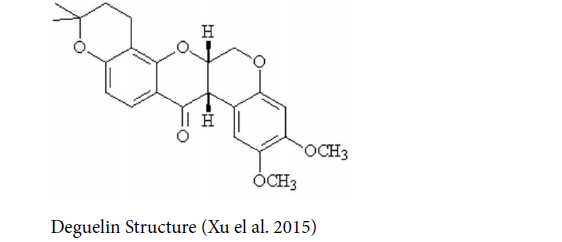
Deguelin: Deguelin is a natural compound of the flavonoid family products extracted from Derris trifoliata Lour. or Mundulea sericea (Leguminosae) (Wang et al. 2013) with the terminal half-life of 9.26 hours (Udeani et al. 2001), having chemopreventive and therapeutic functions in various types of the cancers in vitro and in vivo, such as gastric, breast, colon, rectal, lung, (Xu et al. 2015) and OCs. New researches have shown deguelin is able to repress T-cell migration and invasion of OCs (Bundela et al. 2015). The inhibitory influences of deguelin in low dose levels in OCs are mediated via repressing TNF- α-induced activation of IκB kinase, resulting in the prevention of IκB phosphorylation, NF-κB transcriptional function, and hampering the expression of metalloproteinase-2 (MMP2). Low-dose deguelin can notably prevent tumor growth and invasion capacity without systemic toxicity (Liu et al. 2016) [20-23].
Kaempferol: Kaempferol with the IUPAC name of 3,5,7-trihydroxy- 2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one is a natural flavonoid present in numerous common vegetables and fruits (Ren et al. 2019). Research has shown various properties of kaempferol such as antimicrobial, neuroprotective, antidiabetic, cardioprotective, antioxidant, and anticarcinogenic capacities (Cid-Ortega et al. 2018). Kaempferol has poor absorption, especially regarding oral bioavailability; however, the bioavailability of Kaempferol can be increased if combined with various anti-cancer drugs (Ren et al. 2019). Kaempferol has illustrated antiproliferative influences on oral cavity carcinoma (PCI-13) and pharynx (FaDu), human tongue squamous carcinoma and inhibited clone formation and hampered respectively migration and invasion and induced remarkable apoptosis (Imran et. 2019) [24-29].
Quercetin: Quercetin or 3,3′,4′,5,7-pentahydroxyflavone is a plentiful dietary flavonoid and is found in many fruits, vegetables, leaves, grains, and the highest amounts being found in onions and kale (Russo et al. 2012). The bioavailability of quercetin is associated with its bioaccessibility and thereby solubility in the vehicle used for its administration. Quercetin has lipophilic properties with low water solubility. The little solubility of quercetin and its crystalline form In vivo restricts bioaccessibility and its bioavailability (Kaşıkcı and Bağdatlıoğlu, 2016). Quercetin is widely used to prevent or treat cardiovascular diseases and cancer (Russo et al. 2012). Quercetin with its antioxidant capacity can act as a scavenger of radicals and constitutes complex antioxidant defense systems with the help of metal ions and DNA. Quercetin can affect the ratio of anti-/pro-apoptotic proteins leading to the disability of mitochondria and subsequently the liberation of AIF, Cyto c, and Endo G from mitochondria, and the destruction of the cells via triggering apoptosis. Researches have proven that quercetin can induce the apoptosis process in human OCs. Quercetin can increase the expression of caspase-2, Bak, Bid and Bad, Apaf-1, Endo G, Cyto c, AIF and PARP, caspase-3, caspase-6, caspase-7, the active form of caspase-9, Fas-L, Fas, TRAIL, FADD, and active form of caspase-8, XBP-1, ATF-6β, ATF-6α, IRE-1α, GRP-78 and caspase-4 while decrease the expression of Bcl-x, Bcl-2, and pro-caspase-3 (Ma et al. 2018) [30-33].
Terpene group
Farnesol: Farnesol is acyclic sesquiterpene alcohol mainly found in the essential oils of different plants. Farnesol is an intermediate agent in the sterol biosynthesis pathway in the eukaryotic cells that show anti-cancer, antimicrobial activity, and antioxidant capacity (Intapa et al. 2019). It is roughly insoluble in water leading to a reduction the bioavailability. However, the encapsulation of farnesol in polymer nanoparticles and micelles or loading it into polymer gels enhances its stability and bioavailability (Nowacka et al. 2020). In many tumour cell lines, farnesol can control several tumorigenic proteins and/or control various signal transduction cascades. Moreover, apoptosis and down regulating cell proliferation, angiogenesis, and cell survival processes are induced by farnesol. (Jung et al. 2018). Farnesol can initiate the apoptosis process in oral squamous carcinoma cells, mediated by extruding MRP1 and depleting the intracellular glutathione (Intapa et al. 2014) [34-39].
Triterpene
Brusatol (BT): Quassinoid brusatol (BT), a triterpene lactone compound, was first isolated and recognized from the Brucea sumatrana seeds. Despite effective applications for amebiasis improvement, over the past decades, BT has been quickly identified as a potential anticancer component (Cai et al. 2019; Yu et al. 2020). BT has low aqueous solubility and rapid first-pass metabolism after oral administration, however, pharmacokinetic parameters illustrate that oral bioavailability was greatly ameliorated by brusatol self-microemulsifying drug delivery system (BR-SMEDDS) (Zhou et al. 2017). BT has been recognized as one of the most powerful inhibitors of the Nrf2 signaling pathway, being able to reduce the level of Nrf2 protein. The decrease in Nrf2 protein has been observed in various cancer cell lines without considering the status of Keap1 or Nrf2, which is either mutant or wild (Jaramillo and Zhang, 2013; Ren et al. 2019). Also, it can enhance the cytotoxic effect of chemotherapeutic agents (Sova et al. 2018). BT is a powerful blocker of the STAT3 signaling pathway in several head and neck squamous cell carcinoma cells (HNSCC). Researches have demonstrated that BT leads to initiating cytotoxicity and abrogating the activation of STAT3 and that of upstream kinases such as Src, JAK1, and JAK2. It diminishes the nuclear level of STAT3 and its capability of DNA binding. BT can broaden procaspase-3 and PARP cleavage and decline the regulation of mRNA and various protein expressions such as survivin, Bcl-2 and Bclxl in HNSCC (Sethi et al. 2019) [40-43].
Ophiopogonin D: Ophiopogonin D (OP-D) is a triterpene saponin derived from the Ophiopogon japonicus plant and it is used in traditional medicine in East Asia (Lu et al. 2020). The original form of OP-D had a low bioavailability regardless of route of administration (Chen et al. 2018). OP-D possesses considerable pharmacological influences such as improving learning deficits and dysmnesia, enhancing the body’s resistance to cerebrovascular and cardiovascular diseases, and antitumor, immunoregulatory and inflammatory capacities. OP-D prevents cell proliferation, broadens cytotoxicity, induces apoptosis, and boosts the activity of caspase-3/9 in the larynx carcinoma cells in humans via up regulation of the p38‑MAPK pathway and down regulation of cyclin B1 and MMP‑9 signaling (Yan et al. 2019) [44-49].
Diterpene
Cryptotanshinone: Cryptotanshinone (CPT) or tanshinone C is a natural compound and a cell-permeable diterpene quinone extracted from the root of Salvia miltiorrhiza Bunge (Chen et al. 2013). The bioavailability of CPT is very low when orally administered related to first-pass metabolism and poor intestinal absorption (Chen et al. 2013). CPT has different pharmacological influences such as antiinflammatory, antitumor, and antioxidant properties (Rahman et al. 2016). CPT has shown various anticancer capacities against a variety of human tumors (Chen et al. 2017). Mucoepidermoid carcinoma (MEC) is the most prevalent malignant salivary gland tumor in humans and severely impacts the conditions of the parotid and minor salivary glands. In many cases, surgery results in mostly reduced quality of life because of its severe negative effects on functional and cosmetic cases. CPT can dephosphorylate STAT3; leading to inhibiting the growth of MEC cells and increasing the apoptosis process by way of activating the PUMA protein (Yu et al. 2018) [50-53].

Sesquiterpene
Zerumbone: Zerumbone (ZER) is a natural sesquiterpene from the Zingiberaceae family with various properties including those of antioxidant, antibacterial, antipyretic, immunomodulatory, anti-inflammatory, anti-proliferative activity, and anti-neoplastic. Zerumbone has low oral bioavailability due to poor aqueous solubility and absorption. Still, chemical modification, salt formation, particle size reduction, amorphization, or different formulation development strategies such as molecular complexation, solid dispersions, lipid-based formulations, nanoparticles, and micelles are suitable ways to elevate its solubility and bioavailability. (Girisa et al. 2019). ZER can impress the angiogenesis process as an antitumor drug (Kalantari et al. 2017; Hseu et al. 2019). OSCC cell proliferation, migration, and invasion processes are ceased by blocking the expression of CXCR4, RhoA proteins and suppressing the PI3K-mTOR signalling pathway leading to arrest of the G2/M cell cycle, subsequently starting the apoptotic activity. The prevention of the PI3K-mTOR signalling is related to the repression of Akt and S6 proteins (Zainal et al. 2018) [54-59].
Alkaloid group
Berberine (BBR): Berberine (BBR) is a quaternary ammonium salt that is derived from the protoberberine group of benzylisoquinoline alkaloids. It is found in Chinese goldthread with the scientific name of Coptis Chinensis, Hydrastis Canadensis also called goldenseal, barberry with the scientific name of Berberis vulgaris, and numerous other medicinal plants (Wong. 2018). Poor absorption from the gut and rapid metabolism in the body that caused its low oral bioavailability (Feng et al. 2019) BBR has various pharmacological properties, such as immunomodulatory, antioxidative, cardioprotective, hepatoprotective, and renoprotective (Neag et al. 2018) and anti-tumor effects. BBR has been shown to suppress angiogenesis and cell proliferation via modulating the cell cycle, apoptosis, autophagy, and the tumor microenvironment (Wang et al. 2020). BBB inhibits the growth of different cancers through suppression of epithelial-to-mesenchymal transition (EMT), induces apoptosis and autophagy, arrests cell cycle, and declines the metastatic potential through inhibition of COX-2/ PGE2 or AMPK/ ERK pathways (Lin et al. 2017). Current studies have shown that it has a solid potential to treat OCs. BBR has significant bioactivity against several cancer cell lines (Bundela et al. 2015). BBR can suppress the expression of miR-21 and the subsequent occurrence of oral squamous cell carcinomas-cancer stem cells (OSCC-CSCs). The prevention of endogenous miR-21 due to BBR leads to diminished cases, including self-renewal, migration, invasion abilities and a decrease in the activity of Aldehyde dehydrogenase 1 (ALDH1). The usage of BBR during chemotherapy also has significant beneficial effects on patients (Lin el. 2017) [60-63].
Polyphenol group
Curcumin: Curcumin is a polyphenol compound that is found in the Curcuma longa plant known as turmeric (Song et al. 2019). Curcumin has very poor bioavailability and very low or even undetectable concentrations in blood and extraintestinal tissue related to its poor absorption, rapid metabolism, chemical instability, and rapid systemic elimination (Lopresti. 2018). Aside from its antioxidant capacity, curcumin has anti-inflammatory, antiangiogenic, antimitotic, and anti-metastatic functions. The impressive beneficial effects of curcumin on various cancers, such as oral cancer, have been proven; hence, curcumin seems to be an effective molecule to inhibit and treat cancer in humans. The apoptosis process in cancerous cells is induced by curcumin through various mechanisms, preventing DNA topoisomerase II (Suhail et al. 2015). Curcumin can be used as a chemo preventive agent to broaden the desired effects and reduce the side effects of chemotherapeutic drugs (Panda et al. 2017). The curcumin analog (PAC) repressed cell survival and induced apoptosis and autophagy in oral cancer cells (Semlali et al. 2021) [64-69].
Epigallocatechin gallate (EGCG): Epigallocatechin gallate (EGCG) is one of the many natural polyphenolic components found in green tea (Chu et al. 2017), which possesses antiallergic, antiatherosclerotic, antioxidant, antimutagenic, and anticancer properties. EGCG is transported via serum albumin and has been recognized as an inhibitor of heat shock protein 90 (HSP90) also known as cytoplasmic chaperone (Snitsarev et. 2013). EGCG has low bioavailability but recently produced a nanoemulsion to encapsulate EGCG to elevate its bioavailability, stability, and biological activity (Chen et al. 2020). EGCG can diminish invasion and migration of human oral cancer that is associated with reducing urokinase plasminogen secretion and matrix metalloproteinase-2/9 (Kim et al. 2010). The treatment of cancer cells using EGCG leads to reversed hypermethylation status of the RECK gene and promotes the expression level of RECK mRNA by preventing MMP-2 and MMP-9 gene expression levels. EGCG can repress cancer cell-invasive capability via attenuating the number of invasive foci (Khani et al. 2018). EGCG induces cell cycle arrest and apoptosis in human OSCC cells, resulting in antiproliferative effects in vitro and in vivo (Yoshimura et al. 2019). Also, EGCG suppresses an immunomodulatory protein named Indoleamine 2, 3-dioxygenase via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells (Almatroodi et al. 2020) [70-73].
Honokiol (HK): Honokiol (3’,5-di-(2-propenyl)-1,1’-biphenyl- 2,2’-diol) is a small bioactive natural polyphenol compound that is derived from Magnolia spp (Arora et al. 2012; Costa et al. 2017) HK has anti-cancer activities, affecting various types of cancers via a variety of mechanisms (Huang et al. 2018). HK has low solubility and bioavailability; however, different studies have revealed proper honokiol delivery systems to enhance its pharmacological effectiveness, including the nanoparticles, micelles, and liposomes (Ong et al. 2019). HK can diminish OSCC cell line proliferation by arresting the cell cycle at the G1 stage, which is associated with reducing the regulation of Cdk2 and Cdk4 and promoting the regulation of the cell cycle repressors p21 and p27. Furthermore, HK can start the apoptosis process by way of the caspase and autophagy with the help of autophagosome formation (Huang et al. 2018). Expression levels of iNOS with nitric oxide (NO) secretion are attenuated in OSCC cell lines treated by HK. Endoplasmic reticulum resident protein 44 (ERp44) has a reduction in OSCC cell lines by using HK. HK straightly bound to ERp44 causes knock-down and subsequently prevents cell proliferation of OCs and its colony formation (Cho et al. 2015). HK removes OSCC cells by blocking epithelial-Mesenchymal transition (EMT) by regulating Snail/Slug protein translation. EMT is prevented in human OSCC cells through the destruction of Wnt/β-catenin signaling. (Ong et al. 2020). Also, HK activates the reduction of IL‐6 secretion and STAT3 phosphorylation, which prevents self‐renewal, invasion, and colony formation (Chang el. 2018) [74-79].
Magnolol: Magnolol with the IUPAC name of 5,5’-diallyl-2,2’- dihydroxybiphenyl is a polyphenolic binaphthalene molecule and a constructional isomer of honokiol (Zhu et al. 2012). Magnolol is extracted from the stem bark of the Magnolia officinalis plant. It has low bioavailability, low water solubility, and quick metabolism. Magnolol has a wide range of capacities such as anti-inflammation, anti-angiogenesis, anti-microorganism, anti-oxidation, anticancer, cardiovascular protection, neuroprotection, and lipolysis (Zhang et. 2019). Magnolol induces cell death in OCs associated with Ca2+ signaling by increasing cytosolic Ca2+ via respective stimulation of phospholipase C (PLC)-dependent release of Ca2+ from endoplasmic reticulum organelle and broadening Ca2+ entry through PKC signaling pathway (Hsieh et al. 2018). Magnolol could suppress cancer stemness and STAT3 signaling in oral cancer. However, recent studies have confirmed that magnolol inhibited cell viability in oral cancer cell lines. Magnolol triggers caspase-mediated apoptosis in oral cancer (Chen et al. 2021). To promote the anti-tumor ability of magnolol, a methoxylated derivative of magnolol, namely 2-Omethylmagnolol (MM1), was synthesized. MM1 has a more remarkable ability to suppress invasion, migration, and vimentin expression than magnolol in OSCC cells (Wang et al. 2018) [80-84].
Stilbene
Resveratrol: Resveratrol is a natural stilbene, a non-flavonoid polyphenol, and a phytoestrogen containing anti-oxidant and antiinflammatory capacity, cardioprotective capability, and anti-cancer properties (Ko et al. 2017). The therapeutic application of resveratrol was limited by low bioavailability. However, its derivatives, such as methoxylated, hydroxylated, and halogenated derivatives, show favorable therapeutic potential (Salehi et al. 2018). It has demonstrated activity against several cancers, for instance, OCs (Farooqi et al. 2018). Targeting various molecules by resveratrol denotes that it can control cancer via several pathways such as Nrf2 (Alavi et al. 2021), NF-κB, TGFβ1/SMAD, WNT/β-catenin, TGF/TGFR, SHH/GLI Pathway, NOTCH, TRAIL, STAT, AKT and MAPK, ATM/p53, regulation of MicroRNAs (Farooqi et al. 2018). Its ‘oral cancer score’ is the secondhighest among all the compounds mentioned in the researches. Its physicochemical characteristics are appropriate in the range presented for therapeutically effective drugs. Therefore, resveratrol is a suitable candidate to target small molecules to treat OCs (Bundela et. 2015) [85- 89].
Alkaloids
Indirubin: Indirubin is the natural occurring alkaloid and active component of danggui longhui wan used in East Asian medicine and is found in some plants such as Indigofera tinctoria L. and Isatis tinctoria L (Blažević et al. 2015; Chen et al. 2017). Indirubin has poor water solubility, resulting in low oral bioavailability, and oral usage mostly leads to irritation in the gastrointestinal tract (Chen et al. 2012). Indirubin prevents the growth of different human cancer cells by arresting at the G2/M or G1 phase in the cell cycle during the apoptosis process (Lo and Chang. 2013). Several experiments have shown that indirubin inhibits cyclin-dependent kinases (CDKS) and glycogen synthase kinase 3β (GSK3β) in tumor cells leading to annihilating the progression of the cell cycle. Indirubin can induce the apoptosis process via suppression of Stat3, leading to a decrease in cell proliferation and survival. Indirubin increases antiproliferative influences via controlling growth factor pathways by way of extracellular signal-regulated kinases (Erk), protein kinase B (Akt), and Notch1 and cytokines (Fogaça et al. 2017). Indirubin Derivatives, namely Indirubin-3′-Monoxime (I3M), inhibit oral cancer cell proliferation and induce apoptosis via the arresting cell cycle primarily at the G2/M phase and increase p53 and p21WAF1 expression. I3M inhibits cancer cell migration and invasion through levels of FAK, MMP-9, and uPA play. Also, indirubin can suppress oral cancer tumorigenesis via decreasing survivin gene expression (Lo and Chang. 2013) [90-94].
Vitamin group
γ-Tocotrienol: One of the members of the vitamin E family is tocotrienol that exists in a wide range of vegetable oils, barley, wheat germ, and some types of grains and nuts. Tocotrienols include four types respectively with the name of alpha, beta, gamma, and delta (Ahsan et. 2014). γ-Tocotrienol (GT3) has more powerful antioxidative, anti-hypercholesterolemic, and anticancer than tocopherols, indicating that GT3 has remarkable health benefits. However, the absorption of tocotrienols was found to be low and incomplete via the oral way, and only roughly 9% bioavailability can be watched in vivo. GT3, like all Fat-soluble vitamins, can be better absorbed with the help of surfactants or from emulsified vehicles such as polyethylene glycol (PEG-400) (Deng et al. 2014). γ-tocotrienol exerts considerable anti-proliferative influences in malignant cells, but not in normal cells (Takano et al. 2015). In chemotherapy, GT3 at low doses provides appropriate conditions to annihilate human OC (B88) cells via docetaxel by reducing the expression of the NF-κB-regulated gene related to the prevention of the apoptosis process. Moreover, the initiator caspases such as caspases-8 and -9 and caspase-3 are activated (Kani et al. 2013) [95-99].
Isothiocyanate
Sulforaphane: Sulforaphane is a natural aliphatic isothiocyanate compound and that is derived from cruciferous vegetables like broccoli, cauliflower, and cabbage (Cheung and Kong. 2010; Houghton. 2019) with absolute bioavailability of around 80% (Houghton. 2019). It has many biological influences, such as anti-inflammatory, neuroprotective, anti-angiogenesis, and anticancer effects. Sulforaphane decreases the levels of SCC-9 and SCC-14 markers related to cell motility and reduce invasiveness via the expression of cathepsin. It also escalates the conversion of microtubule-associated protein 1 light chain 3 (LC3), and the knockdown of LC3 via siRNA leads to broadening cell migration capability. This sulfur-rich compound hampers cell motility of oral cancer cells via the extracellular signal-regulated kinase (ERK) (Chen et al. 2018) [100-105].
Drug
Statins: Statins (3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase inhibitors are a broadly prescribed class of drugs to diminish cholesterol. Their function is through the inhibition of HMG-CoA (hydroxy methylglutaryl-coenzyme A) reductase during biosynthesis of cholesterol (Kim et al. 2019; Kim et al. 2019). The bioavailability of statin drugs is relatively low associated with metabolism in the gut wall and following ‘first-pass’ metabolism in the liver. However, some non-oral routes such as parenteral systems and drug delivery increase bioavailability (Korani et al. 2019). Statins have anti-inflammatory actions and decrease interferon-gamma (IFNγ) production, and tumor necrosis factor-alpha (TNF-α) in stimulated T-lymphocytes, and hamper the immune response in the T helper cell (Th-1). Also, it has antioxidant and anti-tumor capacities and is effective on various psychiatric disorders (Kim et al. 2019). It is proved that anticancer influences of the statins and the potential promise of statin compounds as an adjunct to standard treatments available for HNSCC (Islam et al. 2013). Statins hamper the synthesis of isoprenoids essential to localize membrane and following activation of signaling proteins like Ras, Rho, and Rac, leading to raised apoptosis. Also, statins hamper cholesterol synthesis leading to inhibition of the Akt signaling pathway and increase apoptosis in cancer cells (Gehrke et al. 2017). Atorvastatin, simvastatin, lovastatin, pitavastatin, rosuvastatin, fluvastatin, and pravastatin are the common types of statins (Meor Anuar Shuhaili et al. 2017).
Statins have been illustrated to prevent PI3K/AKT, mTOR signaling pathways and mevalonate in OCs. Statins can hamper the action of EGFR and induce the LKB1/AMPK signaling pathway that is enhanced upon ATP depletion in terms of metabolic stress. The statin Lovastatin can cause the launch of pre-G1 dependent apoptosis and simvastatin arrests in OCs cells in the G0/G1 phase (Pavan et al. 2015) [106-111].
2018). In chemotherapy, chemical compounds with cytotoxic capacities target the cancer cells. Chemotherapy remains the primary treatment option for cancer; however, there are severe side effects (Schirrmacher et al. 2019). The chemotherapy drug annihilates the basal cells of the mucosal layer directly but affects their substitute or turnover, leading to mucosal ulceration [112]. The drugs also have indirect toxic influences such as immune cells destruction or myelosuppression happening far from oral cancer. Moreover, salivary enzymes’ action is damaged. Many chemotherapeutic drugs impair the cell DNA leading to chromosomal abnormalities and mutations [113]. Long-term chemotherapy in patients induces risky secondary malignancies like acute leukemia around two to six years after chemotherapy. There are various general chemotherapeutic side effects, such as leukopenia, anemia, defects in spermatogenesis, cardiotoxicity, pericardial thickening or cardiac arrhythmias, thromboembolism, reactivation of hepatitis B, neurologic complications, peripheral neuropathy (CIPN), nausea and vomiting and hair loss (Poulopoulos et al., 2017).The limitation of chemotherapy has been explained mainly by mechanisms that moderate drug resistance at the cellular level or factors intrinsic to tumor microenvironment and the host (da Silva et al., 2012) [114].
After chemotherapy, the oral cavity is a common sight of infections, often caused by bacteria, fungi, and viruses (van Dalen et al., 2016). Mucositis is the most prevalent acute adverse effect experienced by patients undergoing head and neck radiotherapy and chemotherapy. It is characterized by infiltration of the inflammatory cells and epithelial disruption and ulceration with or without pseudomembranes (Napeñas et al.,2007; Tolentino et al., 2011) [115].
Poly (ADP-ribose) polymerase-1 (PARP1) upregulation is a clinically relevant mechanism involved in oral cancer recurrence leading to highly resistant to DNA damage treatment (Wang et al., 2022). PARP inhibitors hamper the repair of radiation-induced single-strand breaks, resulting in replication fork failure and following DSBs in S-phase (Rose et al., 2020). Inhibition of PARP1 overactivation by PARP1 inhibitors causes apoptosis in cancer cells, diminishing mutagenesis, metastasis, necrosis, and tumour-promoting inflammation (Wang et al., 2017). Although despite the practical destructive effects on oral cancer cells, the amount of PARP increases with the antioxidant Quercetin [116].
In radiation therapy, x-rays and gamma rays destroy cancer cells, and Immunotherapy is programmed to stimulate immune cells to invade the cell cancers (Thyagarajan and Sahu. 2018). However, immunotherapy as an indication with survival salvage lead to improved oncologic results in OSCC (Shetty et al., 2022). Radiation therapy also has severe side effects or, in many cases, is not effective on their own. During radiation therapy, a small proportion of cancer cells can survive via enhancing DNA repair and ROS stress response signals, including upregulation of antioxidants with associated molecules p53, SODs [117], glutathione peroxidase 1, sestrin, adaptation to hypoxia, and inhibition of ROS production with associated molecules HIF1, VEGF, PDK1, activation of DNA damage-sensing and repair proteins with associated molecules BRCA1, BRCA2, ATM, γH2AX, DNA-PK, ATR, MDC1, generation of bioactive lipid metabolites with associated molecules COXs, LOXs, HNE (non-protein), Glycolytic reprogramming and mitochondrial malfunction with associated molecules HIF1, PDK1, Activation of UPR signalling with associated molecules PERK, ATF4, ATF6, IRE1, upregulation of cytoprotective autophagy with associated molecules ATGs, ULK1, Beclin-1, and as a result, acquire radioresistance (Kim et al. 2019) [118].
Concerning the high mortality rate, it is necessary to find advanced treatments to eradicate it. However, the combination of therapeutic methods shows better outcomes, but there are already mortality rates, side effects, or recurrent OCs. Most of the mentioned anticancer/ antioxidants, including Apigenin, Genistein, Casticin, Kaempferol, Quercetin, Farnesol, Brusatol, Ophiopogonin D, Cryptotanshinone, Zerumbone, Berberine, Epigallocatechin gallate, Honokiol, Magnolol, Resveratrol, Indirubin, γ-Tocotrienol and Statins including Pitavastatin, Simvastatin, Lovastatin, Lovastatin (+Monensin/+Gefitinib) and Pravastatin target cell carcinoma in OCs by apoptosis via various signaling showing (Table 1) [119].
| Anticancer | Affects Oral Cancer Type (In vivo and/or In vitro) |
Mechanism of Action | Pathway/genes | |
|---|---|---|---|---|
| Apigenin | Oral Squamous Cell Carcinoma Cell Line SCC-25 (DeRango-Adem and Blay, 2021) | Apoptosis (DeRango-Adem and Blay, 2021) | Decreasing the Expression cyclin D1 and E and Inactivation of Cyclin-dependent kinase 1 (CDK1) (Maggioni et al. 2013) | |
| Genistein | Tongue Squamous Cell Carcinoma (Ardito et al. 2017) | Inhibiting efficaciously cell viability, Proliferation, Adhesion, and Migration, and Promoting Apoptosis (Ardito et al. 2017) | Inhibiting survivin and OCT4 (Ardito et al. 2017) | |
| Casticin | Human Oral Squamous Cell Carcinoma (OSCC) UM1 and HSC-3 cells (Xie et al. 2019) | Downregulating β-catenin in Wnt/β-catenin pathway (Xie et al. 2019) |
CTNNB1 Gene (Ramchandani et al. 2020) | |
| OSCC (Chou et al. 2018) | Apoptosis (Chou et al. 2018) | CASP3, CASP8, CASP9, AIFM1 and CYCS (Ramchandani et al. 2020) | ||
| Deguelin | HNSCC cell lines (Yang et al. 2013) |
Autophagy (Yang et al. 2013) |
Activating AMP-activated protein kinase (AMPK), (Ulk1 Gene) (Yang et al. 2013) | |
| HNSCC cell lines (Baba et al. 2015) |
Apoptosis (Baba et al. 2015) |
EGFR-Akt and IGF1R-Akt Pathways (Baba et al. 2015) | ||
| Kaempferol | SCC-4 Cell (Lin et al. 2013) SCC-1483, SCC-25, and SCC-QLL1 (Kubina et al. 2021) |
Inhibiting Cell Migration and Invasion (Lin et al. 2013), Arresting G0/G1 phase (Imran et. 2019), and Apoptosis (Kubina et al. 2021) |
Reducing Matrix Metalloproteinase-2 Expression by Down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways (Lin et al. 2013), Bcl-2, MMP-2, hexokinase-2, and c-Jun genes (Kubina et al. 2021) |
|
| Quercetin | Human Oral Cancer SAS cells (Ma et al. 2018) |
Apoptosis (Ma et al. 2018) | CASP3, CASP8, and CASP9 (Ma et al. 2018) | |
| Human Oral Squamous Carcinoma Cells (HOSCCs) (Scheper et al. 2008) | Apoptosis (Scheper et al. 2008) |
Inducing of Caspases and Inhibiting Survivin (Scheper et al. 2008) |
||
| Farnesol | OSCC9 and OSCC25 (Jung et al. 2018) |
Apoptosis (Jung et al. 2018) |
CASP3 and CASP9 (Jung et al. 2018) |
|
| Brusatol | HNSCCs (Lee et al. 2019) |
Inhibiting Cell Proliferation and Angiogenesis and Inducing Apoptosis (Lee et al. 2019) |
Inhibits the Ability of STAT3 to Bind DNA (Lee et al. 2019) |
|
| Ophiopogonin D | Human Laryngocarcinoma Cells (Yan et al. 2019) |
Inhibits Cell Proliferation and Induces Apoptosis (Yan et al. 2019) |
Downregulation of Cyclin B1 and MMP‑9 and Upregulation of p38‑MAPK Signaling (Yan et al. 2019) | |
| Cryptotanshinone (In Combination with TW-37 ) |
Human Oral Cancer Cell Lines (Yang et al. 2020) |
Apoptosis (Yang et al. 2020) |
Inhibiting STAT3–Mcl-1 Signaling (Yang et al. 2020) | |
| Zerumbone | OSCC (Zainal et al. 2018) |
Apoptosis (Zainal et al. 2018) |
Suppressing Akt and S6 proteins and Inhibiting PI3K-mTOR (Zainal et al. 2018) |
|
| Berberine | Oral Squamous Cell Carcinomas-Cancer Stem cells (OSCC-CSCs) (Lin et al. 2017) | Reduction of Self-Renewal and Metastatic Properties (Lin et al. 2017) | Targeting miR-21 , Regulating the Expression of Phosphatase and Tensin Homolog (PTEN) and Programmed Cell Death 4 (PD4D4) and PTEN/Akt Signaling Pathway (Lin et al. 2017) |
|
| KB human oral cancer cells (Kim et al. 2015) | Suppression of Migration, Inducing FasL-related Apoptosis (Kim et al. 2015) | Downregulation of MMP-2 and MMP-7, and p38 Activation (Kim et al. 2015) | ||
| Curcumin | OSCCs (Zhen et al. 2014) |
Inhibiting Proliferation and Invasion (Zhen et al. 2014) |
EGFR signaling pathways (Zhen et al. 2014) |
|
| OSCCs (Maulina et al. 2019) |
Inhibiting the Epithelial Dysplasia Stage of OSCC (Maulina et al. 2019) |
Suppressing Nuclear Factor Kappa B and Cyclooxygenase 2 Expression (Maulina et al. 2019) |
||
| Epigallocatechin gallate | OSCCs (Almatroodi et al. 2020) |
Inhibiting Cell Migration, Motility, Spread and Adhesion/ Apoptosis/ Antitumor Immunity (Almatroodi et al. 2020) |
Inhibiting HGF-induced phosphorylation of Met and Invasion, cell Growth, and Expression of MMP-2 and MMP-9/ Increasing caspase-7 and -3 activities (Almatroodi et al. 2020) |
|
| Honokiol | OSCCs (Huang et al. 2018) |
Cell Cycle Arrest, Apoptosis, and Autophagy (Huang et al. 2018) |
Arresting the Cell Cycle at G0/G1 Stage, Increasing caspase-3, and Increasing LC3‐II and p62 Expression (Huang et al. 2018) |
|
| Magnolol | Human Oral Cancer Cell Lines (Chen et al. 2021) |
Apoptosis (Chen et al. 2021) |
JNK1/2- and p38-mediated MAPK Pathways (Chen et al. 2021) |
|
| Resveratrol | OSCCs (Xiao et 2021) |
Inhibiting Proliferation and Apoptosis (Xiao et 2021) | Inducing the ZNF750/RAC1 Signaling Pathway (Xiao et 2021) |
|
| Indirubin | Cal-27 and HSC-3 Oral Cancer Cell Lines (Lo and Chang. 2013) |
Apoptosis (Lo and Chang. 2013) |
Arresting Cell Cycle at G2/M Phase and Increasing p53, and Activation of Cytochrome c (Lo and Chang. 2013) |
|
| γ-Tocotrienol | Human Oral Cancer Cell Line (B88) (Takano et al. 2015) |
Apoptosis (Takano et al. 2015) |
caspases-8 and -9 and caspase-3 (Takano et al. 2015). |
|
| Sulforaphane | SCC-9 and SCC-14 Cell Lines (Chen et al.2018) |
Increasing Cell Migration and Inducing Autophagy (Chen et al.2018) |
ERK Signaling Pathway and Suppressing Cathepsin S Expression (Chen et al.2018) |
|
| Statins | Pitavastatin | OSCCs (Lee et al. 2020) |
Apoptosis (Lee et al. 2020) |
Inhibiting AKT and Activating AMPK Leading to FOXO3A Activation and Induction of PUMA (Lee et al. 2020) |
| Simvastatin + Celecoxib | HNSCCs (Gehrke et al. 2017) |
Apoptosis (Gehrke et al. 2017) |
Arresting Cell Cycle at G0/G1Phase (Gehrke et al. 2017) |
|
| Atorvastatin | HNSCCs (Islam et al. 2013) |
Reducing Cell Motility, Invasion, Proliferation, and Colony Formation (Islam et al. 2013) |
Decreasing RhoC and Reducing Activation of ERK1/2 and STAT3 Signaling Cascades (Islam et al. 2013) |
|
| Lovastatin | HNSCC cells (Pavan et al. 2015) |
Apoptosis (Pavan et al. 2015) |
EGFR Pathway (Pavan et al. 2015) |
|
| Lovastatin + Monensin | SCC-9 and SCC-25 cells (Tongue Squamous Cell Carcinoma) (Pavan et al. 2015) |
Apoptosis (Pavan et al. 2015) |
Mevalonate Path Way (Niknejad et al. 2007) Inhibitor p21 and Arresting cell cycle at G1 Phase (Pavan et al. 2015) |
|
| Lovastatin + Gefitinib | SCC-9 and SCC-25 cells (Tongue Squamous Cell Carcinoma) (Ma et al. 2012) |
Apoptosis and Cytotoxicity of Cancer Cells (Ma et al. 2012) |
LKB1/AMPK activation (Ma et al. 2012) |
|
| Pravastatin | SCC-9 (Pavan et al. 2015) |
Apoptosis (Pavan et al. 2015) |
EGFR pathway (Pavan et al. 2015) |
|
Table1: The Function of antioxidants in oral cancers.
Other effects of anticancer/antioxidants on OCs include: Casticin via downregulating β-catenin in the Wnt/β-catenin pathway; Deguelin, Honokiol and Sulforaphane via autophagy; Genistein, Brusatol, Ophiopogonin D, Curcumin, Resveratrol, Atorvastatin from statins via inhibition of cell proliferation; Genistein via inhibition of efficaciously cell viability, adhesion and migration; Kaempferol via inhibition of cell migration, invasion, and arresting G0/G1 phase; Brusatol via inhibition of angiogenesis, Berberine via reduction of self-renewal and metastatic properties; Curcumin via inhibition of Proliferation and invasion and inhibition of the epithelial dysplasia stage of OSCC; Epigallocatechin gallate via inhibition of cell migration, motility, spread, adhesion and antitumor immunity; Honokiol via cell cycle arrest, Sulforaphane via decrease cell migration; Atorvastatin via reduction of cell motility, invasion, proliferation, and colony formation; and Lovastatin via cytotoxicity of cancer cells [120].
Most recently published reviews have given the role of these compounds in different cancers; however, none of the studies have presented an in-depth and comprehensive overview of their dual role of anticancer-antioxidant in oral cancer cells and healthy cells. The experiments demonstrated that the majority of mentioned components have anticancer activity leading to the destruction of OSCCs [121]. The compounds can be used for treatment alone or with other treatments as complementary therapeutic methods. However, more clinical studies are needed to determine the exact therapeutic doses and timing. Unfortunately, there is little accurate statistical data, and there is a need for extensive research on each of the compounds. In each case, the effective dose should be evaluated clinically and statistically. Further, examining the effectiveness of the mentioned compounds in combination with other drugs can also be a breakthrough in treating oral cancers and a salve for the suffering of patients [122].
Conclusion and Remarks
Recent clinical experiments have reported the beneficial effects of mentioned antioxidants on oral cancers. Therefore, it is likely that the compounds mentioned have a dual function, including destructive effects on oral cancer cells and positive protective effects on healthy cells via various signalling pathways.
Acknowledgement
The authors would like to sincerely thank Bahman Rafiei and Robert Earl Nickerson for their support.
Potential Conflict of Interest
The authors have no conflicting financial interests. We declare that there is no conflict of interests regarding the publication of this paper.
References
- Abassi Joozdani F, Yari F, Abassi Joozdani P, Nafisi S (2015) Interaction of sulforaphane with DNA and RNA. PLoS One 10:127-541.
- Alavi M, Farkhondeh T, Aschner M, Samarghandian S (2021) Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int 21:579.
- Ali R, Mirza Z, Ashraf GM, Kamal MA, Ansari SA, et al. (2012) New anticancer agents: recent developments in tumor therapy. Anticancer Res 32:2999-3005.
- Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, et al. (2020) Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 25:3146.
- David AV, Arulmoli R, Parasuraman S (2016) Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn Rev 10:84-89.
- Arango D, Morohashi K, Yilmaz A, Kuramochi K, Parihar A, et al. (2013) Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc Natl Acad Sci 110:2153-2162.
- Ardito F, Pellegrino MR, Perrone D, Troiano G, Cocco A, et al. (2017) In vitro study on anti-cancer properties of genistein in tongue cancer. Onco Targets Ther 10:5405-5415.
- Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, et al. (2012) Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med 12:1244-52.
- Azhar N, Sohail M, Ahmad F, Fareeha S, Jamil S, et al. (2018) Risk factors of Oral cancer- A hospital based case control study. J Clin Exp Dent 10:396-401.
- Baba Y, Fujii M, Maeda T, Suzuki A, Yuzawa S, et al. (2015) Deguelin induces apoptosis by targeting both EGFR-Akt and IGF1R-Akt pathways in head and neck squamous cell cancer cell lines. Biomed Res Int 34:65-71.
- Banerjee S, Li Y, Wang Z, Sarkar FH (2008) Multi-targeted therapy of cancer by genistein. Cancer Lett 269:226-242.
- Bundela S, Sharma A, Bisen PS (2015) Potential Compounds for Oral Cancer Treatment: Resveratrol, Nimbolide, Lovastatin, Bortezomib, Vorinostat, Berberine, Pterostilbene, Deguelin, Andrographolide, and Colchicine. PLoS One 10:141-149.
- Cai SJ, Liu Y, Han S, Yang C (2019) Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci 9:45.
- Mouli PE, Senthil B, Parthiban S, Malarvizhi AE, Priya R, et al. (2012) Antioxidants and its role in Oral Cancer- A Review. Indian J Sci 1:113-115.
- Chang MT, Lee SP, Fang CY, Hsieh PL, Liao YW, Lu MY, et al. (2018) Chemosensitizing effect of honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3 signaling. Environ Toxicol 33:1105-1112.
- Chen BH, Hsieh CH, Tsai SY. Wang CY, Wang CC, et al. (2020) Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci Rep 10:51-63.
- Chen CT, Hsieh MJ, Hsieh YH, Hsin MC, Chuang YT, et al. (2018) Sulforaphane suppresses oral cancer cell migration by regulating cathepsin S expression. Oncotarget 9:17564-17575.
- Chen L, Huang C, Shentu J (2017) Indirubin Derivative 7-Bromoindirubin-3-Oxime (7Bio) Attenuates Aβ Oligomer-Induced Cognitive Impairments in Mice. Front Mol Neurosci 10:393.
- Chen S, Li X, Liu L, Liu C, Han X, et al. (2018) Ophiopogonin D alleviates high-fat diet-induced metabolic syndrome and changes the structure of gut microbiota in mice. FASEB J 32:1139-1153.
- Chen W, Lu Y, Chen G, Huang S (2013) Molecular evidence of Cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents Med Chem 13:979-987.
- Chen YT, Lin CW, Su CW, Yang WE, Chuang CY, et al. (2021) Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways. Biomedicines 9:1295.
- Chen ZQ, Liu Y, Zhao JH, Wang L, Feng NP, et al. (2012) Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int J Nanomedicine 7:1115-1125.
- Chen Z, Zhu R, Zheng J, Chen C, Huang C, et al. (2017) Cryptotanshinone inhibits proliferation yet induces apoptosis by suppressing STAT3 signals in renal cell carcinoma. Oncotarget 8:50023-50033.
- Cheung KL, Kong AN (2010) Molecular targets of dietary phenethy isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J 12:87-97.
- Cho JH, Jeon YJ, Park SM, Shin JC, Lee TH, et al. (2015) Multifunctional effects of honokiol as an anti-inflammatory and anti-cancer drug in human oral squamous cancer cells and xenograft. Biomaterials 53:274-84.
- Chou GL, Peng SF, Liao CL, Ho HC, Lu KW, et al. (2018) Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ Toxicol 33:127-141.
- Chu C, Deng J, Man Y, Qu Y (2017) Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. Biomed Res Int. 8:561-564.
- Cid-Ortega S, Monroy-Rivera JA (2018) Extraction of Kaempferol and Its Glycosides Using Supercritical Fluids from Plant Sources. Food Technol Biotechno l56:480-493.
- Costa A, Facchini G, Pinheiro ALTA, Bonner MY, Arbiser J, et al. (2017) Honokiol protects skin cells against inflammation, collagenolysis, apoptosis, and senescence caused by cigarette smoke damage. Int J Dermatol 56:754-761.
- Deng L, Peng Y, Wu Y, Yang M, Ding Y, et al. (2014) Tissue distribution of emulsified γ-tocotrienol and its long-term biological effects after subcutaneous administration. Lipids Health Dis 13:66.
- DeRango-Adem EF, Blay J (2021) Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front Pharmacol 12:681-687.
- Farooqi AA, Khalid S, Ahmad A (2021) Regulation of Cell Signaling Pathways and miRNAs by Resveratrol in Different Cancers. Int J Mol Sci 19:652.
- Feng X, Sureda A, Jafari S (2019) Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 9:1923-1951.
- Fogaça MV, Cândido-Bacani PM, Benicio LM, Zapata LM, Cardoso PF, et al. (2017) Effects of indirubin and isatin on cell viability, mutagenicity, Genotoxicity and BAX/ERCC1 gene expression. Pharm Biol 55:2005-2014.
- Friedland PL, Bozic B, Dewar J, Kuan R, Meyer C, et al. (2011) Impact of multidisciplinary team in the management in head and neck cancer patients. Br J Cancer 104:1246-1248.
- Gao Y, Wang F, Song Y, Liu H (2018) the status of and trends in the pharmacology of berberine: a bibliometric review. Chin Med 20:15-17.
- Gavin A, Pham JT, Wang D, Brownlow B, Elbayoumi TA, et al. (2015) Layered nanoemulsions as mucoadhesive buccal systems for controlled delivery of oral cancer therapeutics. Int J Nanomedicine 10:1569-84.
- Gehrke T, Scherzad A, Hackenberg S, Ickrath P, Schendzielorz P, et al. (2017) Additive antitumor effects of celecoxib and simvastatin on head and neck squamous cell carcinoma in vitro. Int J Oncol 51:931-938.
- Girisa S, Shabnam B, Monisha J, Fan L, Halim CE, et al. (2019) Potential of Zerumbone as an Anti-Cancer Agent. Molecules 24:734.
- Houghton CA et al (2019) Sulforaphane: It’s "Coming of Age" as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid Med Cell Longev 19:271-180.
- Hseu YC, Chang CT, Gowrisankar YV, Chen XZ, Lin HC, et al. (2019) Zerumbone Exhibits Antiphotoaging and Dermatoprotective Properties in Ultraviolet A-Irradiated Human Skin Fibroblast Cells via the Activation of Nrf2/ARE Defensive Pathway. Oxid Med Cell Longev 2019:409-415.
- Hsieh SF, Chou CT, Liang WZ, Kuo CC, Wang JL, et al. (2018) The effect of magnolol on Ca2+ homeostasis and its related physiology in human oral cancer cells. Archives of Oral Biolog 89:49-54.
- Huang KJ, Kuo CH, Chen SH, Lin CY, Lee YR, et al. (2018) Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J Cell Mol Med 22:1894-1908.
- Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, et al. (2019) Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules. 24(12).
- Intapa C, Rizk MJ (2014) Farnesol-induced apoptosis in oral squamous carcinoma cells is mediated by MRP1 extrusion and depletion of intracellular glutathione. Pathology 46:94-95.
- Islam M, Sharma S, Kumar B, Teknos TN (2013) Atorvastatin inhibits RhoC function and limits head and neck cancer metastasis. Oral Oncol 49:778-86.
- Jaramillo MC, Zhang DD (2013) the emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27:2179–2191.
- Jung YY, Hwang ST, Sethi G, Fan L, Arfuso F, et al. (2018) Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 23(11).
- Ju Z, Sun J, Liu Y (2019) Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules 24:38-41.
- Kani K, Momota Y, Harada M, Yamamura Y, Aota K, et al. (2013) γ-tocotrienol enhances the chemo sensitivity of human oral cancer cells to docetaxel through the down regulation of the expression of NF-κB-regulated anti-apoptotic gene products. Int J Oncol 42:75-82.
- Kaşıkcı MB, Bağdatlıoğlu N (2016) Bioavailability of Quercetin. Curr Res Nutr Food Sci 2:146-151.
- Khani Y, Pourgholam-Amiji N, Afshar M, Otroshi O, Sharifi-Esfahani M, et al. (2018) Tobacco Smoking and Cancer Types. Biomedical Research and Therapy 5:2142-2159.
- Kharbanda OP, Ivaturi A, Priya H, Dorji G, Gupta S, et al. (2019) Digital possibilities in the prevention and early detection of oral cancer in the WHO South-East Asia Region. J Public Health 8:95-100.
- Kim JS, Oh D, Yim MJ (2015) Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol 33:1775-1782.
- Kim JW, Amin ARM, Shin DM (2010) Chemoprevention of Head and Neck Cancer with Green Tea Polyphenols. Cancer Prev Res (Phila) 3(8):900-909.
- Kim SW, Kang HJ, Jhon M, Kim JW, Lee JY, et al. (2019) Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front Psychiatry 10:103.
- Kim JS, Oh D, Yim MJ, Park JJ, Kang KR, et al. (2015) Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol Rep 33:1775-1782.
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, et al. (2017) The Role of Resveratrol in Cancer Therapy. Int J Mol 18(12).
- Korani S, Bahrami S, Korani M, Banach M, Johnston TP, et al. (2019) Parenteral systems for statin delivery: a review. Lipids Health Dis 18:193.
- Lee JH, Kim C, Um JY, Sethi G, Ahn KS, et al. (2019) Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers (Basel) 11:254.
- Lee JH, Rangappa S, Mohan CD (2019) Brusatol a Nrf2 Inhibitor Targets STAT3 Signaling Cascade in Head and Neck Squamous Cell Carcinoma. Biomolecules 9:550.
- Lee N, Pun N, Jang WJ, Bae JW, Jeong CH, et al. (2020) Pitavastatin induces apoptosis in oral squamous cell carcinoma through activation of FOXO3a. J Cell Mol Med 24:7055-7066.
- Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, et al. (2013) Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11:338-378.
- Lingen MW, Kalmar JR, Karrison T, Speight PM (2008)
Citation: Rafiei H, Rafiei M (2023) Oral Cancers: A New Perspective on Anticancer/Antioxidants. J Cancer Diagn 7: 174.
Copyright: © 2023 Rafiei H, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef , Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef , Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef , Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4379
- [From(publication date): 0-2023 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 4014
- PDF downloads: 365
