Overexpression of Programmed Cell Death-Ligand 1 in Small-Cell Lung Cancer and Survival Analysis
Received: 15-Jun-2018 / Accepted Date: 19-Jun-2018 / Published Date: 26-Jun-2018 DOI: 10.4172/2476-2253.1000116
Abstract
Purpose: Programmed cell death-ligand-1 (PD-L1) has identified overexpression in many solid carcinomas. However, the expression in small cell lung cancer (SCLC) remains unclear and the association between the PD-L1 expression and prognosis is not well investigated. Methods: The expression of PD-L1 was evaluated in 136 specimens of SCLC by immunohistochemistry in Huai’an First People’s Hospital. PD-L1 expression was defined as tumors staining in over 5% of tumor cells. Survival analysis was evaluated using the Kaplan-Meier method. Multivariate regression was performed with the Cox proportional hazards model. Results: One hundred and thirty-six patients were enrolled in present study,including 78 of extensive and 58 with limited stage. PD-L1 expression was detected in 54.4% of all the patients (53.8% in extensive and 55.2% in limited stage). Patients with PD-L1 positive expression showed better overall survival (OS) than PD-L1 negative patients regardless of extensive or limited small cell lung cancer (P value were 0.002 and 0.016, respectively). One hundred and twenty-one patients were with recurrence or metastasis. Median progression free survival of first-line chemotherapy in PD-L1 positive patients was 5.30 and 3.50 months in PD-L1 negative patients (P=0.030). PD-L1 remained as significant prognostic factor for better survival with multivariate analyses (HR=0.76; P=0.041). Conclusion: Our results that PD-L1 is overexpressed in 54.4% of SCLC patients. Expression of PD-L1 is correlated with a favorable PFS and OS in SCLC.
Keywords: Programmed cell death-ligand-1; Small cell lung cancer; Prognosis
Introduction
Small cell lung cancer (SCLC) is one of the main subtypes of lung cancer that accounts for approximately 15-20% of all lung cancer [1,2]. More than 60%-70% of SCLC patients were with metastasis at diagnosis. Despite a high rate of response to initial chemotherapy, most of patients may suffer progression [3,4]. New therapeutic method has not emerged currently and with median survival time under one year in extensive SCLC.
Programmed cell death (PD-1/PD-L1) pathway plays an important role to limit the activity of T lymphocyte in tissues at the time of an inflammatory response to infection [5,6]. PD-L1 is broadly expressed in human carcinomas [7,8]. Several preclinical or clinical trials have demonstrated that inhibition of this pathway in solid carcinomas with anti-PD-1 or PD-L1 antibodies exerts a promising effect [9,10]. However, the studies of PD-L1 expression in SCLC have remained unknown. We examined PD-L1 expression in SCLC and investigated its association with clinicopathologic characteristics and prognostic value.
Materials and Methods
Study population
Totally, 136 patients with primary SCLC diagnosed at Huai’an First People’s Hospital from 2009 to 2015 were enrolled in present study. The inclusion criteria were as follows: (1) pathologically proven primary SCLC, and without combined SCLC (2) all patients were staged according to the Veterans’ Administration Lung Study Group staging system [11], (3) disease progression was confirmed using brain MRI, chest/abdomen computed tomography (CT), and ultrasound examination, (4) none of the patients had received chemotherapy or radiotherapy treatment at the time of diagnosis, and (5) Eastern Cooperative Oncology Group performance status (ECOG) of 0 to 2. The study protocol was approved by the Institutional Review Board of Huai’an First People’s Hospital and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). All of the participants gave informed consent before taking part in the present study.
Immunohistochemical analysis of PD-L1 expression
Immunohistochemical (IHC) staining of PD-L1 expression was performed on 4-6 μm thick formalin-fiated, paraffi-embedded tissue. The concentration of rabbit primary antibody that reacts to PD-L1 The concentration of rabbit primary antibody that reacts to PD-L1 (Proteintech Group Inc., Chicago, IL, USA, Catalog number: 66248-1- Ig) was 1:100 in Dako antibody diluent; slides were incubated with this antibody overnight at 4°C. Then, the slides were incubated with Ventana Omni Mapanti-rabbit secondary antibody for 60 min. AVentana Chromo MapKit was used for antibody detection, and then the slides were counterstained with hematoxylin. Next, the slides were dehydrated and cover slipped as per normal laboratory protocol. All slides were examined by two pathologists independently;
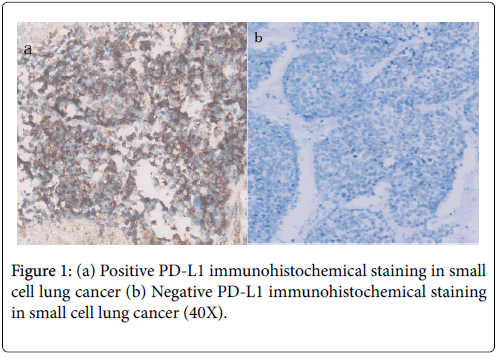
PD-L1 criteria were according to intensity and extent of staining: (1) negative, when staining was absent or detected in <5% of the cells; and (2) positive, when membranous staining was present in ≥ 5% of the cells.
Follow-up
All patients were evaluated progression-free survival (PFS) and overall survival (OS), and none were lost to follow-up. The median follow-up period was 13.5 months (5.6-23); Last follow-up day was Dec 31, 2016.
Statistical analysis
The Chi-squared test was used to evaluate the relationships between clinical characteristics and PD-L1 expression. Survival curves were calculated using the Kaplane-Meier method from the start of confirmed pathology to date of death or last follow-up. The PFS encompassed the time from the start of treatment to documented progression or last follow-up time. Cox regression model was used for multivariate analysis. Statistical analysis was used with the SPSS 18 software (Chicago, IL, US). P<0.05 were considered statistically significant.
Results
Patient characteristics
The clinical and pathological characteristics of the 136 patients are listed in Table 1. Of all patients enrolled, there were 108 males and 28 females with a median age of 58 years (range, 31-78 years). The performance status (PS) was 0-1 in 112 patients (82.4%) and PS 2 accounted for 17.6%. Seventy-eight was in extensive stage and fiftyeight with limited stage on presentation. One hundred and five patients had a history of smoking. One hundred and twenty-one were with recurrence or metastasis and received first-line chemotherapy. Platinum-based first-line chemotherapy was applied in all of the 121 patients. Seventy-six patients received EP (etoposide+cisplatin), 40 with EC (etoposide+carboplatin) regimen and five with other regimens.
| Variable | Number |
|---|---|
| Gender | |
| Male | 108 |
| Female | 28 |
| Age | |
| Range | 31-78 |
| Median | 58 |
| <65 | 105 |
| ≥ 65 | 31 |
| Smoking status | |
| Never | 31 |
| Former/current | 105 |
| Performance status | |
| 0-1 | 112 |
| 2 | 24 |
| Serum NSE level | |
| Normal | 45 |
| Abnormal | 91 |
| Serum LDH level | |
| Normal | 52 |
| Abnormal | 84 |
| Recurrence or metastasis | |
| Yes | 121 |
| No | 15 |
| PD-L1 expression | |
| Positive | 74 |
| Negative | 62 |
| Stage at diagnosis | |
| Limited | 58 |
| Extensive | 78 |
Table 1: Demographic characteristics of the study population.
PD-L1 expression and its correlation with patient characteristics
Seventy-four (54.4%) patients had PD-L1 staining positive. Table 2 showed the correlation between patient characteristics and PD-L1 expression. No significant correlation existed between PD-L1 expression and gender (P=0.45), age (P=0.548), smoking history (P=0.72), PS (P=0.11), serum NSE level (P=0.58), serum LDH level (P=0.55) and stage (P=0.88).
| Variable | PD-L1 positive (n=74) | PD-L1 negative (n=62) | P |
|---|---|---|---|
| Gender | |||
| Male | 57 | 51 | 0.45 |
| Female | 47 | 11 | |
| Age | |||
| <65 | 58 | 47 | 0.72 |
| ≥ 65 | 16 | 15 | |
| Smoking status | |||
| Never | 17 | 14 | 0.96 |
| Former/current | 57 | 48 | |
| Performance status | |||
| 0-1 | 58 | 54 | 0.11 |
| 2 | 16 | 8 | |
| Serum NSE level | |||
| Normal | 26 | 19 | 0.58 |
| Abnormal | 48 | 43 | |
| Serum LDH level | |||
| Normal | 30 | 22 | 0.55 |
| Abnormal | 44 | 40 | |
| Stage | |||
| Limited | 32 | 26 | 0.88 |
| Extensive | 42 | 36 | |
Table 2: Comparison clinical characteristics between the PD-L1 positive and PD-L1 negative patients.
Survival analysis
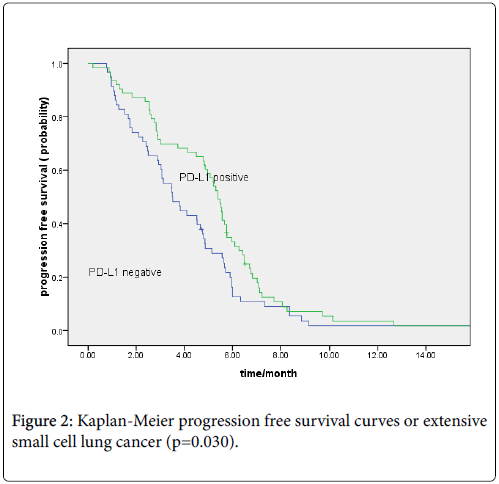
Totally, 121 patients were with recurrence or metastasis. The median overall survivals were 18 and 10.6 months in limited and extensive small cell lung cancer, respectively. Patients with PD-L1 positive expression showed better OS than PD-L1 negative patients (15.40 vs. 11.0 months, P=0.001; regardless of limited (18.0 vs. 12.3 months, P=0.013) or extensive stage (12.4 vs. 8.7 months, P=0.002) (Figures 1a and 1b). The median PFS for first-line chemotherapy for the 121 patients was 4.8 months. The PD-L1-positive group showed longer PFS than the PD-L1-negative group (5.40 vs. 3.50 months, P=0.030; Figure 2).
Univariate analysis demonstrated that PD-L1 expression (P=0.001), good PS (P<0.001), and limited stage (P<0.001) were associated with a favorable OS (Table 3). Multivariate analysis was performed to detect factors that associated with OS. Good PS, PD-L1 expression positive and limited stage were significant favorable predictive factors for OS (Table 4).
| Variable | First-line PFS (n=121) | P | Median overall survival (n=136) | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 4.67 | 0.133 | 12.7 | 0.17 |
| Female | 5.67 | 16.5 | ||
| Age | ||||
| <65 | 4.8 | 0.212 | 15.3 | 0.512 |
| ≥ 65 | 3.07 | 12.6 | ||
| Smoking status | ||||
| Never | 5.4 | 0.322 | 16.5 | 0.089 |
| Former/current | 4.5 | 12.9 | ||
| Performance status | ||||
| 0-1 | 5.07 | <0.001 | 17.7 | <0.001 |
| 2 | 2.83 | 8.6 | ||
| Serum NSE level | ||||
| Normal | 5.25 | 0.212 | 15.5 | 0.087 |
| Abnormal | 4.43 | 10.7 | ||
| Serum LDH level | ||||
| Normal | 5.43 | 0.043 | 14.5 | 0.196 |
| Abnormal | 4.11 | 11.3 | ||
| Stage at diagnosis | ||||
| Limited | 4.97 | 0.95 | 18 | <0.001 |
| Extensive | 4.56 | 10.6 | ||
| PD-L1 expression | ||||
| Positive | 5.4 | 0.03 | 15.4 | 0.001 |
| Negative | 3.5 | 11 | ||
Table 3: Univariate analysis of patients’ first-line progression-free survival and overall survival according to the clinicopathologic characteristics.
| Variable | OS | ||
|---|---|---|---|
| HR | 95% CI | p | |
| Stage (Limited vs. extensive) | 0.45 | 0.27-0.89 | 0.007 |
| Performance status (2 vs. 0-1) | 2.77 | 1.17-3.98 | 0.019 |
| PD-L1 expression (yes vs. no) | 0.76 | 0.56-0.97 | 0.041 |
Table 4: Multivariate survival analysis for overall survival.
Discussion
We demonstrated that PD-L1 was overexpression in 54.4% of SCLC, and that PD-L1 expression was associated with favorable survival regardless of extensive and limited stage patients. Progression free survival of first-line chemotherapy in PD-L1 positive patients was longer than those in PD-L1 negative patients.
An association of PD-L1 expression with clinicopathologic factors has been reported in several studies [12-14]. The differentiation status was identified to be correlated with PD-L1 expression in a metaanalysis revolving the NSCLC [15]. Some driver genes such as EGFR, KRAS have been showed with higher PD-L1 expression in NSCLC in several studies [16,17], while, other reports observed that there was no significant correlation between PD-L1 expression and driver genes [18]. For driver gene is not widely detection in SCLC, the relationship between driver gene of SCLC and PD-L1 expression is not known currently.
Ishii et al. demonstrated that expression of PD-L1 was significantly correlated with a limited stage but not extensive stage disease [19]. However, only 41 patients with limited stage were included in Ishii et al. study. The limited number of patients may influence the results of this report. No clinical factors were found to be associated with PD-L1 expression in current cohort, regardless of limited or extensive stage.
Previous reports have demonstrated that expression of PD-L1 is associated with prognosis in many solid carcinomas. However, the results were conflicting [20,21]. Studies previously have reported that PD-L1 expression was associated with poor survival in patients with several solid carcinomas [20,21]. In contrast, PD-L1 is correlated with a favorable survival in other carcinomas [13,15]. Ishii et al. showed that patients with expression of PD-L1 had significantly better prognosis than those with negative expression in limited SCLC [19]. In current study, the results demonstrated that patients with PD-L1 expression have a better PFS and OS in SCLC, including in limited and extensive stage. To our knowledge, a significant association between PD-L1 expression and better PFS and OS has not been specifically reported previously in extensive SCLC.
There were some limitations in our study. One major limitation was its retrospective nature and with relatively small number patients. Secondly, different antibodies are used in different anti-PD-1 or PD-L1 drugs in clinical trials currently; the choice of antibody and threshold for positivity might be influence the results of different studies. Only one antibody and 5% as threshold were used in our study, different antibodies of PD-L1 needs to be validate in the same sample in future studies.
In summary, the present study is the report with largest number patients’ evaluated PD-L1 levels in SCLC. Our results suggest association between the presences of PD-L1 with overall survival. However, for the limitation of our study with retrospective nature, the value of PD-L1 expression in SCLC as a candidate prognosis factor needs to be validated in the future.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Riaz SP, Lüchtenborg M, Coupland VH, Spicer J, Peake MD, et al. (2012) Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer 75: 280-284.
- Meza R, Meernik C, Jeon J, Cote ML (2015) Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One 10: e0121323.
- Altan M, Chiang AC (2015) Management of Small Cell Lung Cancer: Progress and Updates. Cancer J 21: 425-433.
- Shi Y, Xing P, Fan Y, Zhang X, Hu C, et al. (2015) Current small cell lung cancer treatment in China. Thorac Cancer 6: 233-238.
- Muenst S, Soysal SD, Tzankov A, Hoeller S (2015) The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets 19: 201-211.
- Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X (2015) Human cancer immunotherapy with antibodies to the PD-1 and PD-L1pathway. Trends Mol Med 21: 24-33.
- Qin T, Zeng YD, Qin G, Xu F, Lu JB, et al. (2015) High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 6: 33972-33981.
- Schmidt LH, Kümmel A, Görlich D, Mohr M, Bröckling S, et al. (2015) PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 10: e0136023.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455-2465.
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515: 563-567.
- Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, et al. (2000) Staging small cell lung cancer: Veterans administration lung study group versus international association for the study of lung cancer-what limits limited disease? Lung Cancer 37: 271-276.
- Muenst S, Schaerli AR, Gao F, Däster S, Trella E, et al. (2014) Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 146: 15-24.
- Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, et al. (2014) In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 20: 2773-2782.
- Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, et al. (2015) PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 26: 1488-1493.
- Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, et al. (2015) The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 41: 450-456.
- Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, et al. (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25: 1935-1940.
- D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, et al. (2015) PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 112: 95-102.
- Zhang Y, Wang L, Li Y, Pan Y, Wang R, et al. (2014) Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 7: 567-573.
- Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, et al. (2015) Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 10: 426-430.
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, et al. (2004) Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A101:17174-17179.
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, et al. (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11: 2947-2953.
Citation: Yang S, Cheng G (2018) Overexpression of Programmed Cell Death-Ligand 1 in Small-Cell Lung Cancer and Survival Analysis. J Cancer Diagn 3: 116. DOI: 10.4172/2476-2253.1000116
Copyright: © 2018 Yang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6470
- [From(publication date): 0-2018 - Dec 21, 2025]
- Breakdown by view type
- HTML page views: 5468
- PDF downloads: 1002