Review Article Open Access
Palliative Care for Salivary Gland Dysfunction Highlights the Need for Regenerative Therapies: A Review on Radiation and Salivary Gland Stem Cells
Alejandro Martinez Chibly1, Thao Nguyen1 and Kirsten H Limesand2*
1The University of Arizona Cancer Biology Graduate Interdisciplinary Program, Tucson, AZ 85721, USA
2The University of Arizona Nutritional Sciences Graduate Program, Tucson, AZ 85721, USA
- *Corresponding Author:
- Kirsten H Limesand PhD
The University of Arizona Nutritional Sciences Graduate Program
1177 E 4th Street, Shantz 421 Tucson, AZ 85721, USA
Tel: (520) 626-4517
E-mail: limesank@u.arizona.edu
Received date: May 26, 2014; Accepted date: July 28, 2014 Published date: August 06, 2014
Citation: Chibly M A, Nguyen T, Limesand K H (2014) Palliative Care for Salivary Gland Dysfunction Highlights the Need for Regenerative Therapies: A Review on Radiation and Salivary Gland Stem Cells. J Palliat Care Med 4:180. doi: 10.4172/2165-7386.1000180
Copyright: © 2014 Chibly A M et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Radiotherapy remains the major course of treatment for Head and Neck cancer patients. A common consequence of radiation treatment is dysfunction of the salivary glands, which leads to a number of oral complications including xerostomia and dysphagia, for which there is no existent cure. Here, we briefly describe the current palliative treatments available for patients undergoing these conditions, such as oral lubricants, saliva substitutes, and saliva stimulants. None of these options achieves restoration of normal quality of life due to their limited effectiveness, and in some cases, adverse side effects of their own. Other therapies under development, such as acupuncture and electrostimulation have also yielded mixed results in clinical trials. Due to the ineffectiveness of palliative care to restore quality of life, it is reasonable to aim for the development of regenerative therapies that allow restoration of function of the salivary epithelium following radiation treatment. Adult stem cells are a necessary component of wound healing, and play important roles in preserving normal function of adult tissues. Thus, the present review mainly focuses on the effects of radiation on adult stem cells in a variety of tissues, which may be at play in the response of salivary glands to radiation treatment. This is of clinical importance because progenitor cells of the salivary glands have shown partial regenerative potential in mouse transplantation assays. Therefore, understanding how these progenitor cells are affected by radiation offers potential for development of new therapies for patients with xerostomia.
Keywords
Head and neck cancer; Xerostomia; Salivary gland dysfunction; Radiation; Stem cells
Introduction
The current standard treatment for head and neck cancer utilizes a multimodality approach, which includes surgery, in combination with radiotherapy and chemotherapy [1]. One of the most common side effects of radiotherapy treatment for head and neck cancer is dysfunction of the salivary glands [2]. The degree of dysfunction is dependent on the radiation dose and the amount of glandular tissue that is exposed in the radiation field [2,3]. Histopathological changes include loss of saliva-producing acinar cells, alteration in the epithelium of the ductal compartment, and fibrosis [4,5]. Ultimately, hypofunction of the salivary glands results in xerostomia, a condition characterized by patient reported severe oral dryness [3,4]. Reduction in saliva production and oral dryness can have a devastating effect on oral health that potentially leads to malnutrition and poorer quality of life [2,6,7]. In most cases,xerostomia develops into an irreversible life-long problem despite successful treatment of the cancer [8]. In addition, patients often need a feeding tube as they present with varying degrees of dysphagia, which predisposes to life-threatening pulmonary conditions [9]. Although palliative care exists to alleviate the symptoms of mouth dryness and difficulty swallowing, there are no definitive cures for xerostomia and dysphagia.
Palliative Care for Xerostomia and Dysphagia
The overall goal of palliative care for patients undergoing head and neck radiation is to improve the quality of life for these individuals and allow them to return to a “normal lifestyle” [10]. Common palliative therapies for xerostomia include oral lubricants, saliva substitutes, and saliva stimulants (gum, pilocarpine). Oral lubricants can include a variety of products such as mouthwashes, gels, and toothpastes that could protect the oral mucosa, while saliva substitutes attempt to incorporate all the natural functions of saliva. In general, these therapies are short-lived and have not consistently demonstrated the ability to relieve xerostomia symptoms [11]. Saliva stimulants rely on residual salivary gland function to provide protection to the oral cavity. Pilocarpine is the most commonly prescribed saliva stimulant; however due to a number of adverse side effects (excessive sweating, rhinorrhea, and frequent urination) and contraindications in patients with cardiovascular concerns, its utilization is limited [10,11]. Therapies currently under development include hyperbaric oxygen (HBO) therapy, acupuncture, and electrostimulation. In a retrospective pilot study where all patients underwent hyperbaric oxygen therapy, there were significant improvements in salivary secretions and a decrease in patient reported xerostomia [12]. While the long-term improvements in xerostomia following HBO are unknown, it is a promising treatment option for patients with long-term salivary dysfunction where other treatments have been ineffective [13]. The use of acupuncture for the treatment of xerostomia has had more mixed results [14]. A recent RCT across 7 oncology centers described reduced patient reported outcome measures related to dry mouth when compared to oral care education sessions [15]. Unfortunately the use of acupuncture in this study did not improve objective measurements of salivary output, which could be related to the long standing debate on the appropriate comparator group [14]. Overall a recent Cochrane review has determined that there is insufficient evidence that electrostimulation or acupuncture can consistently alleviate xerostomia [16].
Radiation-Induced Injury in Salivary Glands
It is clear that current palliative care fails to offer a decent quality of life to patients undergoing xerostomia, and better approaches need to be developed. In order to improve current treatments and develop regenerative therapies, it is necessary to better understand the mechanisms behind radiation-induced salivary dysfunction. In general, it is known that ionizing radiation induces DNA damage, either indirectly through the generation of reactive oxygen species (ROS), or directly through the breakage of the DNA double strand, which results in the acquisition of mutations or cell death [17]. Previous studies in animal models have shown that therapeutically relevant doses of targeted radiation to the head and neck induce cell death in the salivary glands [18,19], and chronically decrease the saliva output [4,5,20]. Depending on the cause of injury, cell type, and cellular response, cell death can be mediated by apoptosis and necrosis, while autophagy seems to play a protective role [21,22]. It is likely that all three pathways interact to determine the overall response of the salivary epithelium to radiation damage [21,23]. Findings from our lab demonstrated an early apoptotic response of acinar cells following radiation treatment, which is mediated by induction of p53 [18]. Similar findings were found in cells that are highly radiosensitive, such as thymocytes, lymphocytes, and the small intestine [24-26]. This early apoptotic response seems to be crucial for chronic salivary dysfunction, as several animal studies have achieved radioprotective effects and preservation of salivary function by inhibiting apoptosis [27,28]. Additional mechanisms have been proposed to contribute to salivary hypofunction following radiation, such as activation of the calcium permeable channel TRPM2 (transient potential melastatin-like 2) [29], and a possible loss of salivary progenitors [30].
Even though salivary glands are a slowly proliferative, fully differentiated tissue, work utilizing the ductal ligation technique has shown that salivary glands have some capacity to regenerate following an injury event [31-34]. Osailan et al. [31,34] demonstrated that by ligating the rat submandibular gland with a metal clip, cellular atrophy follows with major loss of acinar cells. When the parasympathetic ganglion (chorda lingual nerve) is excluded from the ligation clip, acinar cell atrophy occurs albeit the extent of atrophy is less severe. However, once the metal clip was removed, the glands underwent a regenerative process leading to restored glandular weight, histological structure and secretory output [31]. While de-ligated salivary glands appear completely restored structurally, a few defects in some enzymatic processes remain. In contrast with ductal ligation, ionizing radiation leads to persistent loss of salivary gland function despite cessation of the radiotherapy treatment [1,2,6]. This clearly indicates that radiation targets a major aspect of glandular regeneration and wound healing, potentially stem or progenitor cells. Nonetheless, restoration of salivary gland function has been achieved by administrating Insulin-like growth factor 1 (IGF-1) to irradiated mice [20]. This resulted in a normal salivary flow and amylase composition of the glands as early as 30 days after treatment [20]. While therapeutic administration of IGF-1 would not be clinically feasible in cancer patients, this study shows that the salivary glands have the capacity to regenerate following irradiation upon administration of the right stimuli. Interestingly, concurrent administration of IGF-1 and radiation to mice bearing head and neck cancer cell xenografts partially modulated tumor growth rates, but when IGF-1 was administered as a post-therapy following radiation, comparable tumor growth delays were observed as in the radiation group [35]. The mechanism by which IGF-1 aids in restoration of function in salivary glands following radiation is not well understood, highlighting the need to deepen our studies in the wound healing processes of the salivary glands.
Effects of Radiation on Adult Stem Cells
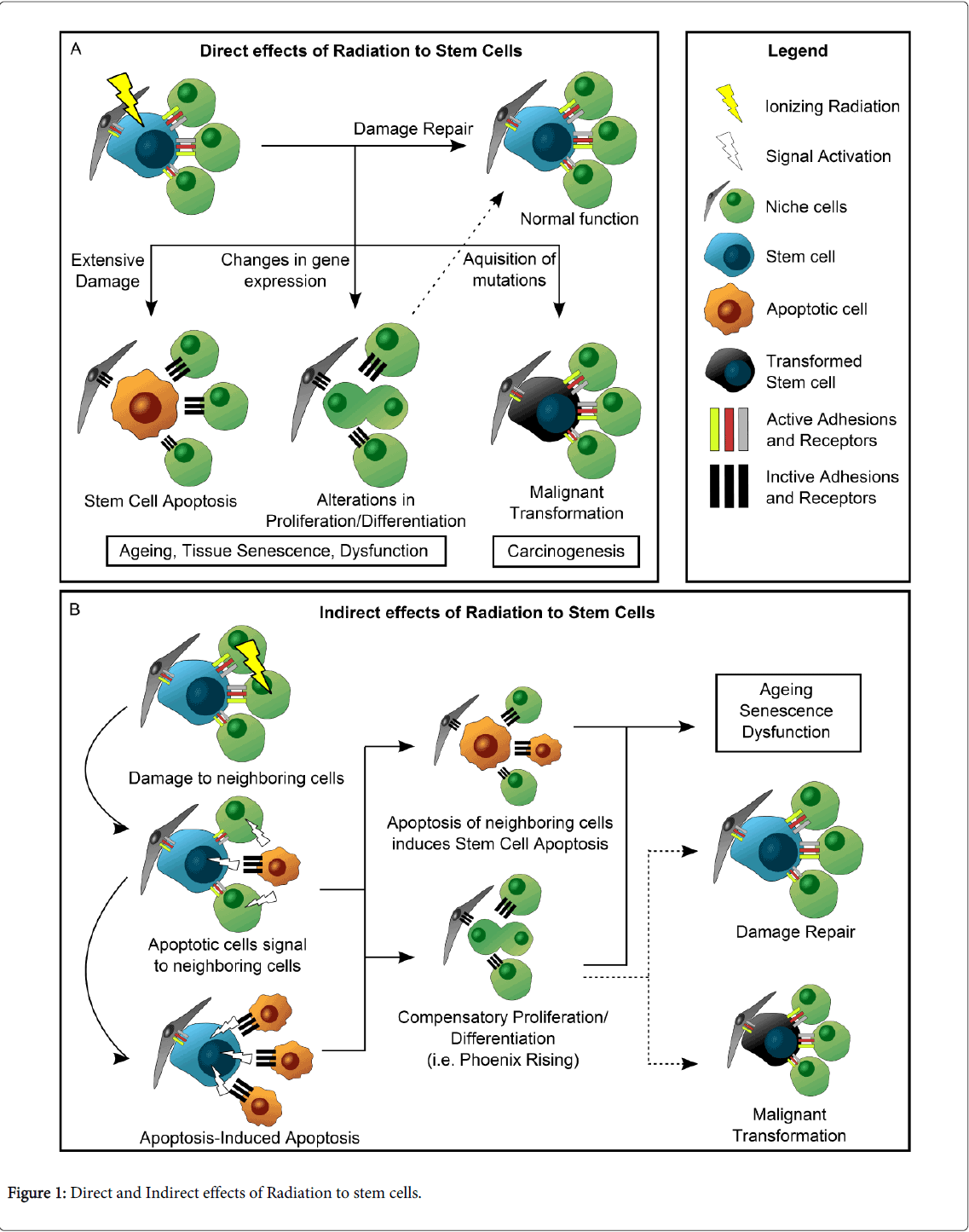
Adult stem cells are a necessary component of wound healing [36-39] and thus it is of great importance to understand the effects radiation treatment has upon these cells, particularly in tissues such as salivary glands, in which radiation-induced damage leads to a progressive and permanent loss of function, and regeneration does not naturally occur [20,40,41]. In the past few years, several studies have uncovered some of the mechanisms by which radiation directly and indirectly affects stem cells in a number of tissues (Figure 1). While such mechanisms cannot be generalized, they may serve as clues for what might happen in other tissues where the interaction between stem cells and radiation is not well understood. This section focuses on how the wound healing response is altered following radiation treatment, such that it allows for regeneration in tissues like the intestine, but fails to regain function in salivary glands or hair follicles.
Figure 1A summarizes the results of DNA damage as a direct mechanism of radiation-induced injury upon stem cells. Unrepaired DNA damage in stem cells is thought to be the cause of mutations that leads to carcinogenesis [38,39]. Additionally, radiation has been shown to modify gene expression in stem cells, altering cell fate decisions and differentiation pathways [38,42-44], which can lead to inappropriate proliferative responses or an abnormal state of quiescence [36,42]. All these changes have the potential to disrupt wound healing; however, stem cells have different ways of coping with DNA damage, and some will be more efficient at repairing it. In the intestinal epithelium, the Lgr5+ crypt base columnar (CBC) stem cells have been shown to be somewhat radioresistant in spite of being actively dividing cells [45]. Following radiation, CBCs undergo cell death due to extensive DNA damage, but their capacity to repair DNA more effectively through homologous recombination (HR), rather than the more inaccurate mechanism of non-homologous end joining (NHEJ), allows for a subset of CBCs to survive [45]. These surviving CBCs are responsible for regenerating the intestinal epithelium. In skin, Keratinocyte stem cells (KSC) and Melanocyte stem cells (MSC) work together to maintain the integrity of epidermis and hair follicles. Upon radiation exposure, KSC are more resilient to radiation-induced apoptosis but become largely quiescent and have reduced colony-forming activity [46]. MSCs, in contrast, are induced to exit their niche, initiate proliferation and subsequently differentiate into melanocytes, which likely depletes the pool of MSCs [42,46,47].
In addition to these direct mechanisms in which stem cell homeostasis is disturbed, radiation can also indirectly impair or induce their ability to regenerate their homing tissue (Figure 1B). Stem cells are highly dependent on their interaction with the cellular niche in order to function properly. They maintain adhesions with their surrounding cells, which are in part responsible for the transmission of signals that regulate stem cell fate decisions [48]. When these neighboring cells are damaged by external factors, signaling changes occur that will dictate stem cell behavior. Li F. et al. [49] described a process called Phoenix Rising, in which the apoptotic cells signal to the neighboring stem cells to begin proliferation and tissue repair. In their study, Caspase 3 and Caspase 7 from apoptotic cells activated calcium-independent Phospholipase A2, which in turn increased the synthesis of arachidonic acid, a precursor of prostaglandin E2 (PGE2). The latter is a known inducer of stem cell proliferation and was found to increase in response to radiation of MEF cells in a Caspase-dependent fashion [49]. In contrast to this study, it was recently reported that apoptotic cells in Drosophila are capable of emitting long-range signals through the JNK pathway to induce apoptosis in distal sites (apoptosis-induced apoptosis) [50]. Whether any of these mechanisms contribute to radiation-induced salivary gland dysfunction remains to be determined.
Regenerative Potential of Salivary gland Stem Cells
In salivary glands, therapeutic doses of radiation are sufficient to induce an apoptotic response in the secretory acinar cells [18]. In turn, increased levels of proliferation take place shortly after apoptosis, and are sustained chronically [28]. Thus, it is feasible that the events depicted in Figure 1 and described above could explain why radiation treatment causes permanent damage to salivary glands. For example, as explained in the previous sections, it is possible that radiation treatment directly targets salivary progenitors, impairing their ability for self-renewal and differentiation; alternatively, it is also possible that radiation causes a disruption in cell-cell interactions, which are required for proper function of stem cells. In fact, it was recently reported that Keratin 5 (K5) progenitors in submandibular gland explants from mice embryos survive after radiation treatment, but epithelial regeneration is greatly impaired nonetheless [51]. In this case, stimulation of the parasympathetic ganglion with neurturin promoted glandular regeneration, suggesting that an interaction between parasympathetic nerves and salivary progenitors must occur in order to restore homeostasis in the salivary glands.
It has been proposed that stem cell therapies have great potential for regeneration of the salivary glands after radiation injury [52]; therefore, it is of clinical importance to determine whether the mechanisms shown in Figure 1 take place in the salivary epithelium. It has been hypothesized that radiation-induced dysfunction of the salivary glands is due to the induction of cell death in salivary gland stem cells [30,53,54], but no scientific evidence exists to support this theory. Nevertheless, a study showed that transplantation of c-kit+ putative salivary gland progenitors to the submandibular gland of irradiated animals allows for partial restoration of function, demonstrating the therapeutic potential of salivary gland stem cells [54]. A major problem that remains in the field is the poor characterization of adult salivary gland stem cells, which does not allow for improvement in transplantation and regeneration therapies. Some markers have been found in salivary gland progenitors, such as Ascl3 [55], Keratin 5 [56], Keratin 14 [57], and c-kit [54], which have important functions in development. However, the relative importance of these individual progenitors in adult salivary glands, as well as their collective role in glandular homeostasis remains elusive. Further studies aimed at the mechanisms that prevent salivary gland progenitors from restoring homeostasis following radiation, will greatly facilitate the development of new therapies for patients with salivary hypofunction.
Conclusion
Radiation-induced salivary gland dysfunction is a common problem in head and neck cancer patients, which leads to adverse conditions such as dysphagia and xerostomia, for which there is no cure. Given the lack of effectiveness of the current palliative care for head and neck cancer patients undergoing radiotherapy, it is crucial that regenerative therapies are further developed, in order to increase quality of life of these patients. It is vital for this purpose that studies are aimed to better characterize salivary gland stem cells to facilitate future mechanistic studies. Regeneration of the salivary glands has been achieved in animal models, but the mechanisms for regeneration are mostly unknown. The use of salivary gland stem cells remains a promising field for developing regenerative therapies, and thus it is of major clinical importance to characterize this population and their involvement in radiation-induced injury to the salivary epithelium.
References
- Collins R, Flynn A, Melville A, Richardson R, Eastwood A (2005) Effective health care: management of head and neck cancers. QualSaf Health Care 14: 144-148.
- Dirix P, Nuyts S, Van den Bogaert W (2006) Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer 107: 2525-2534.
- Valdez IH, Atkinson JC, Ship JA, Fox PC (1993) Major salivary gland function in patients with radiation-induced xerostomia: flow rates and sialochemistry. Int J RadiatOncolBiolPhys 25: 41-47.
- De la Cal C, Fernández-Solari J, Mohn C, Prestifilippo J, Pugnaloni A, et al. (2012) Radiation produces irreversible chronic dysfunction in the submandibular glands of the rat. Open Dent J 6: 8-13.
- Radfar L, Sirois D a: Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surgery, Oral Med Oral Pathol Oral RadiolEndodontology 2003, 96:267–274.
- Jensen SB, Pedersen AM, Reibel J, Nauntofte B (2003) Xerostomia and hypofunction of the salivary glands in cancer therapy. Support Care Cancer 11: 207-225.
- Kakoei S, Haghdoost AA, Rad M, Mohammadalizadeh S, Pourdamghan N, et al. (2012) Xerostomia after radiotherapy and its effect on quality of life in head and neck cancer patients. Arch Iran Med 15: 214-218.
- Jham BC, Reis PM, Miranda EL, Lopes RC, Carvalho AL, et al. (2008) Oral health status of 207 head and neck cancer patients before, during and after radiotherapy. Clin Oral Investig 12: 19-24.
- Jensen SB, Pedersen a ML, Vissink a, Andersen E, Brown CG, Davies a N, et al. (2010)A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 2010, 18:1039–60.
- Goldstein NE, Genden E, Morrison RS (2008) Palliative care for patients with head and neck cancer: "I would like a quick return to a normal lifestyle". JAMA 299: 1818-1825.
- Dost F, Farah CS (2013) Stimulating the discussion on saliva substitutes: a clinical perspective. Aust Dent J 58: 11-17.
- Forner L, Hyldegaard O, von Brockdorff AS, Specht L, Andersen E, et al. (2011) Does hyperbaric oxygen treatment have the potential to increase salivary flow rate and reduce xerostomia in previously irradiated head and neck cancer patients? A pilot study. Oral Oncol 47: 546-551.
- Hadley T, Song C, Wells L, Lehnhardt J, Rogers MW, et al. (2010)Does hyperbaric oxygen therapy have the potential to improve salivary gland function in irradiated head and neck cancer patients? Med Gas Res 3:15.
- Towler P, Molassiotis A, Brearley SG (2013) What is the evidence for the use of acupuncture as an intervention for symptom management in cancer supportive and palliative care: an integrative overview of reviews. Support Care Cancer 21: 2913-2923.
- Simcock R, Fallowfield L, Monson K, Solis-Trapala I, Parlour L, et al. (2013) ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Ann Oncol 24: 776-783.
- Furness S, Bryan G, Mcmillan R, Birchenough S, Hv W: Interventions for the management of dry mouth?: non- pharmacological interventions. Cochrane Libr 2013.
- Suzuki K, Ojima M, Kodama S, Watanabe M (2003) Radiation-induced DNA damage and delayed induced genomic instability. Oncogene 22: 6988-6993.
- Avila JL, Grundmann O, Burd R, Limesand KH (2009) Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J RadiatOncolBiolPhys 73: 523-529.
- Limesand KH, Avila JL, Victory K, Chang H-H, Shin YJ, et al. (2010) Insulin-like growth factor-1 preserves salivary gland function after fractionated radiation. Int J RadiatOncolBiolPhys 78:579–86.
- Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH (2010) Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer 10: 417.
- Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. CurrOpin Cell Biol 16: 663-669.
- Morgan-Bathke M, Hill GA, Harris ZI, Lin HH, Chibly AM, et al. (2014) Autophagy correlates with maintenance of salivary gland function following radiation. Sci Rep 4: 5206.
- Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. BiochimBiophysActa 1833: 3448-3459.
- Belloni P, Meschini R, Czene S, Harms-Ringdahl M, Palitti F (2005) Studies on radiation-induced apoptosis in G0 human lymphocytes. Int J RadiatBiol 81: 587-599.
- Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847-849.
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, et al. (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285: 1733-1737.
- Arany S, Benoit DS, Dewhurst S, Ovitt CE (2013) Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol Ther 21: 1182-1194.
- Limesand KH, Avila JL, Victory K, Chang HH, Shin YJ, et al. (2010) Insulin-like growth factor-1 preserves salivary gland function after fractionated radiation. Int J RadiatOncolBiolPhys 78: 579-586.
- Liu X, Cotrim A, Teos L, Zheng C, Swaim W, et al. (2013) Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat Commun 4: 1515.
- Konings AW, Coppes RP, Vissink A (2005) On the mechanism of salivary gland radiosensitivity. Int J RadiatOncolBiolPhys 62: 1187-1194.
- Osailan SM, Proctor GB, Carpenter GH, Paterson KL, McGurk M (2006) Recovery of rat submandibular salivary gland function following removal of obstruction: a sialometrical and sialochemical study. Int J ExpPathol 87: 411-423.
- Carpenter GH, Osailan SM, Correia P, Paterson KP, Proctor GB (2007) Rat salivary gland ligation causes reversible secretory hypofunction. ActaPhysiol (Oxf) 189: 241-249.
- Correia PN, Carpenter GH, Osailan SM, Paterson KL, Proctor GB (2008) Acute salivary gland hypofunction in the duct ligation model in the absence of inflammation. Oral Dis 14: 520-528.
- Osailan SM, Proctor GB, McGurk M, Paterson KL (2006) Intraoral duct ligation without inclusion of the parasympathetic nerve supply induces rat submandibular gland atrophy. Int J ExpPathol 87: 41-48.
- Victory K, Burd R, Fribley A, Sittadjody S, Arnett D, et al. (2011) Head and neck tumor cell radiation response occurs in the presence of IGF1. J Dent Res 90: 347-352.
- Choi J, Artandi S (2009) Stem cell aging and aberrant differentiation within the niche. Cell Stem Cell 5: 6-8.
- Barker N, Bartfeld S, Clevers H(2010) Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 7:656–670.
- Insinga A, Cicalese A, Pelicci PG (2014) DNA damage response in adult stem cells. Blood Cells Mol Dis 52: 147-151.
- Harfouche G, Martin MT (2010) Response of normal stem cells to ionizing radiation: a balance between homeostasis and genomic stability. Mutat Res 704: 167-174.
- Grundmann O, Mitchell GC, Limesand KH (2009) Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res 88: 894-903.
- Martin KL, Hill GA, Klein RR, Arnett DG, Burd R, et al. (2012) Prevention of radiation-induced salivary gland dysfunction utilizing a CDK inhibitor in a mouse model. PLoS One 7: e51363.
- Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, et al. (2009) Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137: 1088-1099.
- Nicolay NH, Sommer E, Lopez R, Wirkner U, Trinh T, et al. (2013) Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J RadiatOncolBiolPhys 87: 1171-1178.
- SokolovMV, Panyutin IV, Panyutin IG, Neumann RD (2011) Dynamics of the transcriptome response of cultured human embryonic stem cells to ionizing radiation exposure. Mutat Res 709-710: 40-8.
- Metcalfe C,Kljavin NM, Ybarra R, de Sauvage FJ (2014) Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14: 149-159.
- Aoki H, Hara A, Motohashi T, Kunisada T (2013) Keratinocyte stem cells but not melanocyte stem cells are the primary target for radiation-induced hair graying. J Invest Dermatol 133: 2143-2151.
- Chou WC, Takeo M, Rabbani P, Hu H, Lee W, et al. (2013) Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med 19: 924-929.
- Chen S, Lewallen M, Xie T (2013) Adhesion in the stem cell niche: biological roles and regulation. Development 140: 255-265.
- Li F, Huang Q, Chen J, Peng Y, Roop DR, et al. (2010) Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration. Sci Signal 3: ra13.
- Pérez-Garijo A, Fuchs Y, Steller H (2013) Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. Elife (Cambridge) 2: e01004.
- Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, et al. (2013) Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun 4: 1494.
- Lombaert IM, Knox SM, Hoffman MP (2011) Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis 17: 445-449.
- Hai B, Yang Z, Millar SE, Choi YS, Taketo MM, et al. (2010) Wnt/ß²-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev 19: 1793-1801.
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, et al. (2008) Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3: e2063.
- Rugel-Stahl A, Elliott ME, Ovitt CE (2012) Ascl3 marks adult progenitor cells of the mouse salivary gland. Stem Cell Res 8: 379-387.
- Knox SM, Lombaert IM a, Reed X, Vitale-Cross L, Gutkind JS, et al. (2010)Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329:1645–1647.
- Lombaert IM, Abrams SR, Li L, Eswarakumar VP, Sethi AJ, et al. (2013) Combined KIT and FGFR2b Signaling Regulates Epithelial Progenitor Expansion during Organogenesis. Stem Cell Reports 1: 604-619.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 15115
- [From(publication date):
August-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 10489
- PDF downloads : 4626