Research Article Open Access
Pharmacokinetic Study of Zaltoprofen Spherical Agglomerated Dense Compacts Canvassed with Commercial Product
| Hari Krishna E1*, Rama Mohan Gupta V2 and Jyothi S1 | |
| 1Department of Pharmaceutics, CES College of Pharmacy, Kurnool, India | |
| 2Principal, Pulla Reddy Institute of Pharmacy, Dommadugu, Dundigal, Hyderabad, India | |
| Corresponding Author : | Hari Krishna E Associate Professor CES College of Pharmacy Kurnool-18, India Tel: 919948904629 E-mail: elluruharikrishna@gmail.com |
| Received January 08, 2013; Accepted January 28, 2013; Published February 01, 2013 | |
| Citation: Hari Krishna E, Rama Mohan Gupta V, Jyothi S (2013) Pharmacokinetic Study of Zaltoprofen Spherical Agglomerated Dense Compacts Canvassed with Commercial Product. Clinic Pharmacol Biopharm 2:107. doi:10.4172/2167-065X.1000107 | |
| Copyright: © 2013 Hari Krishna E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
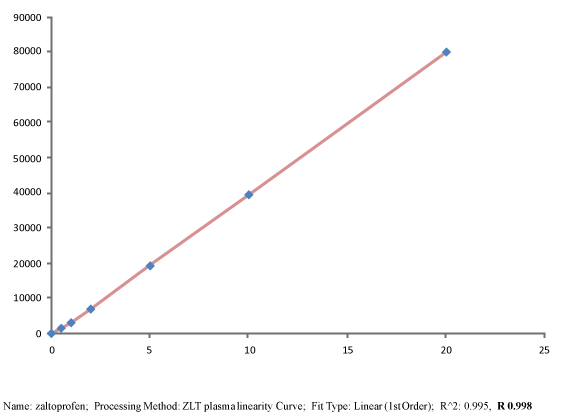
Pharmacokinetic parameters including AUC0-t, AUC0-∝, Cmax, Tmax, T1/2 and Elimination rate constant (Kel) were determined from plasma concentrations of Zaltoprofen spherical agglomerated dense compacts by 24 factorial design canvassed with a commercially available formulation of Zaltoprofen. Foremost spherical agglomerates of Zaltoprofen prepared with PEG 6000 which is hydrophilic polymer and evaluated for different compressibility parameters and in vitro dissolution studies, studies indicated that the drug release can be modulated by using hydrophilic polymers and the best one compressed as dense compacts and appraised for pharmacokinetic parameters in rabbit models. Sensitive and selective high performance liquid chromatographic method was used to determine drug plasma concentrations with good linearity in the range of 0.5 to 20 μg/mL (r2=0.995, n=7). Study ascertained meliorated values of AUC0-24 (15.789 ± 12.9, 97.334 ± 7.478).
| Keywords |
| Pharmacokinetic study; Zaltoprofen; Spherical agglomerates; Spherical crystals; Rabbit model |
| Introduction |
| The conventional tableting method used involves first making granules and then compressing into tablets, but the need in recent years for process validation, GMP and automation of production processes has focused renewal of attention on the direct tableting, which involves few steps [1]. Zaltoprofen, is a novel NSAID, selective COX2 inhibiting drug used in the treatment of rheumatoid arthritis and osteoarthritis and to relieve inflammation and pain after surgery [2]. Zaltoprofen exhibits poor flow and compression characteristics and hence Zaltoprofen selected as suitable candidate for spherical crystallization process to improve flow properties and compressibility. Further, Zaltoprofen shows poor oral bioavailability due to low aqueous solubility [3] hence in such case enhancing bioavailability is a valuable goal to improve therapeutic efficacy. Estimation of Zaltoprofen in plasma by methods like UV-visible spectrophotometric [4], Mass spectrometry in human plasma [5] was reported. |
| In the present study intended to prepare spherical crystals of Zaltoprofen to improve aqueous solubility, dissolution rate and bioavailability besides improving it micromeritic properties using PEG-6000 which is hydrophilic polymer [6] the best formulation compressed into dense compacts and in this study further intended to determine the pharmacokinetic parameters for optimized formulation by factorial design an followed by in vivo study, rabbit as an animal model and comparative to a marketed formulation. |
| Materials and Methods |
| Materials |
| Zaltoprofen (99.3% purity), as a gift sample was received from M.S Hetero pharmaceutical, Hyderabad. Acetonitrile and water were HPLC grade from RFCL Ltd (Mumbai, India). Bath sonicator was used for degassing (PCI Analytics, Mumbai, India). Elico pH meter was used for adjustment. Ten station rotator tablet punching machine used for compression of tablets with 7 mm flat shaped punch (Elite tablet punching machine, Mumbai) REMI R-2 research centrifuge and REM- 101 cyclo mixer were used for extraction of drug from plasma (REMI Dectrotechnik Ltd., Vasai, India). |
| Formulation of Zaltoprofen spherical agglomerates |
| A solution of Zaltoprofen (500 mg) in acetone (3 mL) was added to a solution of Poly ethylene glycol 6000 (1-4% w/v) in 100 mL distilled water. The mixture was stirred continuously using digital mechanical stirrer (IKA motors. Mumbai) at 500 rpm, the bridging liquid (Dichloromethane; 0.5 mL) was added drop wise (Table 1) and stirring was continued for 30 min. The agglomerates were separated by filtration using whatman filter paper (No.1) and dried for 24 hours at room temperature. |
| Compression of dense compacts |
| Amid of all spherical agglomerates, the best performed agglomerates in terms of compressibility properties selected as optimized batch. It was thought of interest to study the effect of super disintegrants such as Ac-Di-Sol (CCS), Polyplasdone XL (CP) and Explotab (SSG) on the drug release of Zaltoprofen. |
| Spherical crystals were made into dense compacts with 4% w/w CCS, CP and SSG separately using Elite tablet punching machine, 10-station rotary punching machine (7 mm punch and die set) at a compression force of 6 tons. |
| In vitro dissolution studies |
| The in vitro dissolution studies [7] were carried out using 8 station USP XXIII dissolution testing apparatus (Electrolab, Mumbai, India). The dissolution medium used was 900 mL, mixture of phosphate buffer solution pH 6.8 and water (1:1) used as dissolution medium. The agglomerates containing 80 mg of Zaltoprofen were weighed and then introduced into the dissolution medium. The medium was stirred at 50 rpm using paddle at 37 ± 0.5°C. The samples were collected, filtered through whatman filter paper (0.45 μM) and analyzed spectrophotometrically at 340 nm. |
| In Vivo Studies |
| In vivo chromatographic conditions of HPLC |
| The HPLC system included system was automatically processed with and fully automated processor with Elite program. The pre column used in HPLC system was Capcell pak and the main analytical column used in the system was Capcell pak C18. Mobile phase A (a 12:88 v/v mixture of Acetonitrile and 10 mM Phosphate buffer) and Mobile Phase B (A 35:65 v/v mixture of Acetonitrile and 10 mM Phosphate buffer, pH 6.8) was pumped into the HPLC system at a flow rate of 500 μL/min and 90 μL/min. The Zaltoprofen concentrations in effluent of the system were discovered with a UV detector at 340 nm and the temperatures in the system of pre and analytical columns were maintained at 40°C and auto sampler chamber was sustained at 4°C. |
| Extraction procedure |
| The Zaltoprofen solutions were added to blank plasma samples of rabbits, 5 mL of ethyl acetate and 50 μL of amyl alcohol were added and adjusted the pH to 7.4 with 0.2 mol NaOH for absolute recovery followed by centrifuge at 2500 r/min at 40 min. The ethyl acetate phase was separated and evaporated under the nitrogen stream, reconstituted with 2 mL of mobile phase and stored at -20°C till analyzed by RPHPLC system. |
| Study Design and Pharmacokinetic Analysis |
| Methods |
| In a single-dose, open-label, crossover study, twelve rabbits bisected randomly in two groups, six rabbits in each group. White Rabbits weighing 1.4-1.6 kg were allowed free access to a normal chow diet and water for at least one week before the experiments. The rabbits were anesthetized with diethyl ether, and the common central ear vein was cannulated with polyethylene tubing for blood sampling. The cannula was flushed and filled with heparinized isotonic saline (100 IU/mL) to prevent the blood from clotting. The rabbits were housed individually in a cage for one day to allow them to recover from surgery. |
| The rabbits were fasted overnight for at least 6 hour before the experiment, with free access to water. The rabbits were assigned to one of two groups each of 6 rabbits in each group. A mini capsule filled with Spherical agglomerates formulation and marked formulation equivalent to 6.5 mg of Zaltoprofen was administered orally with a mini capsule injector. Immediately after oral dosing, 5 mL of distilled water was given to each rabbit to help them swallow the minicapsule. About 1 mL of blood was collected from the each rabbit at 0, 0.25, 0.5, 1, 2, 4, 8, 12, 24 hour after the oral administration of the drug. About 0.2 mL of heparinized 0.9% NaCl solution was let into the cannula just after each bold sample was taken and centrifuged immediately; the plasma was collected and stored at -20°C until HPLC analysis. |
| The pharmacokinetic parameters of spherical crystals formulation and marketed formulation after the oral administration were determined by non-compartmental analysis using Winnonlin software. The pharmacokinetic parameters estimated in this study are area under the plasma concentration time curve from 0-24 h (AUC0-24), area under the plasma concentration time curve from 0 hour to infinity (AUC0-∞), elimination half-life (t1/2), Volume of distribution (Vd) and clearance (Clt), the maximum plasma concentration (Cmax) and the time required to reach Cmax (tmax). |
| WINNONLIN® (WINNONLIN NONCOMPARTMENTAL ANALYSIS PROGRAM 1.0 Core Version 04 Jun 2007) is the industry standard used for Pharmacokinetic, Pharmacodynamic, and noncompartmental analysis. In addition to its extensive library of built-in PK, PD and PK/PD models. |
| Results and Discussions |
| In vitro dissolution studies |
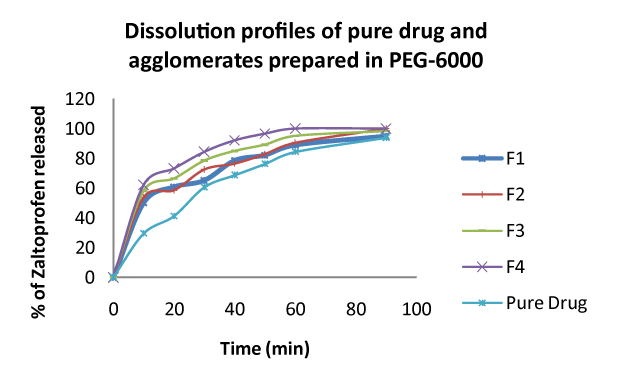
| The results of in vitro dissolution studies are shown in figures 1 and 2, Compressibility parameters in table 2. Pure Zaltoprofen exhibited very less release at the end of 30 min is 66.6%. The drug release at 30 minutes from the spherical crystals prepared in the presence of 1% to 4% w/v PEG 6000 were 65.2, 72.6,78.6 and 84.4% respectively, which indicates that the spherical crystals prepared with 4% w/v PEG 6000 exhibited maximum drug release i.e. 84.4%. |
| This indicates that Zaltoprofen crystals prepared with 4% w/v PEG 6000 exhibit an excellent dissolution and solubility enhancing ability. The mechanism behind the solubility and dissolution rate enhancing effect of Zaltoprofen in crystals form may resemble the solid dispersion mechanism, although the particle size of the crystals is high. This effect may be due to the improved wet ability of the surface of the crystals by the adsorption of PEG 6000 onto the surfaces of crystals. This crystal modification technique can enhance the bioavailability of the drug. Further in vivo studies are continued to study the improved solubility and dissolution rate on bioavailability of Zaltoprofen. |
| Comparison of dissolution of compacts and commercial products |
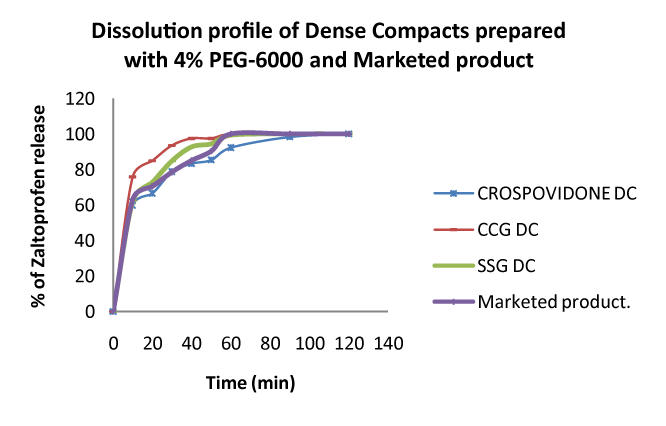
| Out of all spherical crystals, the agglomerates prepared with 4% w/v PEG 6000 have shown maximum drug release in addition to improving the micromeritic properties of the drug, hence selected as optimized batch. The study the effect of super disintegrants such as Ac-Di-Sol(CCS), Polyplasdone XL (CP) and Explotab (SSG) on the drug release of Zaltoprofen was done by compressing agglomerates prepared with 4% w/v PEG 6000 and drug with 4% w/w of above said super disintegrants. |
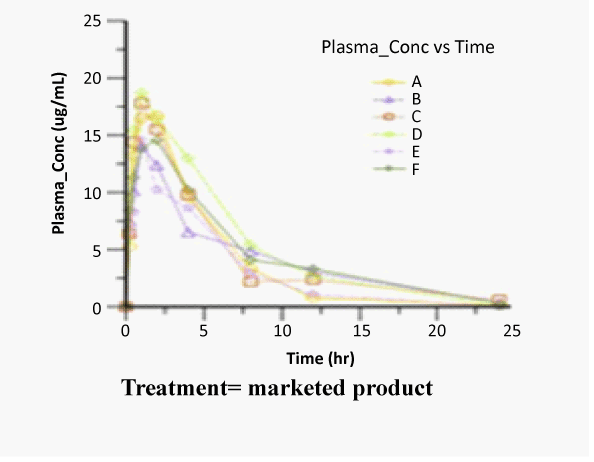
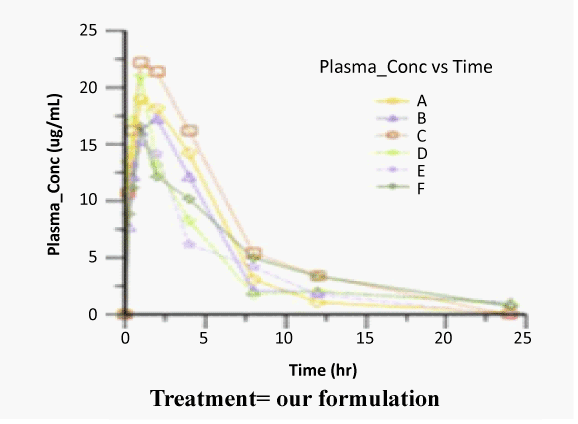
| These spherical agglomerated dense compacts (SADC) along with market product were subjected to dissolution studies and maintained same conditions of the spherical agglomerates. The results of drug release from dense compacts and market product are shown in figures 3-5. The results displayed that the percent release of Zaltoprofen from dense compacts and market product, at the end of 30 minutes were found to be 78.8, 93.6, 84.9, 78.6 for dense compacts prepared with 4% w/w Crospovidone, CCS, SSG and commercial product correspondingly. These results indicate that the efficiency of our formulation i.e. dense compacts prepared with 4% CCS exhibited better drug release is compared to other formulations and market product. |
| Pharmacokinetic analysis |
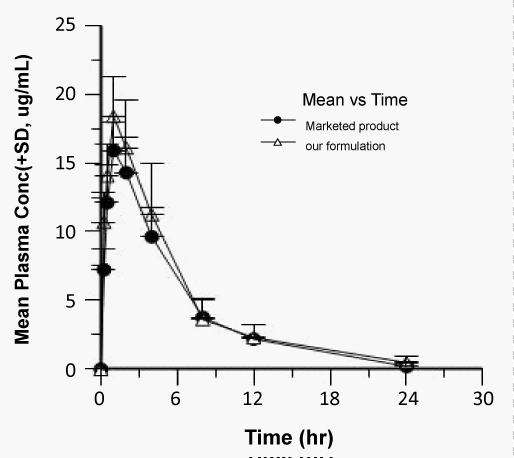
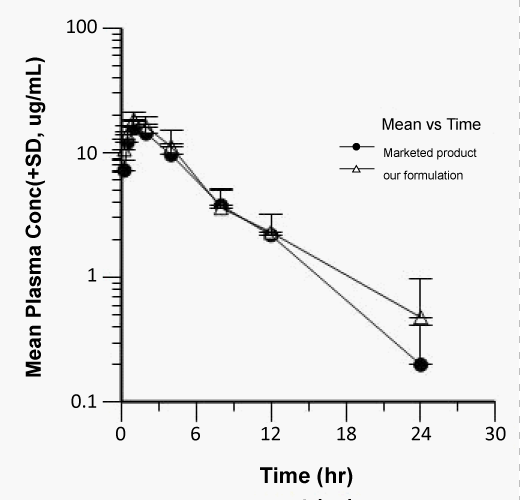
| The concentration of Zaltoprofen in plasma and the respective average values at different time intervals following the oral administration of selected formulation i.e. Zaltoprofen spherical agglomerates prepared in 4% PEG 6000 solution compressed with 4% of Ac-Di-Sol and commercial product were evaluated for their pharmacokinetics using Winnonlin software. The average plasma concentration versus time was shown in figures 4-6 and log concentration versus time curve show in figure 7. Individual and mean pharmacokinetic parameters (AUC0-24, AUC0-∝, Tmax, Cmax, Kel, t1/2 and MRT0-24) calculated from the average serum concentrations from the in vivo experiments are in shown in table 3. |
| The Cmax of zaltoprofen from selected formulation and commercial product were found to be 18.39 ± 2.59 and 15.09 ± 1.87 μg/mL respectively and the values of the commercial product are below the selected formulations. The tmax values from selected formulation and commercial product were found to be 1.16 ± 0.51 and 1.33 ± 0.50 hr respectively and the values of the commercial product are below the selected formulations. The Kel values were found to be -0.807 ± 0.03 and -0.619 ± 0.02 hr-1 after oral administration of selected formulation and commercial product respectively calculated from the slope of the terminal portion of log plasma concentration of zaltoprofen versus time (Figure 8). The corresponding t1/2 values were found to be 0.858 ± 0.03 and 1.11 ± 0.03 hrs respectively. The t1/2 values are almost similar to the reported values. |
| The values of AUC0-24, and MRT0-24 of selected formulation and commercial product were 115.789 ± 12.9, 97.334 ± 7.478 and 5.253 ± 1.02, 5.079 ± 1.162 respectively. |
| The AUC values of selected formulation were higher compared to the commercial product indicating the higher bioavailability of the selected formulation. Thus the results of present study indicated the applicability of spherical crystals of zaltoprofen as directly compressible material in direct tableting. |
| Conclusion |
| This study ascertains that the spherical agglomerated dense compacts were successfully formulated. The use of PEG 6000 in the formulation of spherical agglomerates successfully improved bioavailability of the zaltoprofen compared to commercial product confirmed by in vivo studies in rabbits. There lies a great promise that spherical agglomeration technology with use of hydrophilic polymers like PEG 6000 will hasten the drug dissolution and bioavailability of miserable water soluble drugs. |
| References |
References
- Kawashima Y, Imai M, Takeuchi H, Yamamoto H, Kamiya K (2001) Development of Agglomerated Crystals of Ascorbic Acid by the Spherical Crystallization Technique for Direct Tableting, and Evaluation of Their Compactibilities. J Soc Powder Technology 38: 160-168.
- Ito A, Mori Y (1990) Effect of a novel anti- inflammatory drug, 2-(10, 11-dihydro-10-oxo-dibenzo[b,f]-thiepin-2-yl) propionic acid (CN-100), on the proteoglycan biosynthesis in articular chondrocytes and prostaglandin E2 production in synovial fibroblasts. Res Commun Chem Pathol Pharmacol 70: 131-142.
- Yang HK, Kim SY, Kim JS, Sah H, Lee HJ (2008) Application of column-switching HPLC method in evaluating pharmacokinetic parameters of zaltoprofen and its salt. Biomed Chromatogr 23: 537-542.
- Aher KB, Bhavar GB, Joshi HP, Chaudhari SR (2011) Economical spectrophotometric method for estimation of Zaltoprofen in pharmaceutical formulations. Pharm methods 2: 152-156.
- Lee HW, Seo JH, Kim YW, Jeong SY, Lee KT (2006) Determination of Zaltoprofen in human plasma by liquid chromatography with electrospray tandem mass spectrometry: Application to a pharmacokinetic study. Rapid Commun Mass Spectrom 20: 2675-2680.
- Rowe RC, Sheskey PJ, Owen SC (2006) Hand book of pharmaceutical Exciepients. (5thedn), American Pharmacist Association, 1446-1463.
- Society of Japanese Pharmacopoeia (2007) Pharmaceutical and food safety bureau of Health and welfare. The Japanese Pharmacopoeia. (15thedn), 1243-1244.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14278
- [From(publication date):
March-2013 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 9677
- PDF downloads : 4601
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
