Pharmacological Targeting of Neutrophil Serine Proteases Prevents Lethality in Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice
Received: 17-Jan-2018 / Accepted Date: 02-Feb-2018 / Published Date: 08-Feb-2018 DOI: 10.4172/2161-069X.1000551
Abstract
Background and aims: In recent years the number of patients with inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis has been on the rise. Neutrophils are thought to be first cells to colonize disease-state colon tissue. Neutrophil elastase (NE) and cathepsin-G (Cat-G) are major secretory products from activated neutrophils and a major contributor to tissue destruction in inflammatory diseases. We predicted that inhibiting neutrophils and their proteases would improve ulcerative colitis.
Methods: Acute colitis was induced in mice by oral administration of 2% DSS for 7 days. In the first experiment, an inhibitor of Gr-1, a key neutrophil protein, was administered. In the second, the respective antagonists for NE and Cat-G, proteinases secreted by neutrophils, were administered, and the conditions of DSS-induced colitis were evaluated for improvement. Blood samples were collected and analyzed for levels of cytokines.
Results: The survival rate and pathology were improved in the Gr-1 inhibition experiments. In addition, administration of inhibitors of NE and Cat-G also resulted in clinical improvement. However, elevation of TNF-α, a key cytokine, was not suppressed in either experiment.
Conclusion: The proteolytic environment of neutrophils is associated with colonic inflammation. Disruption of the neutrophil proteases NE and Cat-G might therefore be a strategy for treatment of colitis regardless of TNF-α suppression.
Keywords: Colitis; Neutrophil; Proteases
Abbreviations
Ab: Antibody; DSS: Dextran Sulfate Sodium; CD: Crohn’s Disease; UC: Ulcerative Colitis; FACS: Fluorescence-activated Cell Sorting; IBD: Inflammatory Bowel Disease; TNF: Tumor Necrosis Factor; NE: Neutrophil Elastase; ELISA: Enzyme-linked Immune Sorbent Assay; Cat-G: Cathepsin-G; IL: Interleukin; PBS: Phosphatebuffered Saline; MMP: Matrix Metalloproteinases.
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are the major forms of chronic inflammatory bowel disease (IBD) involving chronic intestinal inflammation. IBD is caused by the interplay of many factors including genetic susceptibility, the external environment, infectious agents, the commensal enteric flora and immune system dysfunction [1,2]. While recent studies have focused mainly on lymphocytes or antigen-presenting cells such as dendritic cells, little is known about the pathogenic role of neutrophils in UC. Neutrophils are not only important in acute inflammatory reactions by early recruitment, but might also be initiators of a second wave of mononuclear cells which are drawn to tissues by neutrophils’ granule proteins [3]. Neutrophil granule components including antimicrobial polypeptides (e.g. LL-37, azurocidin, and human neutrophil peptide) and proteases (e.g. neutrophil elastase [NE], cathepsin G [Cat-G], matrix metalloproteinases-9 [MMP9], and proteinase-3) enhance the immune response [3]. Following migration to inflammatory tissues, neutrophils undergo a burst of transcriptional activity that results in the release of inflammatory cytokines (e.g. tumor necrosis factor-α [TNF-α], interleukin [IL]-1a and b) and chemokines (e.g. monocyte chemotactic protein-1, IL- 8) [3]. We have already shown that inhibition of MMP9 can prevent TNF-α release in mice [4]. Morohoshi et al. showed that an NE-specific inhibitor ameliorated progression of a UC murine model [5]. In addition, NE activated MMP9 in an in vivo model of acute lung injury [6]. N-terminal cleavage of the human neutrophil chemoattractant IL-8 by NE, Cat-G and proteinase-3 releases truncated forms of these chemokines having higher chemotactic activity than the full-length molecules [7]. Thus, these proteolytic environments not only damage inflammatory tissues, but also release pro-inflammatory cytokines and chemokines known to promote the influx of mononuclear cells. Clinically, the efficacy of granulocyte adsorption apheresis therapy to remove granular leukocytes secreted by neutrophils has been reported in active UC patients. Granulocyteadsorptive apheresis therapy induces remission stage, especially in patients with steroid-refractory and steroid-dependent moderate-tosevere UC [8]. Therapeutic drugs for ulcerative colitis have shown remarkable progress in recent years. One of the remarkable new drugs inside is infliximab, which is TNF-α depressant drug. TNF-α is mainly produced by macrophages and T cells. Although anti-TNF-α agents have significantly increased the treatment options for patients with refractory IBD, TNF-α antibody therapy is only effective for 76% of patients [9,10]. In the present study, we focused on inflammatory proteinases secreted by neutrophils rather than macrophages and Tcell- related cytokines. We show that pharmacological inhibition of neutrophils and their proteases prevents progression of IBD in an experimental model of colitis.
Materials and Methods
Animal studies
C57BL/6 mice were purchased from SLC, Shizuoka, Japan. The mice were housed under specific pathogen-free conditions. Animal studies were approved by the Animal Review Board of Juntendo University (Tokyo, JAPAN).
Reagent
Gr-1 antibody (Ab): Mice were depleted of neutrophils using Gr-1 Ab (RB6-8C5) provided by immunology of Juntendo University. It was administered intraperitoneally once a day at a dose of 0.5 mg/mouse every other day for up to 7 days. Non-treated control mice were administered the same amount of IgG without RB6-8C5. Neutrophil elastase (NE) specific inhibitor: The NE-specific inhibitor, ONO-5046, was obtained from Ono Pharmaceutical (Osaka, Japan). It was dissolved in phosphate-buffered saline (PBS) and administered intraperitoneally twice a day at a dose of 50 mg/kg from days 0-7. Nontreated control mice were administered the same amount of PBS without ONO-5046. Cathepsin-G (Cat-G) inhibitor: Mice were injected by Cat-G inhibitor using Z-Gly-Leu-Phe Chloromethyl Ketone (Sigma-Aldrich) once a day at a dose of 50 mg/kg from days 0-7. Non-treated control mice were administered the same amount of PBS without Cat-G.
Induction of DSS colitis model
Female 8-week-old C57BL/6 mice weighing around 20 g were used. Animals were housed under specific pathogen-free conditions. Acute colitis was induced in mice by oral administration of 2.0% DSS (ICN Biomedical molecular weight, 36,000-50,000 daltons; ICN Biomedicals Inc for 7 days. Mice were checked each day for weight, diarrhea, bloody stool and morbidity. Colons were dissected, and colon lengths were measured. Blood samples were collected and analyzed for levels of TNF-α.
Histology
Colon sections were stained with H&E. Histological scoring was performed as described by Cooper et al. [11].
Immunofluorescence
Colon sections were stained with the anti-Gr-1 Ab (clone RB6-8C5; R and D Systems, Minneapolis, MN), anti-F4/80 Ab (clone A3-1; AbD Serotec, Oxford, United Kingdom), or biotin-conjugated anti-CD3e Ab (clone M1-70; BD Pharmingen, San Diego, CA), followed by donkey anti-rat IgG Ab conjugated with 594, donkey anti-rabbit, or goat IgG Ab conjugated with Alexa 488 and streptavidin–Alexa 488 (Invitrogen, Carlsbad, CA). Nuclei were stained using 40,6-diamidino-2- phenylindole.
ELISA
Serum samples from PBS-(control), Gr-1-, ONO5046-, and cathepsin-G-treated colitis model mice were assayed using mousespecific ELISA kit for TNF-α (BioLegend).
Flow cytometry
Bone marrow cells were evaluated for depletion of neutrophils using fluorescence-activated cell sorting (FACS). Collected cells were stained with Ly6G-PE (clone 1A8; BD Pharmingen).
Statistical analyses
Data are expressed as mean values ± SEMs. Simple pair-wise comparisons were analyzed using Student’s t-test (two-tailed distribution with two-sample equal variance). P values <0.05 were considered statistically significant. The Kaplan-Meier survival curve analysis employed a log-rank test.
Results
Neutrophils, macrophages and T cells are associated with DSS-induced enterocolitis
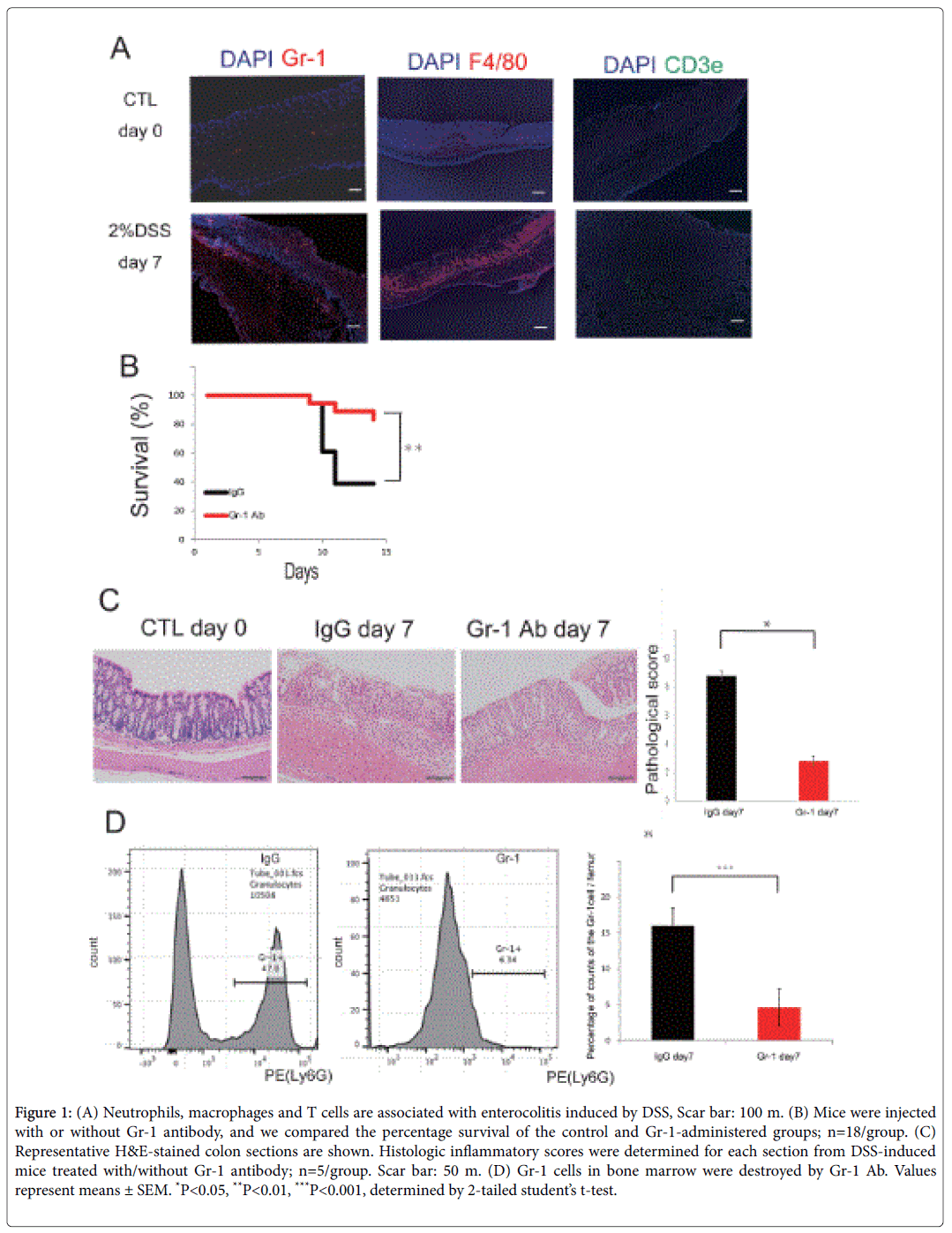
Using a DSS-induced colitis model, we first examined whether neutrophils accumulate in the colon tissues. Induction of neutrophils was found in inflammatory intestinal epithelia compared to the control group (Figure 1A). Similar to neutrophils, we found that T-cells and macrophages were associated with inflammation (Figure 1A). Gr-1 Ab was used to assess the depletion of neutrophils. Inhibition of the Gr-1 Ab group improved the survival rate by inhibiting neutrophils in the UC model mice (Figure 1B). Colon sections of mice given Gr-1 inhibitor showed less severe mucosal damage, loss of goblet cells, and inflammatory cell infiltration as evaluated by histological score (Figure 1C). Gr-1 cells in bone marrow were destroyed by Gr-1 Ab (Figure 1D). These data suggest that neutrophils are important inflammatory cells and therapeutic targets during colon inflammation.
Figure 1: (A) Neutrophils, macrophages and T cells are associated with enterocolitis induced by DSS, Scar bar: 100 m. (B) Mice were injected with or without Gr-1 antibody, and we compared the percentage survival of the control and Gr-1-administered groups; n=18/group. (C) Representative H&E-stained colon sections are shown. Histologic inflammatory scores were determined for each section from DSS-induced mice treated with/without Gr-1 antibody; n=5/group. Scar bar: 50 m. (D) Gr-1 cells in bone marrow were destroyed by Gr-1 Ab. Values represent means ± SEM. *P<0.05, **P<0.01, ***P<0.001, determined by 2-tailed student’s t-test.
Pharmacologic targeting of neutrophil serine proteases prevents DSS-induced colitis progression
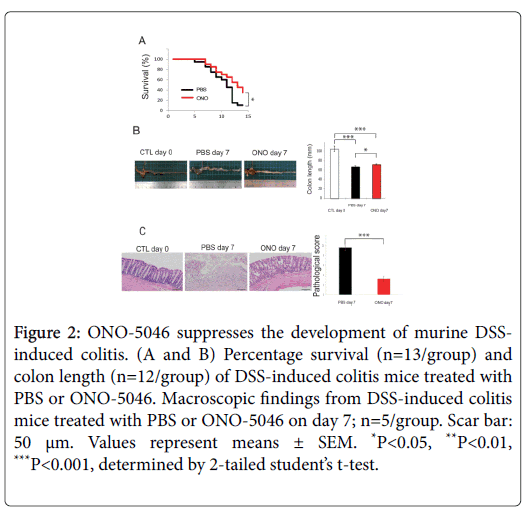
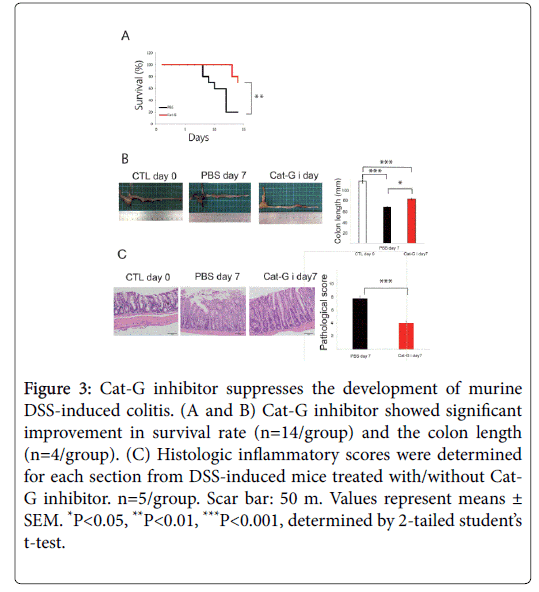
NE and Cat-G are representative proteinases engaged in inflammation. In addition to its protease activity contributing to tissue destruction, it has been reported that NE enhances the migration and adhesion of neutrophils [5]. ONO-5046 has already been clinically used for the treatment of acute respiratory distress syndrome, and no serious adverse effects have been reported. Similarly, Kuwana et al. showed that anti-cathepsin-G antibodies were detected in 40.6% of patients with active UC, and that their prevalence was significantly higher in patients with severe colitis [12]. We investigated whether NE and Cat-G play important roles in DSS-induced colitis by pharmacological inhibition. Elastase inhibition by ONO-5046 treatment protected against DSS-induced colitis-associated lethality and prevented shortening of the colon (Figures 2A and 2B). The shortening of colon length reflects the extent of colon damage of acute DSS-induced colitis. The UC model mice administered ONO showed reduced histological inflammation (Figure 2C). Repeated administration of the Cat-G inhibitor attenuated the severity of DSSinduced colitis, as reflected in survival, colon length and pathological score (Figures 3A-3C).
Figure 2: ONO-5046 suppresses the development of murine DSSinduced colitis. (A and B) Percentage survival (n=13/group) and colon length (n=12/group) of DSS-induced colitis mice treated with PBS or ONO-5046. Macroscopic findings from DSS-induced colitis mice treated with PBS or ONO-5046 on day 7; n=5/group. Scar bar: 50 μm. Values represent means ± SEM. *P<0.05, **P<0.01, ***P<0.001, determined by 2-tailed student’s t-test.
Figure 3: Cat-G inhibitor suppresses the development of murine DSS-induced colitis. (A and B) Cat-G inhibitor showed significant improvement in survival rate (n=14/group) and the colon length (n=4/group). (C) Histologic inflammatory scores were determined for each section from DSS-induced mice treated with/without Cat- G inhibitor. n=5/group. Scar bar: 50 m. Values represent means ± SEM. *P<0.05, **P<0.01, ***P<0.001, determined by 2-tailed student’s t-test.
Inhibiting neutrophils and their proteinases did not impede inflammatory cytokines such as TNF-α
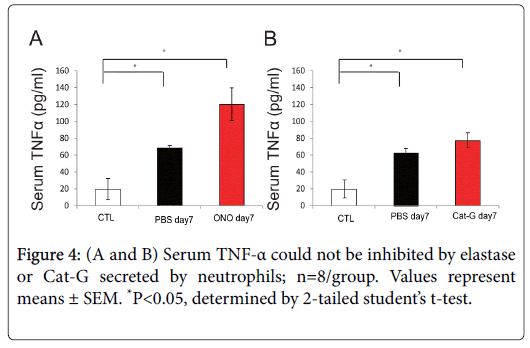
We next examined a typical IBD-associated cytokine in DSSinduced colitis. The pro-inflammatory cytokine TNF-α has been recognized to play a central role in mediating this immune response. However, increase of serum TNF-α was not disrupted by administration of NE and Cat-G inhibitors (Figure 4A and 4B). These data indicate that neutrophil serine proteases are not related to TNF-α suppression.
Discussion
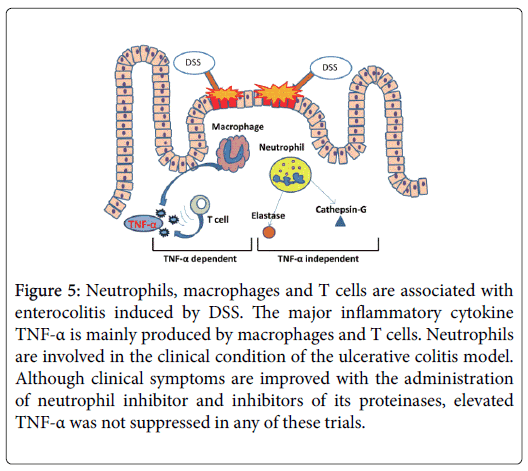
In this study, we provide functional evidence that neutrophil-related protease inhibition prevented progression of DSS-induced colitis, independent of TNF-α. Our findings are summarized in Figure 5.
Figure 5: Neutrophils, macrophages and T cells are associated with enterocolitis induced by DSS. The major inflammatory cytokine TNF-α is mainly produced by macrophages and T cells. Neutrophils are involved in the clinical condition of the ulcerative colitis model. Although clinical symptoms are improved with the administration of neutrophil inhibitor and inhibitors of its proteinases, elevated TNF-α was not suppressed in any of these trials.
Neutrophils are the first cells to arrive at sites of inflammation, and Hall et al. found that from as early as 1 day post-DSS administration the number of neutrophils was significantly increased in morphometry analysis [13]. Neutrophils release their serine proteases to the extracellular environment where they can then do the following: 1) proteolytically modify chemokine activity by releasing CXCchemokine ligand (CXCL) 2; 2) activate and inactivate cytokines CXCL8 and Monocyte chemotactic protein-1; 3) activate specific receptors such as myeloid differentiation primary-response gene 88 and clear translocation of nuclear factor-κB; and 4) potentially participate in neutrophil migration by cleaving adhesion molecules. Neutrophil serine proteases have been shown to interact with several cell-surface adhesion molecules such as intercellular adhesion molecule 1, vascular cell-adhesion molecule 1 and epithelial-cadherin [14].
As far as the NE inhibitor, ONO-5046, it is a selective inhibitor of neutrophil elastase developed and marketed for acute lung injury. In the pathogenesis of acute lung injury, hypercytokinemia can develop, in which neutrophils are activated to accumulate in the lungs and release NE. The NE injures pulmonary vascular endothelial cells and alveolar epithelial cells, causing increased pulmonary vascular permeability. ONO-5046 improves acute lung injury by selectively inhibiting NE [15]. Moreover, we confirmed that ONO-5046 overcame the role of MMP9, but NE was not present on the upstream protease of MMP9 (data not shown). However, as Morohoshi et al. have shown, ONO-5046 might actually have the potential to be a new therapeutic approach for patients with active UC [5].
On the other hand, Cat-G also induced the conversion of proMMP-1 to active MMP-1 in photoaging in hairless mice [16]. Another Cat-G inhibitor was shown to reduce neutrophil influx into sites of inflammation and counteract asthma pathophysiology [17]. We likewise demonstrated that a Cat-G inhibitor ameliorated the DSSinduced inflammation. In addition, NE- and Cat-G-deficient mice showed a decreased response in a rheumatoid arthritis model. This defect was accompanied by a decrease in local production of TNF-α and IL-1β [18]. It is generally accepted that the mTNF-α/sTNF-α ratio is regulated mainly through shedding of cytokines from the cell surface by activated ADAM17. Other proteases present at inflammatory sites, such as Cat-G and NE, may also contribute to TNF-α shedding [19]. Though TNF-α of our colitis model could not be regulated by inhibition of Cat-G and NE, This phenomenon is considered to depend on the particular disease.
In actual clinical trials, while mild cases of IBD may respond to aminosalicylates for induction and maintenance of remission, for management of severe IBD the current situation is far from satisfactory, especially in cases of steroid-dependent or steroidrefractory IBD, or in patients who are intolerant to corticosteroids. The widespread use of anti-TNF-α agents has changed the treatment paradigm in the management of patients with IBD [20]. These therapies are associated with a higher rate of induction of remission, but agents usually reserved for more severe disease have been known to fail in approximately 40% of patients [9,10]. Thus, new target therapy is required such as anti-integrin therapy or inhibition of proinflammatory cytokines such as IL13, IL-17, and IL12/23p40 [9,21,22] in humans but also, for instance, plasmin inhibitor, MMP inhibitor, cannabinoids and palmitoylethanolamide in mice [4,23,24]. In conclusion, our data suggest that serine proteases are activated during colitis progression. This proteolytic environment controls cell infiltration into colonic tissues, but not the production and secretion of the proinflammatory cytokine TNF-α. Our findings suggest that the targeting of NE and Cat-G is an attractive therapeutic candidate for TNF-α Abs-refractory IBD.
Author Contributions
Study concept and design, SM; Data acquisition, HR, SM, TU,; Analysis and interpretation of data, HR, SM, TU; Drafting of the manuscript, SM; Critical revision of the manuscript, SM,HK, MT, YK, YT, KS.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Grant Support
This work was supported in part by grants from the Japan Society for the Promotion of Science (Wakate B grant no. 16K19956 and 15H06603).
Acknowledgements
The authors thank the Division of Molecular and Biochemical Research, Research Support Center (Juntendo University Graduate School of Medicine) for technical assistance.
References
- Baumgart DC, Carding SR (2007) Inflammatory bowel disease: Cause and immunobiology. The Lancet 369: 1627-1640.
- Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347: 417-429.
- Soehnlein O, Weber C, Lindbom L (2009) Neutrophil granule proteins tune monocytic cell function. Trends Immunol 30: 538-546.
- Munakata S, Tashiro Y, Nishida C, Sato A, Komiyama H, et al. (2014) Inhibition of plasmin protects against colitis in mice by suppressing matrix metalloproteinase 9-mediated cytokine release from myeloid cells. Gastroenterology 148: 565-578.
- Morohoshi Y, Matsuoka K, Chinen H, Kamada N, Sato T, et al. (2006) Inhibition of neutrophil elastase prevents the development of murine dextran sulfate sodium-induced colitis. J Gastroenterol 41: 318-324.
- Ferry G, Lonchampt M, Pennel L, de Nanteuil G, Canet E, et al. (1997) Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Letters 402: 111-115.
- Padrines M, Wolf M, Walz A, Baggiolini M (1994) Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett 352: 231-235.
- Sands BE, Sandborn WJ, Feagan B, Lofberg R, Hibi T, et al. (2008) A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology 135: 400-409.
- Danese S (2012) New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 61: 918-932.
- Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, et al. (2005) Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 128: 1805-1811.
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238-249.
- Kuwana T, Sato Y, Saka M, Kondo Y, Miyata M, et al. (2000) Anti-cathepsin G antibodies in the sera of patients with ulcerative colitis. J Gastroenterol 35: 682-689.
- Hall LJ, Faivre E, Quinlan A, Shanahan F, Nally K, et al. (2011) Induction and activation of adaptive immune populations during acute and chronic phases of a murine model of experimental colitis. Dig Dis Sci 56: 79-89.
- Pham CT (2006) Neutrophil serine proteases: Specific regulators of inflammation. Nat Rev Immunol 6: 541-550.
- Polverino E, Rosales-Mayor E, Dale GE, Dembowsky K, Torres A (2017) The role of neutrophil elastase inhibitors in lung diseases. Chest 152: 249-262.
- Son ED, Shim JH, Choi H, Kim H, Lim KM, et al. (2012) Cathepsin G inhibitor prevents ultraviolet B-induced photoaging in hairless mice via inhibition of fibronectin fragmentation. Dermatology 224: 352-360.
- Maryanoff BE, de Garavilla L, Greco MN, Haertlein BJ, Wells GI, et al. (2010) Dual inhibition of cathepsin G and chymase is effective in animal models of pulmonary inflammation. Am J Respir Crit Care Med 181: 247-253.
- Adkison AM, Raptis SZ, Kelley DG, Pham CT (2002) Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 109: 363-371.
- Mezyk-Kopec R, Bzowska M, Bzowska M, Mickowska B, Mak P, et al. (2005) Effects of elastase and cathepsin G on the levels of membrane and soluble TNFalpha. Biol Chem 386: 801-811.
- Arora Z, Shen B (2014) Biological therapy for ulcerative colitis. Gastroenterol Rep (Oxf) 3: 103-109.
- Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, et al. (2012) Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med 367: 1519-1528.
- Danese S, Fiocchi C (2011) Ulcerative colitis. N Engl J Med 365: 1713-1725.
- Pagano E, Capasso R, Piscitelli F, Romano B, Parisi OA, et al. (2016) An orally active cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front Pharmacol 7: 341.
- Borrelli F, Romano B, Petrosino S, Pagano E, Capasso R, et al. (2015) Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol 172: 142-158.
Citation: Ro H, Munakata S, Ueyama T, Komiyama H, Takahashi M, et al. (2018) Pharmacological Targeting of Neutrophil Serine Proteases Prevents Lethality in Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. J Gastrointest Dig Syst 8: 551. DOI: 10.4172/2161-069X.1000551
Copyright: © 2018 Ro H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6281
- [From(publication date): 0-2018 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 5301
- PDF downloads: 980