Research Article Open Access
Physico-Chemical Composition of Saline Lakes of the Gobi Desert Region, Western Mongolia
Bayanmunkh B1*, Sen-Lin T2,3, Narangarvuu D4, Ochirkhuyag B5 and Bolormaa O61Molecular and Biological Agricultural Sciences Program, Taiwan International Graduate Program, Academia Sinica, Taipei, and National Chung-Hsing University, National Unversity of Mongolia Taichung, Taiwan
2Biodiversity Research Center, Academia Sinica, Taipei, Taiwan
3Biotechnology Center, National Chung-Hsing University, Taichung, Taiwan
4Department of Biology, School of Arts and Sciences, National University of Mongolia, Ulaanbaatar 14201, Mongolia
5Department of Environmental Sciences and Engineering, School of Engineering and Applied Sciences, National University of Mongolia, Ulaanbaatar 14201, Mongolia
6Department of Chemistry, School of Arts and Sciences, National University of Mongolia, Ulaanbaatar 14201, Mongolia
- *Corresponding Author:
- Bayanmunkh B
Molecular and Biological Agricultural Sciences
Program, Taiwan International Graduate Program
Academia Sinica, Taipei, Taiwan, and National Chung-Hsing
University, National Unversity of Mongolia, Baga Toiruu-Ulaanbaatar, Mongolia
Tel: 976-95950515
E-mail: analytchem.num@gmail.com
Received date: February 16, 2017; Accepted date: February 24, 2017; Published date: February 28, 2017
Citation: Bayanmunkh B, Sen-Lin T, Narangarvuu D, Ochirkhuyag B, Bolormaa O (2017) Physico-Chemical Composition of Saline Lakes of the Gobi Desert Region, Western Mongolia. J Earth Sci Clim Change 8:388. doi: 10.4172/2157-7617.1000388
Copyright: © 2017 Bayanmunkh B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Mongolian saline lakes have been rarely studied. In particular, physicochemical composition of small shallow lakes of the Gobi Desertregion in western Mongolia is simply unknown. Objectives were to characterize chemical composition and describe biogeochemical characteristics of saline lakes in the Gobi Desertregions and steppe mountains, with an emphasis on environmental parameters associated with heavy metal ions. Major physicochemical parameters were determined in 14 lakes (with varying degrees of salinity) located in western Mongolia and associations with geographic variation and distance were determined. Vertical profiles for temperature, specific conductance, and dissolved oxygen were characterized. Salinity and pH were measured with multiparameter submersible profilers. Heavy metal ions (i.e., As, Pb, and Cd) and microelements (i.e., K, Na, Zn, Cu, Ni, Cr, and Co) were analyzed with Inductively Coupled Plasma-Mass Spectrometry. Hydrogen sulfide, ammonia, nitrite, and nitrate were also determined by commercial kits for elemental analysis. Overall, 43 environmental parameters were determined from 31 water samples from various locations, representing distinct geographic features, including Gobi Desert, mountain range and steppe area. Several of these lakes had harmful concentrations of arsenate (< 0.34 mg/L). Heavy metal concentrations were correlated with selected physico-chemical variables of lake salinity and geographic specificity. We inferred that various environmental features, in addition to environmental pollution and mining activity, may be responsible for increased heavy metal concentrations in the Gobi Desert of the western Mongolia. The outcome of this study not only provided insights into lake chemistry, but should also be valuable for water resource management to mitigate potential poisoning and improve public health.
Keywords
Arsenic; Hydrochemistry; Mongolia; Saline lakes; Water quality
Introduction
Mongolia is abundant in hypersaline or extremely oligotrophic environments [1] Western Mongolia is mainly composed of Gobi Desert, steppe, and high mountains. Based on the official record, there are > 3500 salt lakes and an enormous area of salt lands located in the Altai Mountain and Gobi regions in western Mongolia. However, these salt lakes have had minimal scientific study or human activity [2,3]. Therefore, western Mongolia provides an excellent research environment to explore high-latitude hypersaline environments and to conduct comparative analyses of chemical compositions among salt lakes [4,5].
Physical and chemical properties of major saline lakes have been studied for decades [2]. However, there is limited information regarding the relationship between salinity gradients and heavy metal pollution of brine water lakes in the Gobi region. Consequently, the main goal was a bench-mark survey of heavy metals in high-latitude salt lakes and to compare characteristics among brackish, saline or brine water lakes. Furthermore, we wanted to detect associations between environmental chemistry and heavy metal concentration to provide insights into biogeochemical networks in hypersaline environmental ecosystems, and determine the extent of heavy metal pollution of salt lakes in western Mongolia. It was expected that the new knowledge generated by this study would be useful for evidence-based ways to improve environmental management and public health.
Materials and Methods
Description of sampling sites and sampling procedures
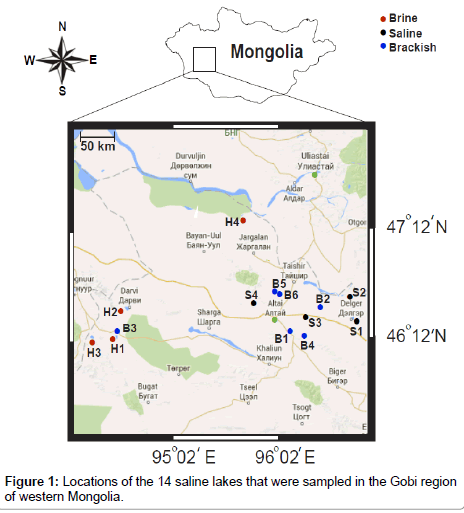
Western Mongolia is a harsh environment; it is extremely dry with a huge range in temperatures (–40°C in winter to +40°C in summer). Moreover, the Gobi is located on a plateau ~910–1520 m above sea level, which further contributes to its extremeness [2-4]. Furthermore, saline lakes in the Gobi region have a high evaporation rate and an average annual rainfall of only ~ 194 mm. Global warming and human activities have caused average temperatures to increase 3 to 5°C in recent years, resulting in increased desertification in inland areas [6], which has decreased river flows and increased salinity in shallow lakes in the Gobi region. Our sampling area was a large compressional basin bounded by the Khangai mountain range to the east, Altai Mountains to the west, the valley of Great Lakes to the north, and the Gobi Desertto the south. Overall, 14 salt lakes located between 45-47 and 93-106°E were studied (Table 1). Water samples were collected from these lakes of the Gobi Desertand high-mountain region of western Mongolia in 2012. Thirty-one water samples were collected at surface and bottom waters of shallow lakes and at various depths in deep lakes. Samples consisted of 3 L of lake water vertically collected from each sampling depth, using a homemade vacuum deep-water sampler. Water samples were retained in sterile 2 L containers and 500 mL polypropylene bottles for analysis of major and trace elements, respectively. Water samples were preserved with high-purity nitric acid (pH 1-2) and directly transported to the field station. In addition, at the same sites, superficial sediment samples (~1 kg of wet sediment) were collected from the lake bottom using a sterile spatula. These samples were sealed in sterile plastic bags and stored at 4°C prior to processing and analysis.
| ID | Lake | Latitude | Longitude | Altitude | Max. length | Max. width | Area | Max. depth | |
|---|---|---|---|---|---|---|---|---|---|
| Brackish | N | E | m | km | km | km2 | m | ||
| B1 | Olon | 46°26' | 96°05' | 2196 | 0.008 | 0.4 | |||
| B2 | Ar gashuun | 46°37' | 96°36' | 1762 | 2.37 | 1 | |||
| B3 | Zegst | 45°11' | 94°55' | 1 | |||||
| Saline | B4 | Galuut | 46°38' | 96°22' | 2130 | 0.052 | 1 | ||
| B5 | Ulaan | 46°45' | 95°53' | 1 | |||||
| B6 | Ulaan Kholboo | 46°41' | 96°52' | 1.5 | |||||
| S1 | Taigam | 47°55' | 106 Ì? 55' | 1767 | 4.5 | 1.6 | 10.1 | 4 | |
| S2 | Kholboo | 46°24' | 97°23' | 1792 | 7.4 | 2 | |||
| Brine | S3 | Mangas | 46°54' | 96°24' | 1786 | 1 | |||
| S4 | Khadaasan | 46°26' | 96°11' | 2065 | 0.75 | ||||
| H1 | Tonkhil | 46°11' | 93°55' | 2064 | 4.6 | 2.6 | 6.2 | 1.6 | |
| H2 | Ikhes | 46°27' | 94°05' | 1600 | 6.4 | 3.9 | 19.7 | 1 | |
| H3 | Khulma | 46°11' | 93°33' | 2234 | 4.2 | 3.4 | 8.81 | 1 | |
| H4 | Duruu | 47°16' | 95°42' | 1429 | 1.86 | 0.5 |
Table 1: Descriptions of the 14 saline lakes that were sampled.
Analysis of physical and chemical parameters
Vertical profiles for pH, electrical conductivity (EC), temperature, dissolved oxygen (DO) and salinity were measured in the field with Hanna HI 9828 submersible profilers (Yellow Springs, Dayton, OH, USA). Before each survey, conductivity and pH sensors were calibrated against a 3 M KCl calibration solution (Hanna).
Conductivity readings of in situ temperatures (Ct) were standardized to specific conductance at 25°C using K25 = Ct × (1+0.0204× (T-25))-1, where T is the in-situ temperature (°C). The relationship between conductivity and salinity for lakes is: S (g L-1) = 1.117 K25 - 7.9716. Concentrations of phosphate were determined in the field with a portable instrument (Yellow Springs, Dayton, OH, USA). For sulphide determination, subsamples were fixed with zinc acetate and determined by a colorimetric method [7]. The following analyses were all conducted as described [8]. Phosphorus (P) and ammonia (NH4+) were determined using routine ammonium molibdate and the Nesserilization method, respectively. Major ions (magnesium, sulfate, chloride, calcium, carbonate and hydrocarbonate) of lake water were measured by titrimetric methods [9,10] at the National University of Mongolia. Analysis of total iron, nitrite and nitrate ions in water were performed with a slight modification of a photoelectric colorimeter KФK-2 (Optic-mechanical factory, Zagorskyi, Russia) method, as described [11]. Regarding the latter, NO2- was determined through formation of purple dye produced by the reaction of NO2- with Gries reagent, as NO3- was converted into NO2- by reduction with the Cd redactor. Furthermore, SO42- was measured with a standard barium chloride method. Trace and minor elements in water were analyzed by ICP-MS (inductively coupled plasma-mass spectrometry) X-Series 2 (Thermo Scientific, Berlin, Germany) in an accredited laboratory (Central Geological Laboratory of Mongolia).
Superficial sediment samples were air-dried under a laminar flow at room temperature for 24 to 48 h, and then initially run through a 2-mm diameter sieve net to remove debris and gravel. Sediment pH and EC were measured in sediment slurries and distilled water (ratio 1:2) using suitable electrodes [12]. The moisture content of lake sediment was determined using a dry oven DHG-9030 (Jiangsu, Zhengji Instruments, China) at 105°C. Weight loss-on-ignition (LOI) was measured with a muffle furnace SX-2.5-12 (Xi'an HEB Biotechnology, China), as described sediment organic carbon was examined by a chromic acid wet oxidation method [13]. Micro elements (aluminum, silicone, total iron, arsenic, manganese, chromium, copper, nickel, molybdenum, lead, zinc, strontium, and cadmium) in sediment samples were measured with wavelength dispersive X-ray fluorescence spectrometry (WDXRF) Axios (PANalytical, Almelo, the Netherlands) at the Central Geological Laboratory of Mongolia. Structural analysis of the sediment samples was determined using an X-ray diffractometry diffraction apparatus 801 Y801 (Enraf-Nonius, Holland) in the Nanotechnology and Material Science Laboratory of Mongolian University of Science and Technology.
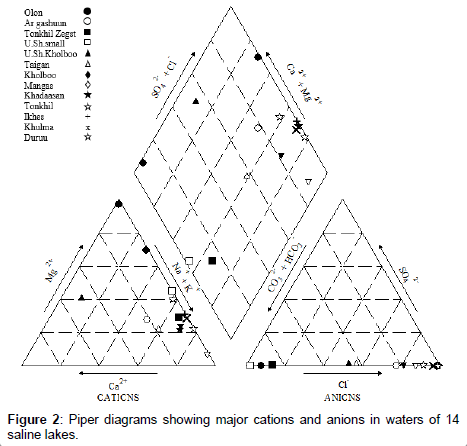
A Piper diagram was described by the GW chart from the U.S. Geological Survey [14]. Hydroparameters are checked for collinearity using Spearman’s Rank correlation, and a set of parameters with low correlations (R<0.5) were selected for Principal Component Analysis (PCA) to explore contributions of environmental parameters to each depth of selected lakes. The PCA was analyzed in R (http://www.Rproject. org/; R Development Core Team). Correlations between individual hydro-parameters and arsenic level were examined using the correlation analyses in JMP software (http://www.jmp.com/).
Results and Discussion
The physical and chemical parameters of saline lakes
Physical and chemical properties of 14 western Mongolian saline lakes are shown (Tables 2 and 3). Although physical and chemical properties of some larger saline lakes have been measured (i.e., Lakes Tonkhil, Khulma, Duruu, Ikhes and Taigam [3,5], morphometric characteristics and environmental parameters of smaller lakes remain unknown [1,2]. All 14 studied lakes were extremely shallow (water depth < 2 m) excluding Lakes Taigam (4 m) and Kholboo (2 m). There was a large range in salinity among lakes (Figure 1 and Table 2), classified into three types: brine (Lakes Tonkhil, Khulma, Duruu and Ikhes); saline (Lakes Taigam, Kholboo, Mangas and Khadaasan); and brackish (Lakes Olon, Ar Gashuun, Zegst, Galuut, Ulaan and Ulaan Kholboo). Brine water has a high salt concentration (usually > 50 g/L), whereas a saline lake has less salt than a brine water lake, but more salt than brackish water. Salt concentrations of brine, saline and brackish water lakes were 51.50 to 335.5, 9.19 to 30.64 and 0.33 to 2.27 g/l, respectively. Brine lakes were considered a more extreme environment than brackish water lakes, due to high concentrations of salt and other chemicals. In general, lakes in arid regions of the Gobi Desert had higher salinity than lakes in steppe and mountain areas (Figure 1). High-salinity lakes in Gobi regions result primarily from high evaporation rate (~1000 mm annually) and uptake of salts from high soil salinization [1,2].
| Depth (m) | pH | Salinity (g/L) | E.C (mS/cm) | D.O (mg/L) | O.R.P (mV) | |
|---|---|---|---|---|---|---|
| Brackish | B1-0 | 9.68 | 0.33 | 0.67 | 1.67 | -16.9 |
| B1-1 | 9.53 | 0.33 | 0.67 | 1.68 | -10.6 | |
| B2-0 | 8.94 | 0.59 | 1.18 | 4.89 | 2.2 | |
| B2-1 | 8.74 | 0.6 | 1.19 | 4.82 | 10.8 | |
| B3-0 | 9.12 | 0.72 | 1.43 | 4.03 | 2 | |
| B3-1 | 9.12 | 0.72 | 1.43 | 10.9 | 1.9 | |
| B4-0 | 9.4 | 1.27 | 2.45 | 2.66 | -20.8 | |
| B4-1 | 9.39 | 1.27 | 2.45 | 2.63 | -82.2 | |
| B5-0 | 9.16 | 1.98 | 3.76 | 6.26 | -1.5 | |
| B5-1 | 9.9 | 1.96 | 3.72 | 6.1 | -6.4 | |
| B6-0 | 9.35 | 2.27 | 4.25 | 6.08 | -14.8 | |
| B6-1 | 9.73 | 2.26 | 4.24 | 5.79 | -13.2 | |
| Saline | S1-0 | 9.64 | 9.19 | 15.66 | 4.11 | -29.3 |
| S1-1 | 9.09 | 9.17 | 15.63 | 4.14 | -9.7 | |
| S1-2 | 8.96 | 9.25 | 15.75 | 4.09 | 8.2 | |
| S1-3 | 9 | 9.25 | 15.75 | 3.49 | 0.8 | |
| S1-4 | 9.13 | 9.34 | 15.9 | 0.15 | -221.2 | |
| S2-0 | 9.62 | 16.94 | 27.5 | 5.69 | -10.7 | |
| S2-1 | 8.99 | 16.93 | 27.48 | 5.54 | -25 | |
| S2-2 | 9.04 | 16.94 | 27.49 | 6.04 | -9.6 | |
| S3-0 | 9.25 | 22.47 | 35.57 | 3.31 | 2.7 | |
| S3-1 | 9.26 | 22.46 | 35.56 | 3.19 | 11.8 | |
| S4-0 | 10.1 | 30.49 | 48.49 | 3.27 | -28 | |
| S4-1 | 9.83 | 30.64 | 48.71 | 2.98 | -10.5 | |
| Brine | H1-0 | 8.31 | 51.5 | 74.96 | 0.6 | 5 |
| H1-1 | 8.06 | 62.69 | 88.56 | 0.65 | 3.8 | |
| H2-0 | 8.03 | 137.42 | 101.6 | 0.67 | 13 | |
| H3-0 | 7.1 | 234.96 | 79.55 | 0.13 | - | |
| H3-1 | 7.1 | 234.96 | 79.55 | 0.13 | - | |
| H4-0 | 7.98 | 335.5 | 116 | 1.79 | -44.5 | |
| H4-1 | 8.22 | 335.5 | 122.3 | 1.35 | -74.5 | |
| F Ratio | 38.8 | 32.5 | 138 | 10.1 | ||
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
D.O – Dissolved oxygen; E.C – Electric conductivity; O.R.P – Oxidation Reduction Potential
Table 2: Physico-chemical parameters in vertical profiles of water samples collected from 14 saline lakes.
| Depth (m) | Na+ g/L | K+ g/L | Ca2+ g/L | Mg2+ g/L | Cl- g/L | HCO3- g/L | SO42- g/L | CO32- g/L | PO43- g/L | NH4+ g/L | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brackish | B1-0 | 0.04 | 0.01 | 0.03 | 0.13 | 0.03 | 0.56 | 0.1 | 12 | 0.2 | 1 |
| B1-1 | 0.04 | 0.01 | 0.03 | 0.13 | 0.03 | 0.57 | 0.1 | 39 | 0.5 | 2 | |
| B2-0 | 0.28 | 0.01 | 0.11 | 0.08 | 0.38 | 0.19 | 0.4 | 0 | 0.5 | 2 | |
| B2-1 | 0.33 | 0.01 | 0.11 | 0.05 | 0.35 | 0.17 | 0.4 | 0 | 0.5 | 5 | |
| B3-0 | 0.66 | 0.03 | 0.04 | 0.16 | 0.03 | 0.26 | 0.7 | 6 | 0.2 | 5 | |
| B3-1 | 0.76 | 0.03 | 0.05 | 0.16 | 0.03 | 0.28 | 0.6 | 6 | 0.2 | 1 | |
| B4-0 | 0.74 | 0.06 | 0 | 0.33 | 0.03 | 1.81 | 0.5 | 126 | 0.2 | 1 | |
| B4-1 | 0.76 | 0.08 | 0 | 0.35 | 0.03 | 1.7 | 0.3 | 147 | 0.2 | 1 | |
| B5-0 | 0.1 | 0.04 | 0.48 | 0.24 | 0.08 | 0.12 | 2 | 0 | 0.5 | 1 | |
| B5-1 | 0.9 | 0.04 | 0.47 | 0.25 | 0.07 | 0.1 | 2 | 0 | 0.5 | 0.2 | |
| B6-0 | 1.3 | 0.03 | 0.36 | 0.26 | 0.1 | 0.13 | 2 | 0 | 0.2 | 1 | |
| B6-1 | 1.2 | 0.03 | 0.35 | 0.27 | 0.1 | 0.13 | 2 | 0 | 0.2 | 0.5 | |
| Saline | S1-0 | 6.7 | 0.11 | 0.5 | 1.22 | 0.74 | 0.29 | 10 | 6 | 0.2 | 0.5 |
| S1-1 | 6.4 | 0.11 | 0.5 | 1.37 | 0.78 | 0.04 | 10 | 6 | 0.5 | 0.2 | |
| S1-2 | 6.3 | 0.11 | 0.5 | 1.37 | 0.82 | 0.16 | 10 | 6 | 0.2 | 0.5 | |
| S1-3 | 6.8 | 0.11 | 0.25 | 1.37 | 0.76 | 0.16 | 12 | 6 | 0.2 | 0.5 | |
| S1-4 | 7.3 | 0.13 | 0.5 | 1.22 | 0.8 | 0.16 | 11 | 6 | 0.2 | 1 | |
| S2-0 | 12 | 0.34 | 0.07 | 0.51 | 1.95 | 0.4 | 16 | 41 | 0.2 | 0.2 | |
| S2-1 | 13 | 0.35 | 0.08 | 0.5 | 1.93 | 0.42 | 15 | 35 | 0.2 | 0.2 | |
| S2-2 | 13 | 0.32 | 0.08 | 0.51 | 1.84 | 0.4 | 16 | 39 | 0.2 | 0.2 | |
| S3-0 | 14 | 0.09 | 1.25 | 2.28 | 3.81 | 0.06 | 6 | 0 | 0.2 | 0.2 | |
| S3-1 | 15 | 0.1 | 1 | 2.13 | 3.79 | 0.06 | 6 | 0 | 0.2 | 0.2 | |
| S4-0 | 25 | 0.56 | 0.75 | 9.12 | 3.23 | 0.39 | 26 | 54 | 0.2 | 0.2 | |
| S4-1 | 92 | 4.1 | 0.75 | 8.97 | 3.23 | 0.39 | 26 | 63 | 0.2 | 0.2 | |
| Brine | H1-0 | 45 | 1.1 | 0.25 | 10.79 | 8.86 | 0.45 | 28 | 63 | 0.2 | 0.5 |
| H1-1 | 66 | 1.6 | 0.25 | 15.96 | 13.26 | 0.58 | 20 | 81 | 0.2 | 0.5 | |
| H2-0 | 79 | 0.98 | 0.5 | 17.02 | 16.84 | 0.7 | 24 | 135 | 1 | 1 | |
| H3-0 | 25 | 0.55 | 1 | 31.31 | 30.13 | 0.78 | 21 | 0 | 0.2 | 0.2 | |
| H3-1 | 110 | 4.5 | 2 | 32.83 | 30.2 | 0.75 | 21 | 0 | 0.2 | 0.5 | |
| H4-0 | 83 | 2.4 | 0.5 | 12.77 | 19.85 | 0.42 | 26 | 36 | 0.2 | 0.2 | |
| H4-1 | 84 | 2.5 | 0.5 | 14.59 | 22.51 | 0.49 | 26 | 24 | 0.2 | 0.2 | |
| F Ratio | 27.98 | 9.3 | 4.89 | 42.51 | 69.03 | 2.1 | 62 | 0.91 | 1 | 6.3 | |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | <0.001 | 0.14 | <0.001 | 0.41 | 0.36 | 0.005 |
Table 3: Vertical profile of macro ions in water samples collected from 14 saline lakes.
Lakes differed in several hydrological parameters (Table 2), including salinity, electric conductivity (E.C), dissolved oxygen and pH (P<0.05). Dissolved oxygen varied from 1.79 to 0.13 mg/L (lowest in brine lakes, due to poor penetration of air into the saline water with high osmotic pressure [15]. In addition, all samples in brackish and saline lakes were alkaline (pH 8.74 to 10.1), with the lowest pH in brine lakes (7.1-8.3). Similarly, pH values averaged 7.9 and 8.9 in freshwater and saline lakes, respectively, in north western Mongolia [16]. Although water salinity may affect pH, salt composition is also critical. The pH is regularly high in Mongolian lakes because the main salts are sulphate and chloride type, which have alkaline properties [1]. In all lakes, oxidation reduction potential (O.R.P) varied among salinity levels (Table 2).
Seven major ions (Na+, Ca2+, K+, Mg2+, HCO3-, SO42- and Cl-) comprised > 99.9% of salt ions [16] (Mitamura et al 2009); variations in chemical properties of major ions depends on concentration and type of dissolved salt in water [17-19]. Concentrations of major anions and cations in this study are shown (Table 3 and Figure 2). There were differences (P<0.05) among brackish, saline and brine lakes with regards to sodium (Na+), potassium (K+), calcium (Ca2+) and magnesium (Mg2+). Higher concentrations of cations, Na+ (up to 110 g/L), K+ (up to 4.5 g/L), Ca2+ (up to 2 g/L), and Mg2+ (up to 32.83 g/L) were detected in brine lakes compared to saline and brackish lakes (Table 3). Furthermore, there were differences (P<0.05) among brackish, saline and brine lakes regarding some anions, including, chloride (Cl-) and sulfate (SO42-), although phosphate (PO43-), hydrogen carbonate (HCO3-), and carbonate (CO32-) were similar among lakes. Higher concentrations of anions Cl- (up to 30.2 g/L) and SO42- (up to 28 mg/L) were detected in brine lakes compared to saline and brackish lakes (Table 3). In addition, ammonium (NH4+) was significantly different between high and low salinity lakes (Table 3). Ammonium is commonly used to assess the impact of human and animal activities on water. Less saline or brackish waters are actually attributed to animal impact in the Gobi region, due to limits of available freshwater supply for wild animals and livestock [3,5,20].
Regarding microelements, only copper (Cu) and arsenic (As) were present in concentrations that exceeded limits of detection (Table 4). It was noteworthy that Cu was detected in all lakes; concentrations were relatively constant (0.07 to 0.09 mg/L) in brackish water lakes, but more variable in saline and brine lakes.
| Depth (m) m | Cu mg/L | As mg/L | Cd mg/L | Cr mg/L | Mn mg/L | Ni mg/L | Pb mg/L | Sr mg/L | Zn mg/L | Al mg/L | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brackish | B1-0 | 0.07 | - | - | - | - | - | - | - | - | - |
| B1-1 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B2-0 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B2-1 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B3-0 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B3-1 | 0.08 | - | - | - | - | - | - | - | - | - | |
| B4-0 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B4-1 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B5-0 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B5-1 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B6-0 | 0.07 | - | - | - | - | - | - | - | - | - | |
| B6-1 | 0.09 | - | - | - | - | - | - | - | - | - | |
| Saline | S1-0 | 0.1 | - | - | - | - | - | - | - | - | - |
| S1-1 | 0.06 | - | - | - | - | - | - | - | - | - | |
| S1-2 | 0.06 | - | - | - | - | - | - | - | - | - | |
| S1-3 | 0.06 | - | - | - | - | - | - | - | - | - | |
| S1-4 | 0.07 | - | - | - | - | - | - | - | - | - | |
| S2-0 | 0.06 | - | - | - | - | - | - | - | - | ||
| S2-1 | 0.07 | 0.06 | - | - | - | - | - | - | - | - | |
| S2-2 | 0.07 | 0.07 | - | - | - | - | - | - | - | - | |
| S3-0 | 0.05 | - | - | - | - | - | - | - | - | - | |
| S3-1 | 0.07 | - | - | - | - | - | - | - | - | - | |
| S4-0 | 0.06 | 0.06 | - | - | - | - | - | - | - | - | |
| S4-1 | 0.14 | 0.08 | - | - | - | - | - | - | - | - | |
| Brine | H1-0 | 0.03 | 0.23 | - | - | - | - | - | - | - | - |
| H1-1 | 0.05 | 0.34 | - | - | - | - | - | - | - | - | |
| H2-0 | 0.06 | 0.21 | - | - | - | - | - | - | - | - | |
| H3-0 | 0.07 | 0.07 | - | - | - | - | - | - | - | - | |
| H3-1 | 0.05 | 0.06 | - | - | - | - | - | - | - | - | |
| H4-0 | 0.04 | 0.1 | - | - | - | - | - | - | - | - | |
| H4-1 | 0.04 | 0.09 | - | - | - | - | - | - | - | - | |
| F Ratio | 2.85 | 20.45 | |||||||||
| P value | 0.07 | <0.01 |
(-) below limit of detection
Table 4: Vertical profile of microelements (mg/L) in water samples collected from saline lakes. Note that concentrations #, #, #, #, #, #, and # were below detection limits in all water samples.
Sediments of saline and brine lakes were similar in terms of pH, but differed in electrical conductivity. Relatively high concentrations (mg/kg) of microelements were detected in sediments of saline and brine lakes (Table 5). Concentrations of microelements in sediments differed between saline and brine lakes, although cadmium (Cd) was not detected in all sediments. Arsenic concentrations were generally higher in brine versus saline lakes (17.0 to 24.0 versus 8.0 to 19.0 mg/kg).
| Saline | Brine | ||||||
|---|---|---|---|---|---|---|---|
| Lake | Taigan | Kholboo | Khadaasan | Tonkhil | Ikhes | Khulma | Duruu |
| ID | S1 | S2 | S4 | H1 | H2 | H3 | H4 |
| Physico-chemical parameters pH EC (mS/cm) | 8.41 | 8.12 | 8.84 | 8.4 | 8.29 | 8.29 | 8.48 |
| 0.97 | 0.75 | 0.65 | 6.87 | 7.3 | 7.1 | 5.23 | |
| Microelements (mg/kg) | |||||||
| As | 19 | 8 | 10 | 20 | 24 | 23 | 17 |
| Cd | - | - | - | - | - | - | - |
| Cu | 42 | 8 | 105 | 84 | 67 | 10 | 33 |
| Cr | 172 | 314 | 148 | 93 | 134 | 75 | 53 |
| Mn | 929 | 464 | 929 | 519 | 945 | 635 | 426 |
| Ni | 80 | 222 | 35 | 68 | 64 | 29 | 15 |
| Pb | 19 | 13 | 13 | <5 | 5 | <5 | <5 |
| Sr | 435 | 449 | 550 | 404 | 326 | 1021 | 5704 |
| Zn | 84 | 47 | 59 | 87 | 82 | 49 | 36 |
| Mineral composition (%) | |||||||
| Quartz | 47.74 | 62.83 | 55.93 | 29.96 | 36.23 | 33.04 | 25.29 |
(-) below the limit of detection
Table 5: Physical parameters, microelements and mineral compositions in superficial sediment samples collected from 14 saline lakes.
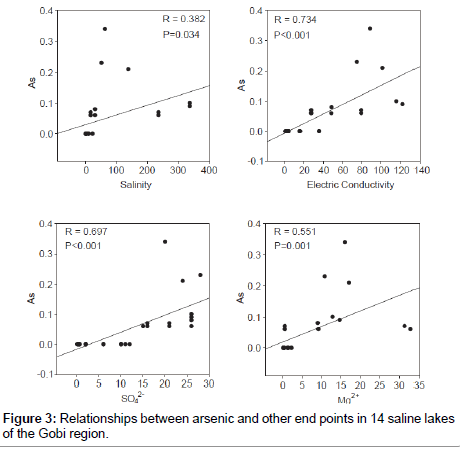
Arsenic concentrations varied among lake types, and were higher (up to 0.34 mg/L) in the water of brine lakes than other saline and brackish lakes. Arsenic was detected in Lakes Kholboo and Khadaasan, but not detected in Lakes Taigan and Mangas (Table 3). High concentrations of arsenic (< 0.25 mg/L) were reported in Lake Shaazgai in north western Mongolia [19]. Regardless, this was apparently the first report of high arsenic concentrations in saline lakes of Mongolia.
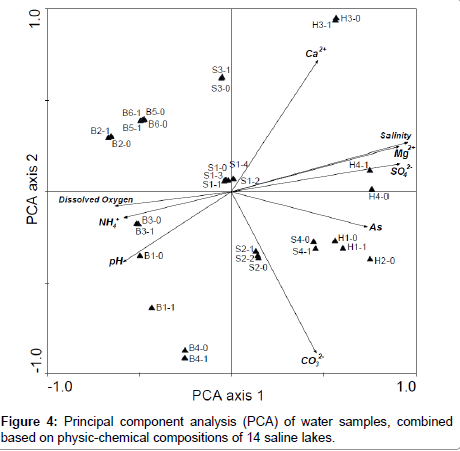
Variation in degree of salinity among salt lakes (Table 2) provided a novel opportunity to study relationships between heavy metals (Table 4) and environmental factors. Concentration of arsenic (As) were positively correlated with salinity, E.C, and concentrations of Mg2+ and SO42 (Figure 3). Based on PCA analysis, chemical composition of brackish, saline and brine water samples were significantly associated with major physical-chemical parameters including pH, dissolved oxygen, salinity, SO42-, As, Mg2+, CO3- and NH4+ (Figure 4). In brine and saline water lakes, salinity was significantly correlated with As, Mg2+, Ca2+, and SO42- (P< 0.05; Figure 4). Furthermore, in the PCA plot, brackish water samples had highers dissolved oxygen, pH and NH4+, but lower As and salinity. Moreover, physico-chemical structures in brine waters were closely associated with Ca2+ (P< 0.05; Figure 4).
Arsenic contamination of lake water is related to both natural and anthropogenic sources [3]. Arsenic is prevalent and widely distributed in Inner Mongolia and the Xinjiang province of China [21-24] these areas have natural arsenic sources and were close to our sampling locations [22,24]. Based on results from other arsenic-contaminated environments [18,22,24-27], we predicted that saline and brine lakes in Gobi region might be affected by geologically based arsenic contamination. However, it was noteworthy that arsenic contamination was not detected in brackish water lakes in the Gobi region. Although causes might be multiple and complex, low salinity brackish water lakes could be a key reason for low arsenic concentrations (Figure 3). It has been reported that a salinity-mediated change in pH might affect arsenic concentration [3].
Conclusion
Although we inferred that high concentrations of arsenic and other heavy metals detected in studied lakes were primarily due to natural geochemical processes, we cannot definitively exclude contributions from anthropogenic sources, including metal mining, coal burning, smelting or livestock activity [3]. Arsenic pollution of surface waters, sediments and soils is commonly reported in mining related areas and heavy metal pollutants are becoming more common in Mongolia due to mining activity [28,29]. Regardless, evaluation of chemistry, particularly for heavy metals, of the salt lakes is critical to understand the environmental condition of the selected salt lakes and provide information for evidence-based food and land management. These increases in arsenic and other heavy metals may cause serious food chain problems [17]. Water chemistry of this study provided insights into the level of pollution and suitability of the water for humans and livestock.
The study focused on the physic-chemical parameters in these salt lakes, including sources and behavior of heavy metals, environmental impacts, and risk management. Furthermore, this study also integrated effects of land usage, human activities, geographical distance, and environmental chemistry to explore relationships between biotic and abiotic factors. More importantly, the study provided important data regarding toxic elements that are important to understanding environmental status and trends in western Mongolia. Finally, this was apparently the first report to characterize various environmental end points in some small lakes in the Gobi region.
Acknowledgments
We thank our Mongolian students (Ankhniibayar, Anudari and Erdenechuluun) from the National University of Mongolia for assistance with providing samples and hydro-parameter data. This study was supported by the thematic project funding of Taiwan-Mongolian Joint Project (NSC101-2923-B-001-003-MY3) from the National Sciences Council of Taiwan and Ministry of Education, Culture, and Science of Mongolia.
References
- Williams WD (1991) Chinese and Mongolian saline lakes: a limnological overview. Hydrobiologia 210: 39-66.
- Egorov AN (1993) Mongolian salt lakes: some features of their geography, thermal patterns, chemistry and biology. Hydrobiologia 267: 13-21.
- Luvsandorj S (1973) Saline lakes of People’s Republic of Mongolia and application possibility of their salts.
- Sumiya J (2011) Salt resources of Mongolia. Salt from present lakes 1.
- Tserensodnom J (2000) Catalogue of the Mongolian Lakes.
- Kasedde H, Kirabira JB, Bäbler MU, Tilliander A, Jonsson S (2014) Characterization of brines and evaporites of Lake Katwe, Uganda. J African Earth Sciences 91: 55-65.
- Volkov I, Zhabina N (1990) The method of determination of inorganic sulfur species in sea water. Okeanologiya 30: 778-782.
- Kalacheva GS, Zhila NO, Volova TG (2002) Lipid and hydrocarbon compositions of a collection strain and a wild sample of the green microalga Botryococcus. Aquatic Ecology 36: 317-330.
- Comecon (1974) Integrated methods of analysis for water quality.
- Reznikov AA, Mulikovskaya EP (1954) Methods of analysis for natural waters. State scientific and technical publishing, Gosgeoltechizdat: Moscow.
- Lurye YY, Rybnikova AI (1963) Chemical analysis of water for industrial use. State scientific and technical publishing, Goskhimizdat: Moscow.
- Bates R (1954) Electronic pH determination. pp: 87-92.
- Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37: 29-38.
- Winston R (2000) Graphical user interface for MODFLOW, Version 4. US Geological Survey Open-File Report 00-315: 1-27.
- Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, et al. (2008) Science and technology for water purification in the coming decades. Nature 452: 301-310.
- Mitamura O, Khadbaatar D, Ishida N (2009) Comparative investigation of chemical and biological characteristics in waters and trophic state of Mongolian lakes. Limnology 11: 17-30.
- Acosta JA, Jansen B, Kalbitz K, Faz A, Martínez-Martínez S (2011) Salinity increases mobility of heavy metals in soils. Chemosphere 85: 1318-1324.
- Bai J, Xiao R, Zhang K, Gao H (2012) Arsenic and heavy metal pollution in wetland soils from tidal freshwater and salt marshes before and after the flow-sediment regulation regime in the Yellow River Delta, China. J Hydrology 450-451: 244-253.
- Isupov VP, Ariunbileg S, Razvorotneva LI, Lyakhov NZ, Shvartsev SL, et al. (2013) Geochemical model of uranium accumulation in Shaazgai-Nuur Lake (Northwestern Mongolia). Doklady Earth Sciences 448: 143-148.
- Pimentel D, Berger B, Filiberto D (2004) Water resources: Agricultural and environmental issues. BioScience 54: 909-918.
- Garelick H, Jones H, Dybowska A, Valsami-Jones E (2008) Arsenic contamination sources. Reviews of Environmental Contamination and Toxicology 197: 17-61.
- Smedley PL, Zhang M, Zhang G, Luo Z (2003) Mobilisation of arsenic and other trace elements in fluviolacustrine aquifers of the Huhhot Basin, Inner Mongolia. Appl Geochem 18: 1453-1477.
- Smidt H, Li P, Wang Y, Dai X, Zhang R, et al. (2015) Microbial community in high arsenic shallow groundwater aquifers in hetao basin of Inner Mongolia, China.
- Sun G (2004) Arsenic contamination and arsenicosis in China. Toxicol Appl Pharmacol 198: 268-271.
- Hudson-Edwards K, Santini J (2013) Arsenic-microbe-mineral interactions in mining-affected environments. Minerals 3: 337-351.
- Vos M, Escudero LV, Casamayor EO, Chong G, Demergasso C (2013) Distribution of microbial arsenic reduction, oxidation and extrusion genes along a wide range of environmental arsenic concentrations.
- Yang N, Welch KA, Mohajerin TJ, Telfeyan K, Chevis DA, et al. (2015) Comparison of arsenic and molybdenum geochemistry in meromictic lakes of the McMurdo Dry Valleys, Antarctica: Implications for oxyanion-forming trace element behavior in permanently stratified lakes. Chemical Geology 404: 110-125.
- Bolormaa O, Baasansuren J, Kawasaki K, Watanabe M, Hattori T (2006) PIXE analysis of heavy metals in water samples from a mining area in Mongolia. 243: 161-166.
- Farrington JD (2005) The impact of mining activities on Mongolia’s protected areas: A status report with policy recommendations. Integ Envir Asse Manag 1: 283-289.
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 5784
- [From(publication date):
February-2017 - Aug 31, 2025] - Breakdown by view type
- HTML page views : 4783
- PDF downloads : 1001